Abstract
The nucleotide sequences of two mitochondrial genes (16S rDNA and COI) were compared among all species of Hediste, including five nominal and two cryptic species (H. atoka and H. diversicolor both consisting of two cryptic species, H. diadroma, H. japonica, and H. limnicola), as well as a newly found undescribed species (H. sp.), to estimate their phylogenetic relationships. The analysis using 16S rDNA sequence supported the monophyly of all five nominal species and H. sp., with no detection of the genetic differentiation between the two cryptic species in both H. atoka and H. diversicolor. However, analysis using COI sequence detected a marked differentiation between the cryptic species, and indicated that the two forms of H. atoka were separated into distinct clades; form A was included in a clade together with H. diversicolor, H. limnicola, and H. sp., whereas form B was included in another clade together with H. diadroma. Based on the topology of our phylogenetic analysis using the combined data set of 16S rDNA and COI, a hypothesis on the evolutionary history of the worldwide speciation in Hediste is proposed. This hypothesis seems to correspond well with the geographical distributions of current species and their morphological differentiation, supporting the previous hypothesis that the unique epitokous swarming and planktic larval development evolved independently in H. diadroma and H. japonica in eastern Asia. We also show that no or few interspecific substitutions have occurred in sequences of nuclear DNA (18S rDNA, 28S rDNA, and histone H3) in Hediste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Estuarine polychaetes of the genus Hediste differ from the closely related genus Neanthes in Nereididae by the presence of simple chaetae derived from falconers in the posterior neuropodia (Fong and Garthwaite 1994; Khlebovich 1996; Sato and Nakashima 2003). The Hediste species show circumboreal distributions in the North Temperate Zone of both the Pacific and Atlantic coasts, and consist of the following five nominal species (Sato 1999, 2004, 2017): H. diversicolor (O. F. Müller, 1776) distributed along both the Northeast and Northwest Atlantic (Smith 1977), H. limnicola (Johnson, 1903) along the Northeast Pacific (Smith 1958), and three species in Asia along the Northwest Pacific, i.e., H. japonica (Izuka, 1908), H. diadroma Sato and Nakashima, 2003, and H. atoka Sato and Nakashima, 2003. Recently, the invasion of the Asian species, H. diadroma, along the North American Pacific coasts was discovered (Nishizawa et al. 2014; Tosuji and Furota 2016). These five species are morphologically very similar to one another. In particular, the three Pacific species, H. diadroma, H. atoka, and H. limnicola are morphologically indistinguishable in sexually immature specimens, whereas H. diversicolor and H. japonica are distinguishable from all other species, even in immature specimens, by their unique chaetal morphology in the neuropodial infra-acicular fascicle: the absence of homogomph spinigers and the presence of homogomph falcigers, respectively (Smith 1958; Sato 2004).
On the other hand, the reproductive and developmental characteristics of the five species of Hediste are markedly diverse (Table 1), showing two contrasting life histories (catadromous and estuary-resident forms) (Sato 2017). The life cycle of the catadromous form is characterized by migration between adult habitats with low salinity and larval habitats with high salinity, with species-specific epitokous metamorphosis in combination with reproductive swarming in adults, and a true planktic larval phase in early development. The typical and non-typical catadromous forms of life cycle are adopted by only two Asian species, H. diadroma and H. japonica, respectively; their epitokous metamorphoses are markedly different from those into the typical heteronereis form prevailing in many marine nereidids (Sato 2017). Another life cycle of the estuary-resident form is characterized by the completion of life cycle within low-salinity regions, without epitokous metamorphosis and reproductive swarming in adults, lacking a true planktic larval phase in early development. This form is widespread in both Pacific (H. atoka and H. limnicola) and Atlantic species (H. diversicolor); H. limnicola, which inhabits not only estuaries but also freshwaters, has very specialized reproductive characteristics such as hermaphroditism, self-fertilization, and viviparity (Smith 1950).
Higher interpopulational genetic differentiation is expected in dioecious species with an estuary-resident life cycle, i.e., H. atoka and H. diversicolor. In fact, this hypothesis has been supported by an electrophoretic study on allozymes of Japanese populations of H. atoka in comparison with those of H. diadroma (Sato and Masuda 1997). Moreover, a recent analysis of the mitochondrial cytochrome oxidase subunit I (COI) DNA sequence suggested cryptic speciation into two parapatric forms had occurred in populations of H. atoka without morphological differentiation: form A constituted all Korean and most Japanese populations except for those in southern Japan, occupied by form B (Tosuji and Sato 2010). A similar degree of interpopulational genetic differentiation has been also revealed in the Atlantic species H. diversicolor, in which the existence of two cryptic species (species A and B) was suggested, by the analyses of allozymes and mitochondrial genes (Hateley et al. 1992; Fong and Garthwaite 1994; Abbiati and Maltagliati 1996; Scaps 2002; Breton et al. 2003; Virgilio and Abbiati 2006; Audzijonyte et al. 2008; Virgilio et al. 2009).
The first study on the phylogenetic relationships among the species of Hediste was carried out by an allozyme electrophoretic analysis, comparing H. diversicolor, H. limnicola, and H. atoka (as H. japonica), showing that H. atoka appears more closely related to H. limnicola than it is to H. diversicolor (Fong and Garthwaite 1994). Thereafter, Sato (1999) and Sato and Nakashima (2003) compared the atokous and epitokous morphology of three Japanese species (H. japonica, H. diadroma, and H. atoka) and proposed the hypothesis that an H. atoka-like species with an estuary-resident life cycle is the ancestral form, from which H. japonica and H. diadroma, with the unique epitoky and catadromous life cycle, were derived independently of each other (see also Sato 2017).
In the present study, we evaluate the phylogenetic relationships between all species of Hediste in the world, based on the nucleotide sequence of parts of two mitochondrial DNA sequences (16S rDNA and COI). We also sequenced three nuclear genes (18S rDNA, 28S rDNA, and histone H3) and show that these molecular markers are generally too conserved to be informative for resolving evolutionary relationships among species of Hediste.
Materials and methods
Collection of specimens
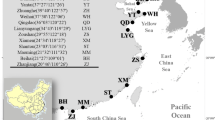
Specimens of Hediste were collected from intertidal flats in estuaries in East Asia (Japan and Korea), North America (USA), and Europe (Denmark, Finland, France, Italy, Germany, Norway, Portugal, and Great Britain) by excavating worms at low tide, fixing the specimens in 75–99% ethanol. These were then used for DNA isolation. As an outgroup species, specimens of Neanthes cf. glandicincta were collected from estuarine mudflats in Kagoshima Bay in southern Japan. Detailed collection data for all materials (256 specimens and one outgroup specimen) are shown in Online Resource 1. Specimens preserved in ethanol were soaked for 30 min in a phosphate-buffered saline (PBS) prior to DNA extraction. Total DNA was extracted using a DNeasy Tissue kit (Qiagen) or Wizard Genomic DNA Purification Kit (Promega). We used a 1–5-mm middle section from each worm for these extractions.
For identification of sexually immature specimens of three Pacific species (H. atoka, H. diadroma, and H. limnicola), which are morphologically indistinguishable, we used the PCR-RFLP method (Tosuji and Sato 2012) and the species-specific multiplex PCR method (Tosuji and Sato 2008).
Primer design, experimental condition, and SNP genotyping for Hediste atoka
The tetra-primer ARMS–PCR procedure (Newton et al. 1989; Ye et al. 2001) was used to detect the genotype of the varieties of H. atoka (forms A and B) at an SNP locus using COI gene sequences (Table 2 and Fig. 1). Primers were designed using the primer design computer program Primer1 (Collins and Ke 2012) and slightly modified. The reaction mixture (5 μL) consisted of 0.125 U Taq DNA polymerase (BioAcademia, Japan), 0.5 μL 10× reaction buffer, 0.4 μL 2.5 mM dNTP mixture, 0.35 μL primers (Table 2), and 0.1–0.5 ng template DNA with/without 0.5 M betaine solution (Sigma-Aldrich). The mixing ratio of the primers was as follows: Fi:Ri1:Ri2:299–325FO1:299–325FO2:590-567RO1:590–567RO2:590–567RO3:590–567RO4 = 40:64:16:4:2:6:6:3:3. The cycling program was as follows: initial denaturation at 95 °C for 2 min, followed by 37 cycles of 95 °C for 1 min, 56.5 °C for 1 min, 72 °C for 1 min, and 72 °C for 2 min. An aliquot of the PCR product was separated by a 5% polyacrylamide gel using TAE buffer and detected by staining with SYBR Gold nucleic acid gel stain (Molecular Probes). The alleles were detected on the basis of the estimated amplicon sizes, 112 bp (form A), 221 bp (form B), and 291 bp (outer amplicon) (Fig. 2).
ARMS primers to detect genotype of the varieties of Hediste atoka (forms A and B). ARMS outer primer sequences are indicated with gray shade, and inner primer sequences are indicated highlighted with shade (arrow for orientation). The primers were designed from accession numbers AB603842–AB603870 (form A) and AB603871–AB603887 (form B)
DNA sequencing
The 16S rDNA fragments were amplified using the primers 16SarL and 16SbrH (Palumbi 1996) or with 12412F primer (present study) combined with 16SbrH primer. The COI gene was amplified either using the LCO1490 and HCO2198 primers (Folmer et al. 1994) or with the LCO1490 primer combined with HCO709 primer (Blank et al. 2008). The amplifications were performed using an Ex Taq (Takara Bio, Japan). The cycling profile was as follows: initial denaturation at 95 °C for 2 min; 38 cycles at 95 °C for 30 s, then at 45 °C for 45 s and at 72 °C for 1 min, with a final extension step at 72 °C for 7 min. All products were verified on a 1% agarose gel and purified with the Plus Gel Elution Kit (GMbiolab). The cycle sequencing reaction was carried out using BigDye 3.1 (Applied Biosystems) as a direct sequencing method with the amplification primers or sequencing primers (12415SF and 12897SR for 16S rDNA, 17SL and 733SH for COI) (present study) and all sequences were checked and corrected by visual inspection. The nuclear 18S rDNA was amplified using the 18SF35 and 18SR1779 primers (Struck et al. 2002). The amplifications were performed using an Ex Taq. The cycling profile was as follows: initial denaturation at 95 °C for 2 min; 38 cycles at 95 °C for 30 s, then at 45 °C for 30 s and at 72 °C for 2 min, with a final extension step at 72 °C for 7 min. The amplicons were verified on a 1% agarose gel and purified with the Plus Gel Elution Kit. Then, they were sequenced using the direct sequencing method with 18SF35, 18F509, 18F997, 18R925, 18R1256, and 18SR1779 primers (Struck et al. 2002) and all sequences were checked and corrected by visual inspection. The nuclear 28S rDNA (D1, D4-7b, and D9-10 regions) and histone H3 were amplified and sequenced as same as the method for 18s rDNA using the following primers; 28SD1F/28SD1R (Brown et al. 1999), 28Srd4.8a/28Srd7b1 (Whiting 2002), 28SD9–10F/28SD9–10R (Hills and Dixon 1991) and H3F/H3R(1) (Colgan et al. 2000). The primers used in this study are displayed in Table 2.
The base-called data obtained from an ABI DNA sequencer were assembled to get contig sequences using the sequence analysis software Genetyx-Mac ver. 19 for manual editing.
The nucleotide sequences were deposited in DDBJ (accession numbers LC323003–LC323104, LC323646–LC323647, LC378710–LC378715, LC380654–LC380663, LC381232–LC381234, and LC381864–LC381865).
Sequence alignment and data analysis
All sequences were aligned using the multiple sequence alignment software MAFFT ver. 7.310 (Katoh and Standley 2013) with L-INS-i method. When samples had the same sequence, the duplicate sequences were removed.
The best-fitting model of nucleotide substitution for a maximum likelihood (ML) tree was selected by ModelFinder Plus (Kalyaanamoorthy et al. 2017) using Akaike information criterion (AIC) (Akaike 1973). This is general time reversible model (GTR) (Tavaré 1986) using a free rate model with two categories (+R2) (Yang 1995; Soubrier et al. 2012) that generalizes the +G model by relaxing the assumption of gamma-distributed rates for the 16S rDNA. The model selected was TPM2 using a discrete gamma distribution (+G) and by assuming that a certain fraction of sites were evolutionarily invariable (+I) for COI.
MrModeltest v. 2.3 (Posada and Crandall 1998) was used to select Bayesian inference (BI) tree. The best substitution models were GTR +G +I, for 16S rDNA and COI genes as determined by AIC and hierarchical likelihood ratio tests (hLRTs). Phylogenetic inference was conducted for sequences of 16S rDNA and COI individually, and this data was combined to create a concatenated data set. Each of the protein-coding and non-coding nucleotide sequence regions was used as separate partitions.
IQ-tree 1.5.4 (Nguyen et al. 2015) was used to obtain phylogenetic trees constructed with ML supported with 1000 bootstrap. MrBayes v. 3.1.6 (Ronquist et al. 2012) was used to obtain BI of phylogenetic trees. Eight Metropolis-coupled Markov chain Monte Carlo algorithms were run, starting with random initial trees and sampling every 100 generations. The analyses were allowed to continue until the average standard deviation of split frequencies (ASDSF) reached below 0.01. As a result of this task, for 16S rDNA, 1,000,000 (ASDSF = 0.009010), for COI, 11,500,000 (ASDSF = 0.008392), for the combined data, 1,000,000 (ASDSF = 0.006127) generations were obtained. The first 25% of the sampled trees were excluded as burn-in samples, and burn-in value for each analysis was assessed using the software Tracer1.6 (Rambaut et al. 2014).
The maximum parsimony (MP) trees were analyzed by PAUP* 4.0 (Swofford 2003) using the heuristic search with 1000 bootstrap replicates. The phylogenetic network was constructed using FigTree ver.1.4.3.
We used Arlequin version 3.512 (Excoffier and Lischer 2010) to calculated pairwise fixation index (FST) values (Weir and Cockerman 1984) to estimate the level of genetic divergence between populations.
Results
Detection of an unknown cryptic species
In both analyses using 16S rDNA and COI sequences, the specimens collected from the Han River System in western Korea (a total of 13 specimens) constituted a clearly separated clade from any other of the previously known species or forms. Therefore, we designated this clade as Hediste sp., which seemed to be an unknown cryptic species.
Phylogenetic analysis using 16S rDNA sequence
The 16S rDNA dataset consisted of 57 nucleotide sequences (haplotypes) from 160 specimens (one of them belonged to the outgroup species) containing 429 characters, of which 322 were conserved across all taxa (75.1%), 99 were variable (23.1%) and 50 were parsimony informative (11.7%). When the outgroup was excluded, the dataset had 344 conserved characters (80.2%), 77 variable characters (17.9%), and 47 parsimony informative characters (11.0%).
In all of the ML, BI, and MP trees, haplotypes from each of all six species (H. atoka, H. diadroma, H. diversicolor, H. japonica, H. limnicola, and H. sp.) constituted independent species-specific clades, supporting the monophyly of these species (Fig. 3). The clade of H. sp. was consistently recovered sister to H. diversicolor including both species A and B. The clade of H. limnicola was recovered sister to H. atoka including both forms A and B, in the ML and BI trees. The clade of H. diadroma was recovered sister to H. japonica in the BI tree.
Maximum likelihood (left), Bayesian inference (middle), and maximum parsimony (right) phylograms for Hediste species derived from the analyses of 429-bp fragment of mitochondrial 16S rDNA sequence. Neanthes cf. glandicincta is used as an outgroup for rooting the tree. In the maximum likelihood and maximum parsimony tree, bootstrap values from 1000 replications are indicated on the branches and only greater than or equal to 50% are shown. In the Bayesian tree, posterior probability values are indicated on the branches and only greater than or equal to 0.5 are shown. The scale bar corresponds to the substitutions per nucleotide site. The letters in brackets (A or B) indicate species A or B for H. diversicolor, and form A or B for H. atoka, respectively. The numbers in brackets indicate the number of individuals (no number means single individual)
In all trees, the large clade of H. atoka was subdivided into several groups, which, however, did not support the monophyly of the forms A and B. The following two common haplotypes were shared by the two forms of H. atoka: haplotype K16-29 (LC323060) obtained from each of four specimens of the forms A and B; haplotype K16-30 (LC323061) from nine specimens of form A and 10 specimens of form B.
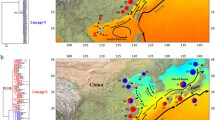
Within H. atoka, all 12 haplotypes of form B, including the two common haplotypes, constituted a monophyletic clade, together with four haplotypes specific to form A (haplotype K16-05, K16-15, K16-19, and K16-27), which were all obtained in southern Japan, from western Kyushu to Okinawa-jima Island, including new records from four islands in the Ryukyu Islands (Tanegashima, Yakushima, Okinawa-jima, and Ishigaki-jima); here, the population of form A belonging to this clade is designated as the southern population of form A of H. atoka. The common haplotypes shared by the two forms were distributed in western and southern Kyushu and the northern and middle Ryukyu Islands in southern Japan, which is within the range of the southern population of form A, mostly overlapping that of form B. On the other hand, the other 14 haplotypes of form A, which were obtained from a wide area in Japan and Korea, from northern Hokkaido to northern Kyushu, formed a paraphyletic group including six subdivided clades in all of ML, BI, and MP trees; here, this group is designated as the northern population of H. atoka (Fig. 4).
Distribution of all haplotypes of the forms A and B of Hediste atoka. Two haplotypes of the mitochondrial 16S rDNA sequence (K16-29 and K16-30) which were shared by the two forms (inset figure). Form B of H. atoka is distributed only in southern Japan (southwestern Kyushu and the northern and middle Ryukyu Islands), whereas form A of this species has a wide distribution covering the whole of Japan (from Hokkaido to the Ryukyu Islands) and Korea. The gray area shows the distributional range of form B of H. atoka based on Tosuji and Sato (2010) and the present study. Dotted line indicates the border between the range of the northern and southern populations of form A of H. atoka. Dashed line indicates the southern limit of the southern population of form A of H. atoka. An asterisk indicates new records of form A of H. atoka in the present study
The clade of H. diversicolor was also subdivided into several groups, which did not support the monophyly of species A and B designated by Audzijonyte et al. (2008).
The values of pairwise FST calculated between all taxa indicated that the differentiation between the form A and B of H. atoka (0.23231) and species A and B of H. diversicolor (0.37637) are extremely low in comparison with all other combinations (0.64027–0.99534). The Korean undescribed species H. sp. was relatively close to form A of H. atoka (0.73724) and species A of H. diversicolor (0.77227) when compared with the other species (0.93003–0.99813) (Table 3).
Phylogenetic analysis using COI sequence
The COI dataset consisted of 113 nucleotide sequences (haplotypes) from 178 specimens (one of them belonged to the outgroup species) with 570 characters, of which 357 were conserved (62.6%), 213 were variable (37.4%), and 171 were parsimony informative (30.0%). When the outgroup was excluded, the dataset had 370 conserved characters (64.9%), 200 variable characters (35.1%), and 171 parsimony informative characters (30.0%).
All of the ML, BI, and MP trees showed almost the same topology (Fig. 5). Haplotypes of each of the four species (H. diversicolor, H. limnicola, H. japonica, H. sp.) constituted an independent species-specific clade, supporting the monophyly of these species, with the basal clade comprising H. japonica. The clade of H. diversicolor was subdivided into two monophyletic groups corresponding to two cryptic species (species A and B designated by Audzijonyte et al. 2008). The clade of H. sp. was a sister clade of H. diversicolor.
Maximum likelihood (left), Bayesian inference (middle), and maximum parsimony (right) phylograms for Hediste species derived from the analyses of the 570-bp fragment of mitochondrial COI sequence. Neanthes cf. glandicincta is used as an outgroup for rooting the tree. In the maximum likelihood and maximum parsimony tree, bootstrap values from 1000 replications are indicated on the branches and only greater than or equal to 50% are shown. In the Bayesian tree, posterior probability values are indicated on the branches and only greater than or equal to 0.5 are shown. The scale bar corresponds to the substitutions per nucleotide site. The letters in brackets (B or D) indicate forms B of H. atoka or H. diadroma, respectively. The numbers in brackets indicate the number of individuals (no number means single individual)
Conversely, haplotypes of each of the two Asian species (H. diadroma and H. atoka) did not constitute a species-specific monophyletic clade, in contrast to the analysis of the 16s rDNA sequence. Haplotypes of the form A of H. atoka constituted a monophyletic clade including several subdivided clades, in which haplotypes of the southern population constituted a monophyletic clade exclusively, whereas those of the northern population constituted a paraphyletic group. The clade form A of H. atoka formed a sister group together with the clade of H. limnicola in the ML tree, though they constituted a paraphyletic group within a large monophyletic clade including both the form A of H. atoka and H. limnicola in the BI and MP trees. Haplotypes of both the form B of H. atoka and H. diadroma constituted another large monophyletic clade. The following four haplotypes were shared by form B of H. atoka and H. diadroma: haplotype KC-034 (AB603872 and AB996698) obtained from three specimens of form B of H. atoka and a specimen of H. diadroma; haplotype KC-039 (AB603887 and AB996709) from a specimen of form B of H. atoka and three specimens of H. diadroma; haplotype KC-044 (AB603878 and AB996712) from a specimen of form B of H. atoka and two specimens of H. diadroma; haplotype KC-049 (AB603876 and AB996700) from a specimen of form B of H. atoka and a specimen of H. diadroma. Most of these common haplotypes were distributed in the western coast of Kyushu, southern Japan, where the distributions of the two species overlap (Fig. 6); all specimens of form B of H. atoka have been obtained from a limited area in southern Japan, from southwestern Kyushu to Okinawa-jima Island in the middle of the Ryukyu Islands, with new records from 4 islands in the Ryukyu Islands (Yakushima, Kikaijima, Tokunoshima, and Okinawa-jima). On the other hand, all specimens of H. diadroma have been obtained from a wide range in Japan, from southern Hokkaido to southern Kyushu, without any records from the Ryukyu Islands as shown in the previous study (Sato and Nakashima 2003).
Distribution of all haplotypes of form B of Hediste atoka and H. diadroma. Four haplotypes of the mitochondrial COI gene (KC-034, KC-039, KC-044, and KC-049) which were shared by form B of H. atoka and H. diadroma in southern Japan (inset figure). Form B of H. atoka is distributed only in southern Japan (southwestern Kyushu and the northern and middle Ryukyu Islands), whereas H. diadroma has a wide distribution covering Honshu and Kyushu in Japan. The gray area shows the distributional range of form B of H. atoka, based on Tosuji and Sato (2010) and the present study. Dashed line indicates the southern border of the range of H. diadroma. An asterisk indicates new records of form B of Hediste atoka in the present study
Three Pacific taxa (form A of H. atoka, H. limnicola, and H. sp.) and an Atlantic species (H. diversicolor) formed a large monophyletic group in the ML and MP trees (Fig. 5).
The values of pairwise FST calculated between all taxa indicated that the differentiation between form B of H. atoka and H. diadroma (0.11177) is extremely low. On the other hand, the differentiation of pairwise FST values between the two forms (A and B) of H. atoka (0.73910) and between the two species (A and B) of H. diversicolor (0.63493) are comparable to those between all other combinations of distinct species (0.62966–0.92664). The Korean undescribed species H. sp. was relatively close to form A of H. atoka (0.75694) in comparison with all other taxa (0.87617–0.96919) (Table 3).
Phylogenetic analysis using the combined data set of COI and 16S rDNA sequences
The combined dataset involved 77 nucleotide sequences (haplotypes) from 101 specimens (both genes from all taxa; one of them belonged to the outgroup species) containing 999 characters, of which 691 were conserved (69.2%), 302 were variable (30.2%), and 223 were parsimony informative (22.3%). When the outgroup was excluded, the dataset had 728 conserved characters (72.9%), 265 variable characters (26.5%), and 221 parsimony informative characters (22.1%).
All of the ML, BI, and MP trees showed essentially the same topology (Fig. 7), which is similar to that of the analysis using the COI gene alone. Haplotypes of each of five species (H. diadroma, H. diversicolor, H. limnicola, H. japonica, H. sp.) constituted species-specific clades, supporting the monophyly of these species, with the basal clade being H. japonica. The clade of H. diversicolor was subdivided into two monophyletic groups corresponding to species A and B designated by Audzijonyte et al. (2008). The clades of H. diversicolor and H. sp. formed a sister group in the ML and BI trees.
Maximum likelihood (left), Bayesian inference (middle), and maximum parsimony (right) phylograms for Hediste species derived from the analyses of the combined data of sequences of the 429-bp fragment of 16S rDNA and 429-bp fragment of COI sequences. Neanthes cf. glandicincta is used as an outgroup for rooting the tree. In the maximum likelihood and maximum parsimony tree, bootstrap values from 1000 replications are indicated on the branches and only greater than or equal to 50% are shown. In the Bayesian tree, posterior probability values are indicated on the branches and only greater than or equal to 0.5 are shown. The scale bar corresponds to the substitutions per nucleotide site. The numbers in brackets indicate the number of individuals (no number means single individual)
Haplotypes of H. atoka did not form a clade. Haplotypes of form A of H. atoka constituted a monophyletic clade (the ML and BI trees) or a paraphyletic group (the MP tree), consisting of a monophyletic clade together with the clade of H. limnicola. In any case, the form A of H. atoka was subdivided into several clades, in which haplotypes of the southern population constituted a monophyletic clade exclusively, whereas those of the northern population constituted a paraphyletic group. On the other hand, haplotypes of form B of H. atoka form a paraphyletic group with respect to H. diadroma.
Three Pacific taxa (form A of H. atoka, H. limnicola, and H. sp.) and two Atlantic taxa (species A and B of H. diversicolor) formed a large monophyletic group in the ML and BI trees.
The values of pairwise FST calculated between all taxa indicated that differentiation between the two species (A and B) of H. diversicolor was lowest (0.58821), and that the differentiation between the two forms (A and B) of H. atoka (0.69496) and between the form B of H. atoka and H. diadroma (0.68331) were relatively low in comparison with all other combinations (0.70350–0.93679) (Table 3). The Korean undescribed species H. sp. was relatively close to the form A of H. atoka (0.77316) in comparison with all other taxa (0.84814–0.96770).
Nucleotide sequence of nuclear DNA
The nucleotide sequences of 18S rDNA were examined for a total of 48 individuals from seven taxa (Online Resource 2). They contained 1682–1683 nucleotides. Most sequences were exactly identical as haplotype K18-01, except sequences in four individuals of form B of H. atoka collected from the same location (Kaminokawa River, Kagoshima, Japan), which belonged to another haplotype K18-02 with only one nucleotide (0.06%) replaced and one nucleotide (0.06%) inserted.
The nucleotide sequences of the D1 region of 28S rDNA were examined for a total of 21 individuals from six taxa (Online Resource 2). They contained 338–339 nucleotides. The sequences from most taxa were identical to haplotype K28D1-01, except sequences from H. limnicola, which belonged to another haplotype K28D1-02 with only one nucleotide (0.3%) inserted.
The nucleotide sequences of the D4–7b region of 28S rDNA was examined for a total of 10 individuals from six taxa (Online Resource 2). They contained 802–804 nucleotides, constituting three haplotypes: the nucleotide sequences of both species A and B of H. diversicolor belonged to haplotype K28D4-7-01; those of form A of H. atoka, H. diadroma, and H. limnicola belonged to haplotype K28D4-7-02; and those of H. japonica belonged to haplotype K28D4-7-03. Only one nucleotide (0.12%) was variable between K28D4-7-02 and K28D4-7-03. K28D4-7-01 had four nucleotide (0.50%) changes and two nucleotide (0.25%) deletions compared to K28D4-7-02.
The nucleotide sequences of the D9–10 region of 28S rDNA was examined for a total of 14 individuals from both forms A and B of H. atoka and H. diadroma (Online Resource 2). They contained 624 nucleotides, constituting two haplotypes: the nucleotide sequences of both forms A and B of H. atoka belonged to haplotype K28D9-10-01; those of H. diadroma belonged to another haplotype K28D9-10-02, with only one nucleotide (0.16%) replaced.
The nucleotide sequences of histone H3 were examined for a total of 41 individuals from seven taxa (Online Resource 2). They contained 291 nucleotides, constituting five haplotypes: the nucleotide sequences of both forms A and B of H. atoka belonged to haplotype KH3-01; those of a part of specimens of H. diadroma belonged to haplotype KH3-02; those of the other specimens of H. diadroma, both species A and B of H. diversicolor and H. limnicola belonged to haplotype KH3-03; those of H. japonica belonged to haplotypes KH3-04 and KH3-05. Only four nucleotides (1.37%) were replaced between the five haplotypes. The amino acid sequences derived from the all haplotypes were identical.
Discussion
Molecular phylogeny of Hediste based on mitochondrial DNA sequences
The present study is the first attempt to estimate the phylogenetic relationship among all species of Hediste currently known in the world, based on the analyses of two mitochondrial DNA sequences. Our analysis using the 16S rDNA sequence supported the monophyly of each of all nominal species established in previous taxonomic studies (e.g., Sato and Nakashima 2003), though it did not support the monophyly of each of two cryptic species in H. diversicolor (Audzijonyte et al. 2008) and two forms of H. atoka (Tosuji and Sato 2010). In another analysis using the COI gene, however, each of the two cryptic species in H. diversicolor formed a monophyletic group as shown in Audzijonyte et al. (2008), and more surprisingly, the two forms of H. atoka were completely separated into distinct clades; form A of H. atoka was included in a large clade together with H. limnicola H. diversicolor, and H. sp., whereas form B of H. atoka was included in another large clade together with H. diadroma. The different results of the two sequences (16S rDNA and COI) analyzed here may be caused by the different rates of nucleotide substitution between the genes; the rate may be faster in COI than in 16S rRNA. This is supported by the fact that the number of haplotypes is larger in the COI than in 16S rDNA in most taxa, with an extreme case in H. diadroma, where the frequency of haplotypes of the COI gene was 4.59 times larger than that of the 16S rDNA sequence (Table 4).
Our data revealed that two common haplotypes of the 16S rDNA sequence were shared by the southern population of form A and the form B of H. atoka, and four common haplotypes of the COI were shared by form B of H. atoka and H. diadroma, showing that most of these interspecific common haplotypes are distributed in southern Japan, from western Kyushu to the Ryukyu Islands. This result suggests that the speciation between the two forms of H. atoka, and the subsequent one between form B of H. atoka, and H. diadroma occurred around western Kyushu or the Ryukyu Islands in southern Japan.
The high values of pairwise FST between the undescribed Korean species H. sp. and the other nominal species indicate that H. sp. is well differentiated genetically from the other species. Hediste sp. is currently known only from the Han River estuary in Korea, and is morphologically indistinguishable from H. atoka in a sexually immature stage, but distinguishable from it in a mature stage, where H. sp. shows a unique epitokous metamorphosis during reproductive swarming (our unpublished data). The taxonomic description of this species will be provided in another paper.
Hypothesis of the speciation in Hediste
We propose here a hypothesis on the probable evolutionary history of the worldwide speciation in Hediste, based on the topology of our phylogenetic analysis using the combined data set of two mitochondrial DNA sequences (COI and 16S rDNA) (Fig. 7), which seems to correspond well to the geographical distributions of current species and the extent of their morphological differentiation (Fig. 8). Our result suggests that the origin of Hediste seems to be located in the Northwest Pacific (i.e., eastern Asia). The oldest differentiation probably occurred in the ancient Yellow Sea dividing the strain of H. japonica from the stem strain. Hediste japonica, currently distributed mainly along the Korean coast of the Yellow Sea, with several isolated distributions in western Japan (Sato and Sattmann 2009; Sato 2017), is clearly distinguishable from all other congeners by the morphology of neurochaetae, neuropodial postchaetal lobes, and a unique epitokous metamorphosis (Sato and Nakashima 2003).
Hypothesis of the phylogeny of Hediste, based on the topology of the phylogenetic analysis using the combined data set of two mitochondrial DNA sequences (16S rRNA and COI). Geographical distributions of the current seven species are shown at the top. AtoA, form A of H. atoka; AtoB, form B of H. atoka; Dia, H. diadroma; DivA, species A of H. diversicolor; DivB, species B of H. diversicolor; Jap, H. japonica; Lim, H. limnicola; U, undescribed species (H. sp.). Dotted and dashed lines indicate the presence of common haplotypes of 16S rDNA and COI sequences, respectively. Stars indicate reproductive characteristics (Sato 1999; Sato and Nakashima 2003; our unpublished data): black star, reproduction with some epitokous metamorphosis with (1) or without (2) addition of epitoke-specific sesquigomph spinigers; white star, reproduction without any epitokous metamorphosis; s, specialized mode with hermaphrodite, self-fertilizing, and viviparity. Rectangles indicate the different morphology in lower neurochaetae of atokes (Smith 1958; Sato and Nakashima 2003): present (black) or absent (white) of homogomph spinigers (a), heterogomph spinigers (b), homogomph falcigers (c); no morphological characteristic of H. sp. is included
Thereafter, the dichotomous differentiation of the stem strain seems to have occurred to produce two stem lineages around southern Japan; the direct descendents of stem lineages 1 and 2 are the forms A and B of H. atoka, respectively. The genetic differentiation between forms A and B is comparable to that between the distinct species, with high pairwise FST value (0.69054) (Table 3). The fact that the southern population of form A and the form B share some common haplotypes of 16s rDNA sequence suggests that the form B was derived from the southern population of form A. No difference has been detected between the two forms of H. atoka in adult morphology, reproduction lacking epitokous metamorphosis, and early development without a true planktic phase, though they may differ slightly in spawning behavior (Sato 2017). The highest similarity in these biological aspects implies that both forms of H. atoka have preserved the original characteristics of the common ancestor Hediste since the separation of the two stem lineages. In the present study, three sympatric habitats of the two forms were newly found in southern Kyushu (Kagoshima Bay) and one was found in the Ryukyu Islands (Okinawa-jima Island) (Fig. 4), and significantly, they were the first sympatric habitats to be found.
From stem lineage 1, the Korean undescribed species (H. sp.) and two circumboreal species (H. diversicolor and H. limnicola), which are distributed in the North Atlantic and the North West Pacific, respectively, seem to be descended as follows: (1) the common ancestor of H. diversicolor which is morphologically distinguishable from any other congener by the absence of homogomph spinigers in lower neurochaetae (Smith 1958), and H. sp. (morphology not yet described) are descended from the Asian stem lineage 1 in an earlier period; then, H. diversicolor was descended from the ancestor that invaded the North Atlantic across the Arctic basin in a period with climatic warming to allow the successful trans-Arctic dispersal, while H. sp. was descended from the resident ancestor in Asia; (2) H. limnicola, which is morphologically indistinguishable from H. atoka, was relatively recently descend from the Asian ancestor that invaded the North East Pacific along the Bering Strait in another period to allow for successful boreal dispersal. In H. limnicola, the most specialized reproductive mode with hermaphrodite, self-fertilizing, and viviparity evolved (Smith 1950). Because this species seems to have originated from a small branching population separated from the large Asian one, such a rapid and drastic differentiation in the reproductive mode may have occurred, accelerated by genetic drift.
The circumboreal speciation caused by the trans-Arctic dispersal has been suggested in various taxa including many familiar taxa such as the algae Laminaria, the bivalves Mytilus and Macoma, the gastropods Littorina and Nucella, and echinoderm Asterias (Vermeji 1991; Väinölä 2003): much of the present fauna in North Atlantic littoral and shallow waters is of Pacific ancestry; following the long-term isolation of the northern Atlantic and Pacific biota from the early Cenozoic period, the opening of the Bering Strait in the Pliocene ca. 3.5 million years ago allowed an exchange of the independently evolved boreal taxa across the current Arctic basin; due to climatic cooling since the Pliocene, the trans-Arctic dispersal rout has been largely closed again, followed by effective isolation between the Atlantic and Pacific populations, which were differentiated as vicarious distinct species.
Conversely, H. diadroma, which is widely distributed throughout Japan except for the northern and southern extremes (northern Hokkaido and the Ryukyu Islands, respectively) (Sato and Nakashima 2003; Tosuji and Sato 2012), seems to have been descended from stem lineage 2 in an area around the western coast of Kyushu most recently; this species is so closely related with form B of H. atoka that these taxa are indistinguishable by the comparison of COI gene alone in the present study; they are also almost indistinguishable in the morphology of sexually immature worms (atokes), but H. diadroma shows a unique epitokous metamorphosis (Sato and Nakashima 2003).
Our hypothesis based on the present molecular data corroborate the previous hypothesis on the phylogeny of three Asian species (H. japonica, H. diadroma, and H. atoka) based on their characteristic morphology and reproductive and developmental modes: an H. atoka-like species with an estuary-resident life cycle is the ancestral form, from which H. japonica and H. diadroma with the unique epitoky and catadromous life cycle were derived independently of each other (Sato 1999; Sato and Nakashima 2003; Sato 2017). At present, we conclude that the epitoky of Hediste, which is markedly different from the typical heteronereis form prevailing in many marine nereidids, seems to have evolved independently three times in H. japonica, H. diadroma, and H. sp. only in Asia.
Interspecific differentiation in nuclear DNA sequences of Hediste
There is no previous study on the interspecific comparison of sequences of nuclear DNA in Hediste. Our result demonstrates that five nuclear DNA sequences (18S rDNA, three regions of 28S rDNA and histone H3) are almost invariable, with very few inter- and intra-specific differences detected. This result is in contrast to that marked interspecific differences were detected in the sequences of 18S and 28S rDNA between congeneric species in Spionidae (Abe et al. 2016).
Our analyses of 28S rDNA and histone H3 could detect some interspecific differences. The sequences of D9–10 region of 28S rDNA showed a slight but consistent difference between H. atoka (both forms A and B) and H. diadroma in agreement with our result on the sequences of the mitochondrial gene of not COI but 16S rDNA.
On the other hand, according to our result on D4–7b region of 28S rDNA, the following three groups were distinguishable: H. diversicolor, H. japonica, and the others (H. atoka, H. diadroma, H. limnicola). This grouping is inconsistent with the result on D9–10 region.
The sequences of histone H3 were most variable, showing a total of five haplotypes. A consistent difference between H. atoka (both forms A and B) and H. diadroma was detected as the result on D9–10 region.
Glasby et al. (2013) demonstrated that the sequences of histone H3 could detect the interspecific difference between morphologically very similar congeneric species in three genera of Nereididae (Nereis, Pseudonereis, Perinereis), as well as the sequences of mitochondrial COI, though the mitochondrial COI gene appears to have evolved more rapidly than nuclear histone H3 gene. However, our result indicates that the sequences of histone H3 and other regions of nuclear DNA are much less differentiated between species of Hediste in comparison with other genera of Nereididae and Spionidae. Therefore, it seems that we cannot estimate the phylogenetic relationship between species of Hediste by the data of nuclear DNA sequences.
References
Abbiati M, Maltagliati F (1996) Allozyme evidence of genetic differentiation between populations of Hediste diversicolor (Polychaeta: Nereididae) from the western Mediterranean. J Mar Biol Ass UK 76:637–647
Abe H, Kondoh T, Sato-Okoshi W (2016) First report of the morphology and rDNA sequences of two Pseudopolydora species (Annelida: Spionidae) from Japan. Zool Sci 33:650–658
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csáki F (eds) 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, USSR, September 2–8, 1971. Akadémiai Kiadó, Budapest, pp 267–281
Audzijonyte A, Ovcarenko I, Bastrop R, Väinölä R (2008) Two cryptic species of the Hediste diversicolor group (Polychaeta, Nereididae) in the Baltic Sea, with mitochondrial signature of different population histories. Mar Biol 155:599–612
Bartels-Hardege HD, Zeeck E (1990) Reproductive behavior of Nereis diversicolor (Annelida: polychaeta). Mar Biol 106:409–412
Blank M, Laine AO, Jürss K, Bastrop R (2008) Molecular identification key based on PCR/RFLP for three polychaete sibling species of the genus Marenzelleria, and the species’ current distribution in the Baltic Sea. Helgoland Mar Res 62:129–141
Breton S, Dufresne F, Desrosiers G, Blier PU (2003) Population structure of two northern hemisphere polychaetes, Neanthes virens and Hediste diversicolor (Nereididae), with different life-history traits. Mar Biol 142:707–715
Brown S, Rouse G, Hutchings P, Colgan D (1999) Assessing the usefulness of histone H3, U2 snRNA and 28S rDNA in analyses of polychaete relationships. Aust J Zool 47:499–516
Christensen B (1980) Animal cytogenetics, vol 2, Annelida. Gebrüder Borntraeger, Berlin
Colgan DJ, Ponder WF, Eggler PE (2000) Gastropod evolutionary rates and phylogenetic relationships assessed using partial 28S rDNA and histone H3 sequences. Zool Scr 29:29–63
Collins A, Ke X (2012) Primer1: primer design web service for tetra-primer ARMS-PCR. Open Bioinforma J 6:55–58
Dales RP (1950) The reproduction and larval development of Nereis diversicolor O. F. Müller. J Mar Biol Ass UK 29:321–361
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Res 10:564–567
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplication of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Fong PP, Garthwaite RL (1994) Allozyme electrophoretic analysis of the Hediste limnicola–H. diversicolor–H. japonica species complex (Polychaeta: Nereididae). Mar Biol 118:463–470
Fong PP, Pearse JS (1992) Photoperiodic regulation of parturition in the self-fertilizing viviparous polychaete Neanthes limnicola from central California. Mar Biol 112:81–89
Glasby CJ, Wei NV, Gibb KS (2013) Cryptic species of Nereididae (Annelida: Polychaeta) on Australian coral reefs. Invertebr Syst 27:245–264
Hateley JG, Grant A, Taylor SM, Jones NV (1992) Morphological and other evidence on the degree of genetic differentiation between population of Nereis diversicolor. J Mar Biol Ass UK 72:365–381
Hillis DM, Dixon MT (1991) Ribosomal DNA: molecular evolution and phylogenetic inference. Q Rev Biol 66:411–453
Izuka A (1908) On the breeding habit and development of Nereis japonica n. sp. Annot Zool Jpn 6:295–305
Johnson HP (1903) Fresh-water nereids from the Pacific coast and Hawaii, with remarks on fresh-water Polychaeta in general. Mark anniversary volume. New York, Henry Holt, 205–223, P.XVI–XVII
Kagawa Y (1955) Note on the optimum salinities, studied in the adult and larva of the brackish-water polychaete worm, Nereis japonica. J Gakugei Coll Tokushima Univ Nat Sci 6:11–16 (in Japanese with English summary)
Kalyaanamoorthy S, Minh BQ, Wong TFK, von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780
Khlebovich VV (1996) Fauna of Russia and neighbouring countries. Polychaetous annelids. Volume III. Polychaetes of the family Nereididae of the Russian seas and the adjacent waters. St. Petersburg: Nauka Publishing House (in Russian with English summary)
Müller OF (1776) Zoologiae Danicae. Prodromus, seu animalium daniae et norvegiae indigenarum characteres, Nomina, et Synonyma. Imprimis Popularium, Copenhagen
Newton CR, Graham A, Heptinstall LE, Powell SJ, Summers C, Kalshekerl N, Smith JC, Markham AF (1989) Analysis of any point mutation in DNA. The ampliflcation refractory mutation system (ARMS). Nucleic Acids Res 17:2503–2516
Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274
Nishizawa R, Sato M, Furota T, Tosuji H (2014) Cryptic invasion of Northeast Pacific estuaries by the Asian polychaete, Hediste diadroma (Nereididae). Mar Biol 161:187–194
Palumbi SR (1996) Nucleic acids II: the polymerase chain reaction. In: Hillis DM, Moritz C, Mable BK (eds) Molecular systematics, 2nd edn. Sunderland: Sinauer, Sunderland, pp 205–247
Posada D, Crandall KA (1998) MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818
Rambaut A, Suchard MA, Xie D, Drummond AJ (2014) Tracer v1.6 (computer program)
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Sato M (1999) Divergence of reproductive and developmental characteristics and speciation in Hediste species group. Hydrobiologia 402:129–143
Sato M (2004) Diversity of polychaetes and environments in tidal flats: a study on the Hediste species group (Nereididae). Fossils 76:122–133 (in Japanese with English abstract)
Sato M (2017) Nereididae (Annelida) in Japan, with special reference to life-history differentiation among estuarine species. In: Motokawa M, Kajihara H (eds) Species diversity of animals in Japan. Springer Japan, Tokyo, pp 477–512
Sato M, Ikeda M (1992) Chromosome complements of two forms of Neanthes japonica (Polychaeta, Nereididae) with evidence of male-heterogametic sex chromosomes. Mar Biol 112:299–307
Sato M, Masuda Y (1997) Genetic differentiation in two sibling species of the brackish-water polychaete Hediste japonica complex (Nereididae). Mar Biol 130:163–170
Sato M, Nakashima A (2003) A review of Asian Hediste species complex (Nereididae, Polychaeta) with descriptions of two new species and a redescription of Hediste japonica (Izuka, 1908). Zool J Linnean Soc 137:403–445
Sato M, Sattmann H (2009) Extirpation of Hediste japonica (Izuka, 1908) (Nereididae, Polychaeta) in central Japan, evidenced by a museum historical collection. Zool Sci 26:369–372
Sato M, Tsuchiya M (1987) Reproductive behavior and salinity favorable for early development in two types of the brackishwater polychaete Neanthes japonica (Izuka). Benthos Res 31:29–42
Sato M, Tsuchiya M (1991) Two patterns of early development in nereidid polychaetes keying out to Neanthes japonica (Izuka). Ophelia Suppl 5:371–382
Scaps P (2002) A review of the biology, ecology and potential use of the common ragworm Hediste diversicolor (O. F. Müller) (Annelida: Polychaeta). Hydrobiologia 470:203–218
Smith RI (1950) Embryonic development in the viviparous nereid polychaete, Neanthes lighti Hartman. J Morph 87:417–466
Smith RI (1958) On reproductive pattern as a specific characteristic among nereid polychaetes. Syst Zool 7:60–73
Smith RI (1964) On the early development of Nereis diversicolor in different salinities. J Morph 114:437–464
Smith RI (1977) Physiological and reproductive adaptations of Nereis diversicolor to life in the Baltic Sea and adjacent waters. In: Reish DJ, Fauchald K (eds) Essays on polychaetous annelids, in memory of Dr. Olga Hartman. University of Southern California, Los Angeles, Allan Hancock Foundation, pp 373–390
Soubrier J, Steel M, Lee MS, Der Sarkissian C, Guindon S, Ho SY, Cooper A (2012) The influence of rate heterogeneity among sites on the time dependence of molecular rates. Mol Biol Evol 29:3345–3358
Struck T, Hessling R, Purschke G (2002) The phylogenic position of the Aeolosomatidae and Parergodrilidae, two enigmatic oligochaete-like taxa of the ‘Polychaeta’, based on molecular data from 18S rDNA sequences. J Zool Syst Evol Res 40:155–163
Swofford DL (2003) PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sinauer, Sunderland (Computer program)
Tavaré S (1986) Some probabilistic and statistical problems in the analysis of DNA sequences. Lectures Math Life Sci 17:57–86
Tosuji H, Furota T (2016) Molecular evidence for the expansion of the Asian cryptic invader Hediste diadroma (Nereididae: Annelida) into the Northeast Pacific habitats of the native H. limnicola. Zool Sci 33:162–169
Tosuji H, Miyamoto J, Hayata Y, Sato M (2004) Karyotyping of female and male Hediste japonica (Polychaeta, Annelida) in comparison with those of two closely related species, H. diadroma and H. atoka. Zool Sci 21:147–152
Tosuji H, Sato M (2006) Salinity favorable for early development and gamete compatibility in two sympatric estuarine species of the genus Hediste (Polychaeta: Nereididae) in the Ariake Sea, Japan. Mar Biol 148:529–539
Tosuji H, Sato M (2008) Identification of three Asian Hediste species (Polychaeta: Nereididae) by PCR-RFLP analysis of the mitochondrial 16S rRNA gene. Plankton Benthos Res 3:50–52
Tosuji H, Sato M (2010) Genetic evidence for parapatric differentiation of two forms of the brackish-water nereidid polychaete Hediste atoka. Plankton Benthos Res 5(suppl):242–249
Tosuji H, Sato M (2012) A simple method to identify Hediste sibling species (Polychaeta: Nereididae) using multiplex PCR amplification of the mitochondrial 16S rRNA gene. Plankton Benthos Res 7:195–202
Tosuji H, Togami K, Miyamoto J (2010) Karyotypic analysis of the hermaphroditic viviparous polychaete, Hediste limnicola (Polychaeta: Nereididae): possibility of sex chromosome degeneration. J Mar Biol Ass UK 90:613–616
Vermeij GJ (1991) Anatomy of an invasion: the trans-Arctic interchange. Paleobiology 17:281–307
Virgilio M, Abbiati M (2006) Temporal changes in the genetic structure of intertidal populations of Hediste diversicolor (Polychaeta: Nereididae). J Sea Res 56:53–58
Virgilio M, Fauvelot C, Costantinif F, Abbiati M, Backeljau T (2009) Phylogeography of the common ragworm Hediste diversicolor (Polychaeta: Nereididae) reveals cryptic diversity and multiple colonization events across its distribution. Mol Ecol 18:1980–1994
Väinölä R (2003) Repeated trans-Arctic invasions in littoral bivalves: molecular zoogeography of the Macoma balthica complex. Mar Biol 143:935–946
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Whiting MF (2002) Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zool Scr 31:93–104
Yang Z (1995) A space-time process model for the evolution of DNA sequences. Genetics 139:993–1005
Ye S, Dhillon S, Ke X, Collins AR, Day INM (2001) An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 29:e88
Acknowledgments
We thank Toshio Furota (Toho University), Minoru Ikeda, Takao Suzuki (Tohoku University), Sayumi Isaka (Hokkaido University), Gen Kanaya (National Institute for Environmental Studies), Hajime Saito (Japan International Research Center for Agricultural Sciences), Takeshi Sonoda (Tokyo University of Agriculture), Kenji Toda (Shimane Environment & Health Public Corporation), Hiroaki Tsutsumi (Prefectural University of Kumamoto), Taichi Wada (Osaka), Shintaro Yamada (Miyazaki Prefectural Museum of Nature and History), Takumi Ebihara, Kotaro Kan, Akiyuki Nakashima, Ryogo Nishizawa, and Takeru Sakaguchi (Kagoshima University) for assistance in collecting animals. Our thanks are also due to Bo Causer (Kagoshima University) for reviewing the manuscript. This research project was supported by JSPS KAKENHI (JP17K07538 and JP17H01913), and Marine Biotechnology Program by Ministry of Oceans and Fisheries, Korea (Grant Number 20170431).
Funding
This study was funded by JSPS KAKENHI (Grant Numbers JP17K07538 and JP17H01913) and Marine Biotechnology Program by Ministry of Oceans and Fisheries, Korea (Grant Number 20170431).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by K. Kocot
Electronic supplementary material
Online Resource 1
(XLSX 33 kb)
Online Resource 2
(XLSX 11 kb)
Rights and permissions
About this article
Cite this article
Tosuji, H., Bastrop, R., Götting, M. et al. Worldwide molecular phylogeny of common estuarine polychaetes of the genus Hediste (Annelida: Nereididae), with special reference to interspecific common haplotypes found in southern Japan. Mar Biodiv 49, 1385–1402 (2019). https://doi.org/10.1007/s12526-018-0917-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-018-0917-2