Abstract
Early development was examined, under various salinities, for two sympatric nereidid polychaetes, Hediste japonica and H. diadroma, which participated in a simultaneous reproductive swarming in an estuary of the Omuta-gawa River in Ariake Sea, Japan. The eggs of both species were isotonic to the medium of 27.5–30 psu salinity. The egg diameter in the isotonic salinity was 180–205 μm in H. japonica, and 130–160 μm in H. diadroma. Successful development of most embryos was observed in a salinity range of 22.5–30 psu in both species, while successful fertilization occurred in wider ranges of salinity, i.e., 10–34 psu in H. japonica and 10 to 30 psu in H. diadroma. In both species, free-swimming larval life started from the indistinct hatching of trochophores out of a jelly layer capsule. The lecithotrophic development appeared to run to the 4-setiger nectochaetes in H. japonica, while to 3-setiger nectochaetes in H. diadroma, resulting in a shorter pelagic larval life in H. japonica. In a comparison of larval morphology among Hediste species, we found a definite negative correlation between the prostomium width, which represents the larval size and depends on egg size, and relative length of chaetae to the prostomium width: the relative length of chaetae was the longest in H. diadroma (with the smallest egg size and long pelagic life), intermediate in H. japonica (intermediate egg size, short pelagic life), and the shortest in H. atoka (largest egg size, no true pelagic life). We also examined the possibility of hybridization between H. japonica and H. diadroma through cross-insemination experiments. The gametes of the two species were reciprocally compatible, and viable hybrid offspring were produced by the laboratory crosses. The hybrid larvae expressed intermediate phenotypes, but with a greater maternal influence in characteristics such as the relative length of chaetae and the lecithotrophic larval duration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hediste species (Nereididae, Polychaeta, Annelida) dominate in macrobenthic fauna in shallow brackish waters in the North Temperate Zone (Sato 1999). The Asian Hediste worms had previously been regarded as a single species, H. japonica. However, they have recently been found to be a sibling species complex consisting of three distinct species (H. japonica sensu stricto,H. diadroma, and H. atoka) (Sato and Nakashima 2003; Tosuji et al. 2004). A comparison of their life histories may be important to clarify the ecological and evolutionary significance of sympatric or parapatric distribution of these closely related species.
H. diadroma and H. atoka, which are commonly distributed in a wide range of Japanese estuaries, have contrasting life histories (Sato and Tsuchiya 1987, 1991; Sato 1999). There have been few studies on the life history of H. japonica (Izuka 1908; Sato and Nakashima 2003), which is distributed in limited areas, i.e., the inner part of the Ariake Sea in Japan and the Korean coast of the Yellow Sea (Sato and Nakashima 2003).
Both H. japonica and H. diadroma inhabit the intertidal flats in an estuary of the Omuta-gawa River, located at the edge of the geographic range of H. japonica in Ariake Sea, Kyushu Island, Japan (Sato and Nakashima 2003). Recently, we found that sexually mature adults of both species participate in simultaneous reproductive swarming there from December to February (our unpublished data). Previous studies showed that early development in H. diadroma is characterized by a small egg size (130–170 μm in diameter), a long pelagic larval life (about 1 month) and a high optimum salinity for the development (19–34 psu) (Kagawa 1955; Inamori and Kurihara 1979; Sato and Tsuchiya 1987, 1991; Sato 1999). On the contrary, H. japonica has a large egg size (180–210 μm, Sato and Nakashima 2003) and a short pelagic larval life (about 10 days, Izuka 1908). Salinity favorable for the early development of H. japonica was hitherto unknown.
In the present study, we compare early development of H. japonica and H. diadroma in their sympatric habitat with special reference to salinity favorable for development. We also examine the possibility of hybridization between these two species through cross-insemination experiments, and discuss a possible mechanism for their reproductive isolation.
Materials and methods
Sexually mature worms of H. japonica and H. diadroma were collected with a scoop net at a site 2.5 km upstream from the mouth of the Omuta-gawa River in Ariake Sea (Fukuoka Prefecture, Japan) from reproductive swarms near the surface in December 2003, January 2004 and January 2005. The mature adults were preserved in a refrigerator (ca. 4°C) before experiments.
Gametes were obtained by pressing adult worms with a forceps. Unfertilized eggs were quickly transferred into Petri dishes containing Jamarin-U artificial seawater (Jamarin Lab., Osaka, Japan), which was diluted with distilled water to a series of (12) salinities (5, 7.5 10, 12.5, 15, 17.5, 20, 22.5, 25, 27.5, 30, 34 psu). Salinity was measured with a handheld salinity, conductivity and temperature system (Model 30, YSI, OH, USA). The diameter of 175 unfertilized eggs (25 eggs from each of four H. japonica and three H. diadroma females) was measured on enlarged micrographs taken after about 1 h holding in the preceding 12 salinities.
The eggs from six females of H. japonica and three females of H. diadroma were inseminated with conspecific sperm suspension (final sperm concentration: 10−5–10−6 dilution of dry sperm) under the series of salinities to identify the optimum salinity for early development. Five experiments of cross-insemination in reciprocal combination (i.e., H. japonica egg versus H. diadroma sperm, H. diadroma egg versus H. japonica sperm) were carried out together with control experiments of conspecific insemination in seawater diluted to 27.5 psu, which was in the optimum range for early development of both species (see results). Different parents were used for each of the experiments. The index used for successful fertilization was jelly-layer formation followed by ooplasmic segregation. The index used for normal development (progress of cleavage and cilia formation at proper timing) was presence of cilia movement at about 24 h after insemination.
In each of the three experiments of the cross-insemination, about 250 successfully fertilized embryos (a day after insemination) obtained from each of four gamete combinations (two reciprocal heterospecific combinations and two conspecific controls) were transferred to a 24-well tissue culture plate (well size: 15 mm in diameter × 10 mm in depth), each well containing 2 ml of diluted seawater (27.5 psu) and 10–17 embryos. Their survivals were daily examined throughout 23 days. Developmental stages of the all survivors were examined 23 days after insemination.
The 3-setiger and later nectochaetes were fed on the commercial fish food “Tetramin Flakes” (Pfizer, NY, USA). To compare the nectochaeta morphology among H. japonica,H. diadroma and their hybrids, the width of prostomium and the length of the longest chaeta in setiger 2 were measured on enlarged photographs of the 3-setiger larvae (about 5 days after insemination). To avoid fixation artifacts, the embryos and larvae were photographed without fixation or immediately after weak fixation in 0.5–1% formalin. All experiments were carried out at controlled room temperature of 15±0.5°C.
Results
Egg size and inferred ooplasmic salinity
The egg diameter varied according to the salinity of the medium (Fig. 1). The eggs swelled, developing a spherical shape in the hypotonic media (hypotonic relative to the ooplasm), while they shrank into irregular shapes in the hypertonic media. The long axis of such eggs was measured. When depressions on the surface become barely detectable, the medium surrounding the eggs may be assumed to be nearly isotonic to the ooplasm.
Hediste spp. Egg diameter in four females of H. japonica (filled circle) and four females of H. diadroma, (filled square) in various salinities. Average and SD bar from data of 25 eggs from each female are shown. The arrows indicate the salinity where the eggs have barely discernable depressions on their surface (i.e. nearly isotonic to the ooplasm)
The eggs of both H. japonica and H. diadroma were isotonic to the medium of 27.5–30 psu. The egg diameter in 27.5 psu was 180–205 μm in H. japonica, and 130–160 μm in H. diadroma.
Effects of salinity on early development
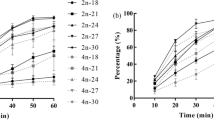
The effects of salinity on fertilization and successful early development were examined (Fig. 2). In H. japonica, most eggs (>80% average over six experiments) were fertilizable in a wide range of salinities (10–34 psu), and developed successfully to embryos with ciliary movement in 22.5–30 psu. Few embryos (<30%) developed to swimming larvae in less than 17.5 psu.
In H. diadroma, most eggs (>97% in average in three experiments) were fertilizable in a range of 10–30 psu. The upper limit of the range was lower than that in H. japonica. Many eggs (>60%) developed successfully to embryos with ciliary movement in a range of 22.5–30 psu, though the rates of successful development varied greatly among batches from the different females. From these experiments, the optimum salinity for early development of both species was judged as around 27.5 psu.
Early development in optimum salinity
Hediste japonica
Mature eggs were turquoise green in color. The ooplasm was relatively opaque, and contained 40–50 lipid droplets surrounding a germinal vesicle (Fig. 3a). Within 30 min after insemination, each fertilized egg secreted a jelly layer (about 150 μm thick). During the period of polar body formation and cleavage, lipid droplets gradually migrated to the vegetal pole and fused (Fig. 3b–f). A band of cilia (prototroch) appeared 18–24 h after insemination, and the embryos (trochophores) began to rotate within the jelly layer capsule (Fig. 3g, h). Several large lipid droplets were situated below the prototroch. The trochophores began swimming freely after hatching out (40–48 h after insemination). The hatching process was not a distinct event, because the jelly layer gradually collapsed and disappeared. About 3 days (65–70 h) after insemination, the larvae became metatrochophores, which were slightly elongated posteriorly and had two pairs of bundles of chaetae (Fig. 3i). Both trochophores and metatrochophores showed positive phototaxis.
Hediste japonica. Early development of H. japonica in 27.5 psu. a Unfertilized egg. b First polar body formation (90 min after insemination). c Second polar body formation (120 min). d Two-cell stage (180 min). e Four-cell stage (210 min).f Cleavage stage (6 h). g Early trochophore rotating within a jelly layer capsule (24 h). h Trochophore just before hatching (48 h). i Metatrochophore (lateral view, 70 h). j Anterior view of same stage as i. k Early stage of 3-setiger nectochaeta (96 h). l Late stage of 3-setiger nectochaeta (110 h). m 4-setiger nectochaeta (8 days). Scale bar: 100 μm
About 4 days after insemination, the larvae became 3-setiger nectochaetes (Fig. 3k). The prototroch remained in the larval prostomium, and lipid droplets moved to the middle part of the larval body. During this stage, the prostomial tentacle, the peristomial cirri and the anal cirri began to grow (Fig. 3l). Nectochaetes developed the fourth setiger a week after insemination, when the lipid droplets disappeared and the larvae began to feed (Fig. 3m). Thereafter, larval stage and body size were highly variable probably depending on available food and individual feeding ability. Cannibalism was commonly observed. Larval settlement to the bottom was not a distinct event: nectochaetes with four or more setigers seem to settle gradually, showing crawling behavior on the bottom together with swimming behavior in surface and bottom water. The chronology of early development is shown in Table 1.
Hediste diadroma
The mature eggs were green or yellow-green in color. The ooplasm was relatively transparent, and contained 20–40 lipid droplets surrounding a germinal vesicle (Fig. 4a). Within 30 min after insemination, each fertilized egg secreted a jelly layer (about 150 μm thick). During the period of polar body formation and cleavage, lipid droplets migrated to the vegetal pole and fused (Fig. 4b–g). A band of cilia (prototroch) appeared 24 h after insemination, and the embryos (trochophores) began to rotate within the jelly layer capsule (Fig. 4g). Several large lipid droplets were situated below the prototroch. The trochophores began swimming freely after hatching out (36–40 h after insemination) (Fig. 4h). As in H. japonica, the hatching process was not a distinct event. About 3 days after insemination, the larvae became metatrochophores, which were slightly elongated posteriorly and had two pairs of bundles of chaetae (Fig. 4i). Both trochophores and metatrochophores showed positive phototaxis.
Hediste diadroma. Early development of H. diadroma in 27.5 psu. a Unfertilized egg. b First polar body formation (100 min after insemination). c Second polar body formation (130 min). d Two-cell stage (210 min). e Four-cell stage (250 min). f Cleavage stage (6 h). g Early trochophore rotating within a jelly layer capsule (24 h). h Free-swimming trochophore (48 h). i Metatrochophore (lateral view, 3 days). j Anterior view of same stage as i. k Early stage of 3-setiger nectochaeta (4 days). l Late stage of 3-setiger nectochaeta (22 days). m 4-setiger nectochaeta (27 days). Scale bar: 100 μm
About 4 days after insemination, the larvae became 3-setiger nectochaetes (Fig. 4k). The prototroch remained in the larval prostomium, and lipid droplets migrated to the middle part of the larval body. During this stage, the prostomial tentacle, the peristomial cirri and the anal cirri began to grow, lipid droplets disappeared and the larvae began to feed (Fig. 4l). The period of the 3-setiger nectochaeta stage of H. diadroma (>12 days) was much longer than that of H. japonica (ca. 3 days). Cannibalism was commonly observed. Nectochaetes developed the fourth setiger 16–30 days after insemination (Fig. 4m). Nectochaetes with four or more setigers showed crawling behavior on the bottom together with swimming behavior in surface and bottom water. As in H. japonica, larval settlement to the bottom was not a distinct event.
Gamete compatibility between Hediste japonica and H. diadroma
Fertilization rates of heterospecific crosses in both reciprocal combinations were variable (50–98%) depending on which eggs or sperm were used, while high fertilization rates (88–100%) were always observed in control experiments of conspecific controls (Fig. 5). However, the fertilization rates were not significantly different among them (ANOVA, F 3,16=2.7, P=0.08). Fertilization rates of two crossing experiments (50–64%) with “old” gametes (4 days after collection of mature adults) were lower than those of three crossing experiments (64–98%) with “fresh” gametes (within 1 day of collection).
Early development of the hybrids produced by successful cross-fertilization was observed (Figs. 6, 7). The polar body formation and early cleavage progressed slower in hybrid embryos (Table 2) than in normal embryos (Table 1). In hybrid larvae from both combinations of reciprocal crossing, lipid droplets disappeared during the 3-setiger nectochaeta and the larvae began to feed about a week after insemination (Fig. 6c, 7c).
Hediste spp. Hybrid larvae obtained from H. japonica eggs fertilized by H. diadroma sperm in 27.5 psu. a Trochophore (lateral view, 3 days after insemination).b Early stage of 3-setiger nectochaeta (4 days). c Late stage of 3-setiger nectochaeta (5 days).d 4-setiger nectochaeta (10 days). Scale bar: 100 μm
Hediste spp. Hybrid larvae obtained from H. diadroma eggs fertilized by H. japonica sperm in 27.5 psu. a Trochophore (lateral view, 3 days after insemination). b Early stage of 3-setiger nectochaeta (4 days). c Late stage of 3-setiger nectochaeta (6 days). d 4-setiger nectochaeta (23 days). Scale bar: 100 μm
Survival rates through 23 days after fertilization were not significantly different among hybrids obtained from two reciprocal crosses and normal offspring obtained from two conspecific combinations (ANOVA, F 3,8=1.5, P=0.28 at 10 days after insemination; F 3,8=0.59, P=0.64 at 23 days after insemination) (Fig. 8). Developmental stages of the survivors were compared at 23 days after fertilization (Fig. 9). Though normal offspring of H. japonica reached the 4- to 10-setiger stages with the highest proportion in the 5-setiger stage (average: 40.4%) (Fig. 9a), hybrids obtained from H. japonica eggs fertilized by H. diadroma sperm reached the 3- to 7-setiger stages with the highest proportion in the 4-setiger stage (63.5%) (Fig. 9b). The difference in proportions of six developmental stages (3-, 4-, 5-, 6-, 7-, and more setiger stages), where data from three experiments were pooled and low individual numbers at the 8- to 10-setiger stages were combined, was significant between them (χ2=189.2, df=5, P<0.0001). Whereas the hybrids obtained from H. diadroma eggs fertilized by H. japonica sperm reached the 3- to 4-setiger stages with the highest proportion in the 3-setiger stage (94.7%) (Fig. 9c), while normal offspring of H. diadroma reached the 3- to 4-setiger stages with the highest proportion in the 3-setiger stage (99.3%) (Fig. 9d). The difference in proportions of two developmental stages (3- and 4-setiger stages) was significant between them (χ2=11.3, df=1, P=0.0008).
Hediste spp. Percentage of eight developmental stages (3- to 10-setiger stages) of the surviving offspring at 23 days after insemination. a Normal offspring of H. japonica (J). b Hybrids obtained from H. japonica eggs fertilized by H. diadroma (D) sperm. c Hybrids obtained from H. diadroma eggs fertilized by H. japonica sperm. d Normal offspring of H. diadroma. Averages and SD bars from three experiments are shown. The number on each datum indicates pooled number of individuals obtained from the three experiments
Comparison of morphology of the 3-setiger nectochaetes among Hediste japonica,H. diadroma and their hybrids
The width of prostomium (an index for the whole body size of nectochaetes) was significantly greater in H. japonica than in H. diadroma (ANOVA and post hoc test of Scheffé’s F, F 3,196=199.5, P<0.0001) (Fig. 10, Table 3), corresponding to the difference in egg size between them (Fig. 1). The width of prostomium in hybrids obtained from H. japonica eggs fertilized by H. diadroma sperm was not significantly different from that in H. japonica proper (P=0.46). The width of prostomium in hybrids obtained from H. diadroma eggs fertilized by H. japonica sperm was not significantly different from that in H. diadroma proper (P=0.17).
Hediste spp. Relationships between the width of prostomium and the length of the longest chaeta for all individual data (a) and between the width of prostomium and the relative length of the longest chaeta (length of the longest chaeta/width of prostomium) for average data with SD bars (b) in 3-setiger nectochaetes of H. japonica (J) proper (open circle, n=50), H. diadroma (D) proper (filled circle, n=50) and the hybrids, which were obtained from H. japonica eggs fertilized by H. diadroma sperm (open triangle, n=50), or H. diadroma eggs fertilized by H. japonica sperm (filled triangle, n=50)
The actual length of chaetae was significantly greater in H. diadroma than in H. japonica (F 3,196=27.6, P<0.0001) (Fig. 10a). The relative length of chaetae (length of longest chaeta/width of prostomium) was also significantly greater in H. diadroma than in H. japonica (F 3,196=164.5, P<0.0001) (Fig. 10b). The actual and relative length of chaetae in hybrids obtained from H. japonica eggs fertilized by H. diadroma sperm was not significantly different from those in H. japonica proper (P=0.35 and 0.15, respectively). Whereas the actual and relative length of chaetae was significantly smaller in the hybrids obtained from H. diadroma eggs fertilized by H. japonica sperm than those in H. diadroma proper (P=0.0001 and <0.0001, respectively). The actual length of chaetae in the hybrids obtained from H. japonica eggs was not significantly different from that in the hybrids obtained from H. diadroma eggs (P=0.2), while the relative length of chaetae was significantly greater in the latter (P<0.0001).
Discussion
Comparison of early development among three Asian species of the genus Hediste
Salinities isotonic to ooplasm of H. japonica and H. diadroma in the Omuta-gawa River were similar, ranging from 27.5 to 30 psu. These values are consistent with 27–30 psu in H. diadroma (=small-egg type of Neanthes japonica) collected from Kagoshima, Hiroshima, and Miyagi Prefecture, and contrast with much lower value (around 15 psu) isotonic to ooplasm of H. atoka (=large-egg type of Neanthes japonica) (Sato and Tsuchiya 1987).
Corresponding to the similar salinity isotonic to ooplasm in H. japonica and H. diadroma, salinity favorable for early development was also similar between them, ranging from 22.5–30 psu, and roughly consistent with that of previous report (19–34 psu) in H. diadroma collected from various localities, and contrasting with that in H. atoka (9–21 psu) (Kagawa 1955; Inamori and Kurihara 1979; Sato and Tsuchiya 1987). Our result shows that embryos of H. japonica are more tolerant of higher salinity (more than 30 psu) than those of H. diadroma. This difference may cause the different adult distributions between the two species in the estuary of the Omuta-gawa River to some extent: benthic adults of H. japonica occupy mainly the lower reaches with higher salinity, and those of H. diadroma usually inhabit the upper reaches with lower salinity, though their distributions are overlapped in a wide range (Hanafiah et al., in preparation).
Previous studies showed that early development in H. diadroma is characterized by a small egg size (130–170 μm in diameter) and a long pelagic larval life (about 1 month in the field) after hatching out at trochophore stage (Kagawa 1955; Inamori and Kurihara 1979; Sato and Tsuchiya 1987, 1991; Sato 1999). Our result on developmental characteristics of H. diadroma in the Omuta-gawa River is the same as those of the previous findings.
As for H. japonica, a few previous studies indicated a large egg size (180–210 μm, Sato and Nakashima 2003) and a short pelagic larval life (about 10 days, Izuka 1908) after hatching out at trochophore stage. Our results support these previous findings.
The lecithotrophic larval periods are different between H. japonica and H. diadroma. The amount of maternal nutrient (yolks and lipid droplets) contained in unfertilized eggs is more abundant in H. japonica than in H. diadroma, corresponding to the different egg size. Lecithotrophic development runs to the 4-setiger nectochaete stage in H. japonica, and to the 3-setiger nectochaete stage in H. diadroma. In both species, later larval development (stage and body size) is strongly affected by the availability of food in the environment.
The lecithotrophic larval duration and the commencement of larval feeding in the hybrids seem to be intermediate between those in the two species. Development to 4-setiger nectochaetes was completely lecithotrophic in H. japonica proper, whereas partially planktotrophic and therefore somewhat delayed in the hybrids obtained from H. japonica eggs fertilized by H. diadroma sperm. Development from 3-setiger to 4-setiger nectochaetes was planktotrophic in H. diadroma eggs, and somewhat faster in the hybrids obtained from H. diadroma eggs fertilized by H. japonica sperm than H. diadroma proper. Our data on relative length of chaetae in 3-setiger nectochaete larvae show that the hybrids (at least those obtained from H. diadroma eggs fertilized by H. japonica sperm) tended to express phenotypes intermediate to conspecific ones, but showing more of maternal characteristics than paternal ones. Similar results were obtained in asteroid hybrids (Byrne and Anderson 1994).
In contrast to H. japonica and H. diadroma in the present study, H. atoka produce the largest eggs (200–250 μm in diameter), which develop directly to benthic juveniles without a true planktonic phase (Sato and Tsuchiya 1991; Sato 1999). The lecithotrophic development seems to run to the 4-setiger nectochaete or further in H. atoka (Sato and Tsuchiya 1991).
Our data on the chaetal length and the prostomium width in 3-setiger nectochaetes of H. japonica and H. diadroma (populations of the Omuta-gawa River in Fukuoka Prefecture) in southern Japan were compared with data from Sato and Tsuchiya (1991) on H. diadroma and H. atoka (populations of the Nanakita-gawa River in Miyagi Prefecture and the Niida-gawa River in Aomori Prefecture, respectively) in northern Japan (Table 3, Fig. 11). The width of prostomium was significantly different between all combinations of the four populations (ANOVA and post hoc test of Fisher’s PLSD: F 3,130=151.1, P<0.0001), corresponding to the difference in egg size between them. Populations with larger egg size produced larger larvae. This result also shows geographical variation in larval size depending on egg size in H. diadroma. The Nanakita-gawa River population produced larger eggs (140–170 μm in diameter, Sato and Tsuchiya 1991) and larger larvae (average width of prostomium: 110 μm) than the Omuta-gawa River population (egg diameter: 130–160 μm, average width of prostomium: 100 μm). We found a tendency for larger larvae to have shorter chaetae (both in absolute length and relative to the width of the prostomium). This tendency was very clear when the relative length of chaetae was considered (Fig. 11). The average relative length of chaetae (RL) was significantly different between all combinations of the four populations (ANOVA and post hoc test of Fisher’s PLSD: F 3,130=263.4, P<0.0001), and negatively correlated with the average width of the prostomium (WP) according to the regression formula: RL=−0.021 WP+4.0 (r 2=0.998, P=0.0002). The relative length of chaetae was the longest in H. diadroma (with a long pelagic life), the shortest in H. atoka (without a true pelagic life), and intermediate in H. japonica (with a short pelagic life). This corresponds with the difference between the larval types of the spionid polychaete, Streblospio benedicti found by Levin (1984), in which long chaetae are present in planktotrophic larvae with a long pelagic life, but reduced or absent in lecithotrophic larvae with a short pelagic life. Long chaetae seem to be evolutionarily adaptive for pelagic larval life due to some function such as defensive mechanisms against predators (Pennington and Chia 1984).
Hediste spp. Relationship between the width of prostomium and the relative length of the longest chaeta in 3-setiger nectochaetes of four populations of three Hediste species. Averages and SD bars are shown based on data of Table 3
Reproductive isolation between Hediste japonica and H. diadroma
The gametes of H. japonica and H. diadroma were reciprocally compatible, and hybrids between them were viable at least until 23 days after fertilization. This result indicates that neither gamete incompatibility nor hybrid inviability appears to ensure reproductive isolation between these species, though sympatric distribution of these species is not common and at present known only in the Omuta-gawa River, located at the edge of the narrow geographic range of H. japonica (Sato and Nakashima 2003).
For many free-spawning marine invertebrates, gamete incompatibility is considered an important mechanism of reproductive isolation among different species (e.g., Palumbi and Metz 1991; Marsden 1992). However, instances of complete or near-complete gamete compatibility in one or both reciprocal crosses between sympatric congeners are also known in polychaetes (Pernet 1999), echinoderms (Strathmann 1981; Lessios and Cunningham 1990; Byrne and Anderson 1994), and corals (Wallace and Willis 1994; Miller and Babcock 1997). In these cases, other pre- or postzygotic mechanisms may act as barriers to gene exchange between species in the field: For example, prezygotic isolation by ecological or habitat segregation and/or temporal separation in breeding was suggested in the asteroids (Patiriella spp., Byrne and Anderson 1994) and the polychaetes (Arctonoe spp., Pernet 1999). Postzygotic isolation by hybrid inviability was shown in the corals (Montipora spp., Hodgson 1988). Whereas in some corals (Acropora spp., Wallace and Willis 1994; Platygyra spp., Miller and Babcock 1997), bivalves (Mytilus spp., Gardner 1994), echinoids (Strongylocentrotus spp., Strathmann 1981) and asteroids (Asterias spp., Schopf and Murphy 1973; Leptasterias spp., Kwast et al. 1990), gene exchange between closely related species may occur through production of viable hybrids in the zone where their distributions overlap.
There is no evidence showing prezygotic isolation between H. japonica and H. diadroma in the Omuta-gawa River. Their breeding periods largely overlapped in a period from December to February, when mature males and females of both species participate in simultaneous reproductive swarming just after high-tide at night during the spring tides, and multiple-species spawning occurred (Hanafiah et al., in preparation). In some nereidids which perform reproductive swarming after a metamorphosis to the typical epitokal (heteronereis) form, prezygotic isolation may be ensured by formation of pairs of conspecific partners of the opposite sex, which swim rapidly in tight circles around each other (nuptial dance) prior to the release of gametes as is well-documented in Nereis succinea (=N. limbata) (Lillie and Just 1913; Clark 1961), Platynereis dumerilii (Boilly-Marer 1974) and Perinereis nuntia var. brevicirrus (Hardege and Bartels-Hardege 1995). This behavior is controlled by sex pheromones (Zeeck et al. 1990; Hardege 1999). Bartels-Hardege et al. (1996) identified a sex pheromone, which increased the swimming activity of mature adults, from the coelomic fluid of swarming females of Asian Hediste species (probably H. diadroma judging from its locality, Qingdao, China, and reproductive period, March, Sato and Nakashima 2003). However, mature adults of both H. japonica and H. diadroma do not metamorphose to the typical epitokal form, and their gametes are released in a diffuse group of many males and females without nuptial dance of pairing partners (Bartels-Hardege et al. 1996: Sato and Nakashima 2003). Therefore, potential exists for gametes of the two species to mix in the water column in the Omuta-gawa River as shown in mass spawning of corals (Wallace and Willis 1994; Miller and Babcock 1997) and echinoderms (Pearse et al. 1988).
Salinities in bottom water in the Omuta-gawa River, where mature adults of H. japonica and H. diadroma were collected during simultaneous reproductive swarming, were 24–30 psu (Hanafiah et al., in preparation), which was in the optimum range (around 27.5 psu) for development in both species. Therefore, it is probable that successful hybridization between H. japonica and H. diadroma occurs even in the field. Successful hybridization may be affected by difference of egg fertilizability between conspecific and heterospecific sperm when both sperm are mixed in water column. Our result shows that early developmental processes such as polar body formation and early cleavage were delayed in hybrid embryos, suggesting that the first step of fertilization (i.e., sperm penetration and formation of male pro-nucleus) requires more time in heterospecific crosses (Table 2). This suggests that sperm competition may function as a prezygotic isolation.
Our next objective is to determine whether Hediste hybrids are produced in the field, and whether they participate in reproduction. Because H. japonica and H. diadroma were clearly distinguishable in all mature adults used in the present study by a morphological diagnosis in posterior neuropodial ligules (Sato and Nakashima 2003) without any intermediate forms, few, if any, hybrids may survive to sexual maturity in the field. Postzygotic isolation such as hybrid sterility also should be considered. However, the reproduction of H. diadroma starts unusually early and lasts long in the Omuta-gawa River (from December to April), consequently allowing it overlap considerably the reproduction of H. japonica (our unpublished data), while H. diadroma reproduces from February to April in Kagoshima, Kyushu, where H. japonica is not distributed (our unpublished data). This fact may suggest that gene flow through hybridization between the two Hediste species is not completely prevented in the Omuta-gawa River.
References
Bartels-Hardege HD, Hardege JD, Zeeck E, Muller C, Wu BL, Zhu MY (1996) Sex pheromones in marine polychaetes V: a biologically active volatile compound from the coelomic fluid of female Nereis (Neanthes) japonica (Annelida Polychaeta). J Exp Mar Biol Ecol 201:275–284
Boilly-Marer Y (1974) Etude experimentale du comportement nuptial de Platynereis dumerilii (Annelida: Polychaeta): chemoreception, emission des produits genitaux. Mar Biol 24:167–179
Byrne M, Anderson MJ (1994) Hybridization of sympatric Patiriella species (Echinodermata: Asteroidea) in New South Wales. Evolution 48:564–576
Clark RB (1961) The origin and formation of the heteronereis. Biol Rev 36:199–236
Gardner JPA (1994) The structure and dynamics of naturally occurring hybrid Mytilus edulis Linnaeus, 1758 and Mytilus galloprovincialis Lamarck, 1819 (Bivalvia: Mollusca) populations: review and interpretation. Arch Hydrobiol 99(Suppl):37–71
Hardege JD (1999) Nereidid polychaetes as model organisms for marine chemical ecology. Hydrobiologia 402:145–161
Hardege JD, Bartels-Hardege H (1995) Spawning behaviour and development of Perinereis nuntia var. brevicirrus (Annelida: Polychaeta). Invertebr Biol 114:39–45
Hodgson G (1988) Potential gamete wastage in synchronously spawning corals due to hybrid inviability. Proc 6th Int Coral Reef Symp 2:707–714
Inamori Y, Kurihara Y (1979) Analysis of the environmental factors affecting the life of the brackish polychaete, Neanthes japonica (Izuka). III. The effects of the environmental factors on fertilization, cleavage and postlarval development. Bull Mar Biol Stn Asamushi 16:113–121
Izuka A (1908) On the breeding habit and development of Nereis japonica n. sp. Annot Zool Jpn 6:295–305
Kagawa Y (1955) Note on the optimum salinities, studied in the adult and larva of the brackish-water polychaete worm, Nereis japonica. J Gakugei Coll, Tokushima Univ, Nat Sci 6:11–16 (in Japanese with English summary)
Kwast KE, Foltz DW, Stickle WB (1990) Population genetics and systematics of the Leptasterias hexactis (Echinodermata: Asteroidea) species complex. Mar Biol 105:477–489
Levin LA (1984) Multiple patterns of development in Streblospio benedicti Webster (Spionidae) from three coasts of North America. Biol Bull 166:494–508
Lessios HA, Cunningham CW (1990) Gametic incompatibility between species of the sea urchin Echinometra on the two sides of the Isthmus of Panama. Evolution 44:933–941
Lillie FR, Just EF (1913) Breeding habits of the heteronereis form of Nereis limbata at Whitstable Massachusetts. Biol Bull 24:147–160
Marsden JR (1992) Reproductive isolation in two forms of the serpulid polychaete Spirobranchus polycerus in Berbados. Bull Mar Sci 51:14–18
Miller K, Babcock RC (1997) Conflicting morphological and reproductive species boundaries in the coral genus Platygyra. Biol Bull 192:98–110
Palumbi SR, Metz EC (1991) Strong reproductive isolation between closely related tropical sea urchins (genus Echinometra). Mol Biol Evol 8:227–239
Pearse JS, McClary DJ, Sewell MA, Austin WC, Perez-Ruzafa A, Byrne M (1988) Simultaneous spawning of six species of echinoderms in Barkley Sound, British Columbia. Invertebr Reprod Dev 14:279–288
Pernet B (1999) Gamete interactions and genetic differentiation among three sympatric polychaetes. Evolution 53:435–446
Pennington JT, Chia FS (1984) Morphological and behavioral defenses of trochophore larvae of Sabellaria cementarium (Polychaeta) against four planktonic predators. Biol Bull 167:168–175
Sato M (1999) Divergence of reproductive and developmental characteristics in Hediste (Polychaeta: Nereididae). Hydrobiologia 402:129–143
Sato M, Nakashima A (2003) A review of Asian Hediste species complex (Nereididae, Polychaeta) with descriptions of two new species and a redescription of Hediste japonica (Izuka, 1908). Zool J Linn Soc 137:403–445
Sato M, Tsuchiya M (1987) Reproductive behavior and salinity favorable for early development in two types of the brackish-water polychaete Neanthes japonica (Izuka). Benthos Res (Japan) 31:29–42
Sato M, Tsuchiya M (1991) Two patterns of early development in nereidid polychaetes keying out of Neanthes japonica (Izuka). Ophelia 5(Suppl):371–382
Schopf TJM, Murphy LS (1973) Protein polymorphism of the hybridizing seastars Asterias forbesi and Asrerias vulgaris and implications for their evolution. Biol Bull 145:589–597
Strathmann RR (1981) On barriers to hybridization between between Strongylocentrotus droebachiensis (O.F. Müller) and S. Pallidus (G.O. Sars). J Exp Mar Bio Ecol 55:39–47
Tosuji H, Miyamoto J, Hayata Y, Sato M (2004) Karyotyping of female and male Hediste japonica (Polychaeta, Annelida) in comparison with those of two closely related species, H. diadroma and H. atoka. Zool Sci 21:147–152
Wallace CC, Willis BL (1994) Systematics of the coral genus Acropora: Implications of new biological findings for species concepts. Annu Rev Ecol Syst 25:237–262
Zeeck E, Hardege J, Bartels-Hardege H (1990) Sex pheromones and reproductive isolation in two nereid species, Nereis succinea and Platynereis dumerilii. Mar Ecol Prog Ser 67:183–188
Acknowledgments
We would like to thank Hidetoshi Nakashima of the Omuta Zoo for assistance in collecting animals, and Simon P. Varnam (Shizuoka, Japan) for English advice. This research was supported by a grant from the Research Institute of Marine Invertebrates (RIMI), Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Tosuji, H., Sato, M. Salinity favorable for early development and gamete compatibility in two sympatric estuarine species of the genus Hediste (Polychaeta: Nereididae) in the Ariake Sea, Japan. Marine Biology 148, 529–539 (2006). https://doi.org/10.1007/s00227-005-0079-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0079-1