Abstract
A phyllodocid polychaete belonging to the genus Eulalia is reported from Nuevo Gulf, Patagonia (South-western Atlantic Ocean) with abundant populations thriving in the intertidal zone. Morphological and molecular data allowed assigning this population to Eulalia clavigera (Audouin & Milne-Edwards, 1834), a species typically occurring along the north-eastern Atlantic coast. The absence of genetic structuring between north-eastern and south-western Atlantic E. clavigera strongly supports a non-native origin of the Patagonian population. Conversely, the majority of the Mediterranean Eulalia cf. clavigera analysed in this study turned out to belong to a different, probably undescribed species, suggesting that the diversity and taxonomy of green Eulalia is more complex than previously supposed. The high adaptation capabilities to stressed environments showed by E. clavigera, along with its possible high impact on native assemblages through predation, compel to carefully monitor its spread along the Patagonian coasts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of non-native species represents a major threat to natural ecosystems, especially when the introduced forms become invasive and eventually affect ecosystem functioning and human activities (Vilà et al. 2010), thus producing relevant economic losses (Pimentel et al. 2001). In the last decades, biological invasions in marine environments have dramatically increased, chiefly due to the technical and logistic improvements of maritime trade and the development of the transport network (Hulme 2009). The effects of non-native species colonising a new environment are often unpredictable, and even in the instance of an initial economical gain, the invasion process has usually a negative ecosystem impact, possibly leading to a complete change in natural assemblages (Molnar et al. 2008; Jeschke et al. 2014). For this reason, monitoring of biological invasions is essential for environmental management, allowing to plan impact reduction and, where possible, mitigation strategies (Wittenberg and Cock 2001). Monitoring plans aimed at early tracking of non-native species should concentrate on some areas that are particularly prone to biological invasions, among them, aquaculture facilities and commercial ports, as well as artificial canals connecting different basins or oceans (Molnar et al. 2008). However, the study of biological invasions in marine ecosystems is greatly hindered by the lack of knowledge regarding a large part of geographic areas. In particular, a close monitoring of species, of their spreading and of their impact on native communities is commonly realised only in European waters (Leppäkoski et al. 2002), in the Mediterranean Basin (Occhipinti-Ambrogi and Savini 2003), along the North American shores (Carlton 1989; Lodge et al. 2006) and in Australia (Pollard and Hutchings 1990a, b), whereas in other geographic areas the knowledge about non-native species is fragmentary and most likely their impact is greatly underestimated (Rilov and Crooks 2009). Moreover, even in well-monitored geographic areas, the majority of studies focus on a few taxa, such as fishes, crustaceans and molluscs, whereas information about the occurrence of the majority of non-native forms belonging to other phyla is anecdotic and fragmentary, and their effects on ecosystem functioning are scarcely known (Marchini et al. 2015).
In vertebrates and a few invertebrate groups, such as insects, echinoderms, or molluscs, it is relatively easy to assess whether a species is native or not. Instead, for several taxa, this question is far more complex to tackle: species with uncertain or unknown origin are considered cryptogenic and are particularly common in unicellular eukaryotes as well as in several macroinvertebrate groups, chiefly annelids and amphipods (Carlton 1996). This poses an additional difficulty to alien species management, in particular in marine environments (Carlton 1996). Even if cryptogenic species are usually treated as non-native in environmental management (Ojaveer et al. 2013), issues related to the interpretation of newly reported species may have consequences on environmental management practices, and on ecological status assessment in marine environments (Borja et al. 2005; Vilà et al. 2010). Thus, works aimed at solving cryptogenic species issues have high relevance.
Among marine animals, polychaete worms are characterised by a high frequency of cryptogenic species (Carlton 1996). This is due to a poor understanding of the diversity of some groups, which may lead to misidentifications, the inclusion of different biological species under the same name, and lack of information about the biogeography of several species (Giangrande 2003; Çinar 2013). Fifty out of the 292 alien polychaete species listed by Çinar (2013) are considered cryptogenic, at least in some areas; this high percentage (approximately 17%) shows that the occurrence of cryptogenic species in polychaetes is substantial. Moreover, alien and cryptogenic species are not evenly distributed among polychaete families. According to Çinar’s (2013) review, groups that have been subject of thorough taxonomic revisions, such as Nereididae, Sabellidae and Serpulidae, show a high occurrence of alien species, and conversely, a lower number of cryptogenic ones, whereas groups with a more uncertain taxonomy, and putative occurrence of cryptic species, such as Spionidae, comprise a relatively low number of confirmed alien species, and a higher number of cryptogenic ones. Molecular techniques are often necessary to unravel the distribution and dispersal path of alien polychaetes (Blank et al. 2008), as well as the occurrence of cryptic species within traditionally recognised morphospecies with a wide geographic range (Barroso et al. 2010; Carr et al. 2011; Nygren 2014). Cryptogenic species issues can be solved with the appropriate use of such techniques as well (Blakeslee et al. 2008; Bolton et al. 2011), even though they have rarely been used to clarify the status of cryptogenic polychaete species (Carrera-Parra and Salazar-Vallejo 2011; Sun et al. 2017). A number of non-native polychaetes are known to have a strong impact on native communities. This is particularly evident for non-native polychaetes that are also ecosystem engineers and are known to strongly alter the invaded habitats and induce dramatic structural and functional changes in the native assemblages (Crooks 2002; Holloway and Keough 2002; Orensanz et al. 2002; Tovar-Hernández et al. 2011), but polychaete species might also have strong impacts on native assemblages by competing with, and eventually replacing, native species (Çinar and Altun 2007). Phyllodocidae are relatively large polychaetes that commonly occur in shallow-water assemblages; as the majority of them are carnivores or scavengers, they are expected to have a strong impact on the overall assemblages of colonised environments. In fact, Schimmenti et al. (2016) and Bertocci et al. (2017) suggest that the phyllodocid Eulalia ornata Saint-Joseph, 1888, occurring at high densities on Mediterranean Sabellaria reefs, may have a remarkable effect on ecological dynamics of this habitat through predation on other invertebrates.
In recent years, a phyllodocid polychaete belonging to the genus Eulalia has been reported with remarkably high densities on shallow rocky environments along the Patagonian coast in South America. In Patagonia, this homogeneously green phyllodocid was found crawling on rocks or in tide pools and Lobo Orensanz, who participated in the collecting trip, indicated he had not seen the species during the 1970s when he collected polychaetes along Argentinian shores (J.M. Orensanz pers. com. to MED and SSV, 2013). The species had been preliminarily identified as an undescribed species belonging to the Eulalia viridis/clavigera complex, but its sudden appearance along the Patagonian coast raised doubts about the native status of the species. This work has the purpose to clarify the identity of the Eulalia species spreading in Patagonia, to assess its eventual non-native status and to understand and discuss possible introduction paths and vectors.
Material and methods
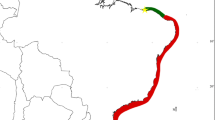
Specimens of Eulalia sp. from Patagonia were collected at tide level in environments along the coast of Puerto Madryn (42°49.79′S; 64°53.1′W) in localities Cerro Avanzado, Punta Cuevas and Ambrosetti as indicated below (Fig. 1), during several years (2007–2013). Material for comparison from European waters was collected at tide level among mussels in Plymouth (50°40.45′N; 4°27.12′ W) NW Atlantic during years 2006 and 2011 and at 0.2–0.5 m depth among calcareous algae at Capraia Island (43°1.77′N; 9°50.61′E), Mediterranean Sea, and in the port of Stintino, N Sardinia (40°56.13′N; 8°13′47.86″E), Mediterranean Sea, during year 2013 (Fig. 1). Along with this material, we studied also other museum specimens identified as Eulalia clavigera (Audouin & Milne-Edwards, 1834), collected in Bretagne, France, in 1994, and in Brazil in 2008. The examined material has been preserved in the institutional collections of El Colegio de la Frontera Sur, Chetumal, México (ECOSUR) and of the Laboratorio de Parasitología of the Instituto de Biología de Organismos Marinos, Puerto Madryn, Argentina (CNP), in the Los Angeles County Natural History Museum (LACM) and in the Natural History Museum of the University of Pisa, Italy (MSNP).
Sampling localities of Eulalia clavigera in western and eastern Atlantic Ocean: Amb Ambrosetti, BaF Beg an Fry, Cap Capraia Island, Cas Cassino, CAv Cerro Avanzado, PCu Punta Cuevas, Ply Plymouth, Sti Stintino. Black stars: populations analysed from the molecular and morphological point of view; white stars: populations analysed from the morphological point of view only
Specimens from Cerro Avanzado (Puerto Madryn), Plymouth, Capraia Island and Stintino were directly fixed and preserved in 96 or 70% ethanol until DNA extraction. DNA extraction was carried out using the GenElute™ Mammalian Genomic DNA Miniprep Kit distributed by Sigma-Aldrich, following the manufacturer’s instructions. For molecular identification and phylogenetic reconstruction, we amplified the mitochondrial gene coding for the subunit I of the cytochrome c oxidase (COI) using the universal primers LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al. 1994). Polymerase chain reaction (PCR) amplifications were carried out in 20 μL solutions using 1.5 mM of MgCl2, 0.2 mM of each dNTP, 0.1 μM of each primer, 1 U of DreamTaq DNA polymerase (Thermo Scientific), and ∼ 2.5 ng of template DNA. The PCR profile was set as follows: initial denaturing step at 94 °C for 3 min; 34 cycles of denaturing at 94 °C for 45 s, annealing at 54 °C for 1 min, and extending ay 72 °C for 1 min and a final extending step at 72 °C for 7 min. A negative control was included in each reaction. PCR products were precipitated with sodium acetate and absolute ethanol and sent to Macrogen Europe for sequencing. The obtained sequences were compared with sequences of E. clavigera from Banyuls-sur-Mer (Gulf of Lion, Mediterranean Sea) and Plymouth (Northeastern Atlantic, English Channel) retrieved from the GenBank database. Moreover, we employed COI sequences of Eulalia quadrioculata Moore, 1906; Eulalia gracilior (Chamberlin, 1919), Eulalia levicornuta Moore, 1906 and E. ornata and Eulalia viridis (Linnaeus, 1758) obtained from the GenBank database (Table 1) for phylogenetic reconstruction.
Sequences were aligned with ClustalX 2.1 (Larkin et al. 2007), and alignments were edited in BIOEDIT version 7.2.5 (Hall 1999). Measurement of the genetic differentiation was based on the Kimura-two-Parameter (K2P) model (Kimura 1980). Unrooted Neighbour-Joining (NJ; Saitou and Nei 1987) trees were built using the software MEGA 7 (Kumar et al. 2016), considering reliable nodes supported by a high proportion (> 90%) of replicates in the bootstrap analysis (Felsenstein 1985). The bootstrap test, along with reciprocal monophyly, was used to determine whether a species-like cluster was well supported. The Automatic Barcode Gap Discovery (ABGD) method, generated on the K2P pair-wise distances, was used to support the grouping of the sequences into species. Based on the barcode gap model, this test identifies whenever the average divergence among sequences within species is lower than the average divergence inter-species (Puillandre et al. 2012).
Estimates of Eulalia densities were obtained in an abrasion platform located in Cerro Avanzado (42°49.79′S, 64°53.1′W) through the sampling of 50 randomly placed 8 × 8 cm quadrats. The number of specimens in each quadrat was counted using the program ImageJ and divided by the area.
Results
Systematics
Eulalia clavigera (Audouin & Milne-Edwards, 1834) (Figs. 2, 3, and 4)
Phyllodoce clavigera Audouin and Milne-Edwards 1834: 226–228, Pl. 5A, Figs. 9–13
Eulalia clavigera: Bonse et al. 1996: 40–45, Fig. 14 (redescr., syn.); Alós 2004: 193–196, Fig. 69 (SEM photographs)
? Eulalia viridis: Morgado and Amaral 1984: 51 (non Linnaeus, 1767)
Material examined
Morphology and genetics
South-western Atlantic. Argentina. Cerro Avanzado (42°49.79′S, 64°53.1′W) ten individuals (MSNP: P/3892-P/3901), 15 February 2015, coll. T. Vega Fernández and F. Badalamenti. North-western Atlantic. Great Britain, UK. Plymouth (50°40.45′N; 4°27.12′W), tide level, rocky shore, five specimens complete, 18 March 2006, coll. F. Pleijel; tide level, rocky shore with holdfast of Laminaria, six specimens complete, 21 Mar. 2011, coll. F. Pleijel and A. Nygren. Mediterranean Sea. Capraia Island, Italy. Capraia Island (43°1.77′N; 9°50.61′E), 0.5 m, rocky shore with calcareous algae, five specimens (MSNP: P/3024; P/3136; P/3902; P/3903), 18 March 2013, coll. C. Ravaglioli and F. Bulleri. Northern Sardinia, Italy. Stintino (40°56.13′N; 8°13′47.86″E), 0.5 m, rocky shore with calcareous algae, endolithic, five specimens (MSNP: P/3456; P/3904; P/3905; P/3906; P/3907), 15 May 2013, coll. J. Langeneck and M. Casu.
Morphology
South-western Atlantic. Argentina. Cerro Avanzado (42°49.79′S, 64°53.1′W), five specimens (CNP INV 993), two complete, others fragmented, 13 August 2007, coll. unknown; 27 specimens; 6 ethanol-preserved specimens (ECOSUR-OH-P702–706), 2 complete, and 21 formalin-preserved specimens (ECOSUR-P2903), 14 complete, and 7 other anterior fragments, Northern rocky platform, 19 February 2013, coll. E. González, J.M. Orensanz and S.I. Salazar-Vallejo. Punta Cuevas (42°46′S, 64°54′W), Puerto Madryn, nine specimens, six complete, among mussels, 3 December 2009, coll. B. Trovant and J.M. Orensanz. Ambrosetti (42°50.00′S, 64°50.00′W), ten specimens (CNP INV 964), five complete, others fragmented, 15 February 2010, coll. unknown. Brazil. Cassino (32°11′S, 52°9′W), five specimens (CNP INV 1647), three complete, others fragmented, 9 March 2008, coll. unknown. North-eastern Atlantic. Bretagne, France. Beg an Fry, Guimaëc (48°40′04″N, 03°42′27″W), 1 m, rocky shore, two specimens (LACM, formerly SMF 4639, id. Eulalia viridis (Linnaeus 1767)), complete, 13 March 1994, coll. D. Fiege.
Description. Complete specimens with up to 168 chaetigers and 78 mm total length, for 3 mm maximum width (smallest 13 mm long, 1 mm wide, 94 chaetigers). Prostomium triangular, almost as long as wide; antennae, palps and tentacular cirri tapered, but tips can be modified due to preservation. Median antenna slightly ahead of eyes, reaching prostomial anterior margin; longest tentacular cirri reach chaetigers 7–8 (6–9 in smaller specimens) (Fig. 3a, d). Pharynx with densely packed papillae, covering its whole surface; 22–30 marginal papillae, with varying shape depending on the degree of its extension (Fig. 3b, e). Dorsal cirri lanceolate, blunt, about twice longer than wide, becoming longer in posterior segments. Dorsal cirrophores narrower than dorsal cirri in anterior chaetigers; progressively wider, as wide as dorsal cirri in posterior chaetigers. Chaetal lobes bilobed, each lobe rounded. Ventral cirri ovate, about twice longer than wide, blunt, usually smaller than chaetal lobes (Fig. 4a, c, f), or as long as them (Fig. 4d–e). Parapodia uniramous, with thick bundles of heterogomph compound chaetae, handle slightly swollen distally, denticulate, hinge teeth blunt, 2–3 times longer than wide, blades tapered, finely denticulate, 6–8 times longer than wide.
Pigmentation, Living specimens are deep green (Fig. 2), including the pharynx; once preserved, the pigment fades off into a greenish hue (Fig. 3e, b). Aged specimens (Fig. 3c–e) turn into brownish or pale brownish, and the pharynx is paler. Darker, glandular spots are present laterally from prostomium, in dorsal cirri, along posterior segmental margins but missing in mid-dorsal regions and basally in parapodial bases. These spots become better defined once the green pigment fades off. Unlike the greenish pigmentation which fades off in aged specimens (Fig. 3), dark brown or blackish, possible glandular spots are present in dorsal cirri, in dorsal cirrophores and in the lower part of parapodial lobes. In dorsal cirri, they can be arranged in irregular series in median chaetigers (Fig. 4b–e), or as an irregular transverse series (Fig. 4f), or not visible (Fig. 4a).
Eulalia clavigera (Audouin & Milne-Edwards, 1834). a, b Cerro Avanzado, Puerto Madryn, Argentina, freshly preserved specimens (ECOSUR-OH). c–e Bretagne, France, aged specimens (LACM). a Anterior end, dorsal view. b Same, showing fully everted pharynx (inset: close-up of pharynx margin). c Two complete specimens, one with fully exposed pharynx. d Anterior end of above specimen. e Same, dorsal view, pharynx exposed. Scale bars—a 0.25, b 1.4, c 3.5, d 0.3, e 1 mm
Eulalia clavigera (Audouin & Milne-Edwards, 1834). a–c Cerro Avanzado, Puerto Madryn, Argentina, freshly preserved specimens (ECOSUR). d–f Bretagne, France, aged specimens (LACM). a Chaetiger 10, right parapodium, posterior view. b Chaetiger 50, right parapodium, posterior view. c Chaetiger 110, right parapodium, posterior view. d Chaetiger 10, right parapodium, posterior view. e Chaetiger 50, right parapodium, posterior view. f Chaetiger 110, right parapodium, posterior view. Scale bars—a 100, b, c 150, d 135, e 200, f 180 μm
Remarks
Specimens from the south-western and north-eastern Atlantic perfectly match as regards size, colour pattern and morphological features; Mediterranean specimens are often slightly smaller, with yellowish-green (rather than bright green) colour alive, slightly more elongate prostomium and more pointed dorsal cirri. Kato et al. (2001: 387, Table 1) compiled several Eulalia species having green pigmentation, but the only one having uniform pigmentation was Eulalia viridis (Linnaeus, 1767). Bonse et al. (1996) redescribed E. viridis and reinstated E. clavigera (Audouin & Milne-Edwards, 1834), but this paper was apparently overlooked by Kato et al. (2001). These two species have slight differences in prostomial, parapodial and pharynx papillation features that allow their distinction. According to Bonse et al. (1996), the length-to-width ratio of dorsal cirri is the most useful character to distinguish between E. viridis and E. clavigera.
Ehlers (1901) described Eulalia strigata from Puerto Madryn, Argentina. He hesitated about the generic placement because his specimen had its pharynx invaginated; he indicated that the body was brownish with a distinct mid-dorsal, longitudinal band, and that the median antenna was placed between the eyes. Since some specimens of E. clavigera become darker, sometimes brownish, a comparison with Ehlers’ (1901) description is needed. However, the drawing of a parapodium of E. strigata (Plate 7, Fig. 18) shows that it is very different from those found in E. clavigera: in E. strigata, dorsal cirri are oval to rounded, slightly tapered distally, whereas ventral cirri are rounded but markedly longer than the neurochaetal lobe. On the contrary, in E. clavigera, dorsal cirri are markedly tapered, and the ventral cirri are oval, slightly pointed and about as long as the neurochaetal lobes. A somehow similar species, Eulalia magalhaensis was described by Kinberg (1866, 1910) from Buket Island, Magellan Strait, in shallow subtidal environments. It also has a greenish body and similar prostomial and parapodial features, but dorsal and ventral cirri are lanceolate, acute, not blunt as in E. clavigera. All native species of Eulalia are therefore clearly different from the introduced E. clavigera.
Changes in pigmentation after fixation have been highlighted in the original description. Audouin and Milne-Edwards (1834: 228) indicated that “the overall colour of Phyllodoce clavigera is bright green but, through the action of alcohol, changes to metallic brown” (“La couleur générale de cette Phyllodocé clavigère est d’un vert brilliant qui, par l’action de l’alcool, passe au brun métallique”). The redescription indicated a homogeneous pigmentation but living animals are paler ventrolaterally. The pharynx distal papillae change their shape depending on the sample treatment. Non-relaxed specimens have globose, low papillae whereas osmotic shocked specimens have them thin, better defined.
Distribution
The species is naturally present in the UK, France to the Mediterranean Sea (Alós 2004) and southwards to the Canary Islands (Núñez et al. 2005; Núñez et al. 2010). It is now being recorded as an exotic species in Puerto Madryn, Argentina; the examination of southern Brazilian material highlighted the occurrence of individuals morphologically corresponding to this species, even if molecular data are not available. It is likely that individuals identified as E. viridis in southern Brazil by Morgado and Amaral (1984) also belong to E. clavigera, but we could not study their material. In Argentina, it has been found in intertidal rocky or mixed bottoms, among mussels and barnacles and spionid tube masses (Fig. 2). The mean density recorded on rocky bottoms at Cerro Avanzado was of approximately 90 individuals/m2, with a maximum density recorded of 468 individuals/m2.
Phylogenetic reconstruction and species delimitation
We obtained for our material 592 bp COI sequences (GenBank accession numbers MG253792 to MG253802). The unrooted NJ tree obtained (Fig. 5) showed that all specimens from Patagonia belonged to a highly supported clade including Eulalia clavigera from the north-eastern Atlantic Ocean (PLY) and a single individual from the northern Mediterranean Sea (BAN). These specimens should therefore be identified without any ambiguity as E. clavigera. Moreover, individuals from Portugal identified as Eulalia cf. viridis by Lobo et al. (2016) turned out to belong to the Eulalia clavigera clade, and their identification should be changed accordingly. Intraspecific K2P distances detected within this group ranged from the 0 to the 1.2%, and conversely, no trace of geographical differentiation was detected, with specimens from Patagonia, Portugal, Great Britain and the Gulf of Lion showing extremely low distances. By contrast, the remaining Mediterranean specimens examined, originally identified as E. clavigera, turned out to be the sister group of the “true” E. clavigera, showing similar intraspecific distances (0.2–0.7%) but a high interspecific distance towards E. clavigera (19–22%). All other species employed in the phylogenetic reconstruction are clearly distinct from the two lineages, with high interspecific distances (18–28%). The ABGD test univocally identified all divergent lineages as different species, suggesting that Eulalia cf. clavigera from the Mediterranean Sea actually represents a different, undescribed species.
Tree inferred using the Neighbour-Joining method on Kimura-2-Parameter COI distances. The tree is drawn to scale. Only significant bootstrap values (> 90%) are shown next to the nodes. *herein sequenced. BAN Banyuls-sur-Mer, Mediterranean Sea; CAP Capraia Island, Mediterranean Sea; PAT Puerto Madryn, Patagonia, SW Atlantic Ocean; PLY Plymouth, NE Atlantic Ocean; POR Portugal, NE Atlantic Ocean; STI Stintino, Mediterranean Sea
Discussion
Morphological and molecular characterisation unambiguously demonstrates the occurrence of Eulalia clavigera in shallow-water environments of Patagonia. The examined specimens do not show any difference from European material both from the molecular and the morphological point of view; on the other hand, morphological features allow a sure distinction from native Eulalia species occurring in infralittoral and upper subtidal environments of Patagonia. E. clavigera is known to effectively resist dehydration (Kensler 1967), has been recorded from salt-marshes (Nicol 1935) and is often seen crawling among barnacles in European waters (Evans 1949). This ability to thrive in different environments characterised by large variation in chemical and physical parameters could explain why this species has been capable to establish abundant populations in the upper intertidal, among Spartina root mats, as it was observed in Puerto Madryn, Argentina. The absence of differentiation between specimens from the north-eastern and south-western Atlantic Ocean strongly supports the hypothesis of a recent introduction of E. clavigera in south-western Atlantic waters; the introduction date is currently unknown, but this species has not been recorded in intensive surveys carried out in the 1970s (J. M. Orensanz, pers. comm.) and therefore, it is likely that its introduction occurred in the last three decades. The relatively recent development of aluminium industry (1970), that led to a substantial expansion of the small town of Puerto Madryn, and to a significant increase of its international naval connections, is strongly consistent with the hypothesis of a recent unintentional introduction of E. clavigera with shipping, either with ballast waters or in fouling communities (Schwindt et al. 2014). On the other hand, specimens from Great Britain, Portugal and northern Mediterranean Sea did not show any trace of genetic differentiation, and intraspecific distances detected were always very low. This suggests a high connectivity among populations of E. clavigera and prevents a more precise reconstruction of the origin of the introduced population of E. clavigera recorded in the present study.
This study highlights also some uncertainties in the taxonomy of green Eulalia species. The distinction at specific level between E. clavigera and E. viridis was confirmed only recently by Bonse et al. (1996), and Kato et al. (2001) still considered E. viridis as the only species with uniform green pigmentation. The distinction between E. clavigera and E. viridis is difficult, especially in environmental monitoring surveys, and the two species are often confused: for instance, E. viridis is still reported from the coast of Portugal (Rodrigo et al. 2015), but sequences obtained by Lobo et al. (2016) from Portuguese specimens, and deposited in GenBank as E. viridis, actually belong to E. clavigera (Fig. 5). Alós (2004) suggested, in agreement with Bonse et al. (1996), that E. viridis is a northern boreal and sub-arctic species, and it does not occur in the majority of the European Atlantic coastline, whereas E. clavigera is a temperate species, widespread in the Atlantic and in the Mediterranean Sea. The occurrence of E. clavigera in the Mediterranean Sea is confirmed by the sequence from Banyuls-sur-Mer in our phylogenetic reconstruction; however, specimens from shallow environments of the Mediterranean Sea sequenced in this work turned out to be only distantly related to both E. clavigera and E. viridis and should probably be assigned to a currently undescribed species. Possible morphological differences of the new species towards both E. clavigera and E. viridis are still uncertain and might take into account fine differences in the shape of prostomium and cirri, and in the live colour pattern, that is nonetheless still completely green, without any trace of contrasted drawings. Considering that the Gulf of Lion represents one of the coldest areas in the Mediterranean Sea, showing the occurrence of several Atlantic relict species, it is possible that E. clavigera is a relict species in the Mediterranean Sea, whereas the majority of the shallow-water green Eulalia in the Mediterranean Sea should be assigned to one, or more, different species. Recent studies on the genus Eulalia highlighted that different species are almost impossible to distinguish based on features of the fixed individual, whereas live colour represents one of the most important features in the taxonomy of this genus (Schimmenti et al. 2016). It is very likely, therefore, that further studies will highlight in Mediterranean Eulalia cf. clavigera a previously unexpected diversity, as already shown for other Phyllodocidae (Nygren and Pleijel 2011), and that the distribution of the “true” E. clavigera in Mediterranean environments will turn out to be distinctly narrower.
Taxonomic uncertainties tend to hinder the research about polychaete introductions, and several allegedly alien polychaetes should actually be considered cryptogenic (Çinar 2013). In several cases, new records of polychaete species are merely the consequence of taxonomic revisions (D’Alessandro et al. 2016) or of more detailed studies on poorly known environments (Simboura and Zenetos 2005), and several allegedly alien species have been demonstrated to represent misidentifications of native, often undescribed species (Faulwetter et al. 2008). Molecular identification techniques represent in this frame a powerful tool to disentangle cryptogenic species issues, but until now, their use for polychaetes has been restricted to few taxa, often with implication for human economic activities (Sun et al. 2017), whereas the majority of cryptogenic species cases still remain unsolved. Taxonomic uncertainties most likely prevent also a correct identification of non-native species in the family Phyllodocidae: Çinar (2013) listed only four alien Phyllodocidae, and it is noteworthy that two of them are Lessepsian immigrants (one might be cryptogenic—see Alós 2004) and the remaining two species are currently considered as species complexes, thus of uncertain taxonomy and origin. The complex taxonomy of the genus Eulalia, and more specifically of the apparently uniform group of the “green Eulalia”, could account for the late identification of E. clavigera as a new successful invader in Patagonia; however, the combination of morphological and molecular approaches confirmed the identity of these specimens. The present work shows that specimens from Patagonia actually belong to E. clavigera and have been most likely introduced in Patagonia from the north-eastern Atlantic Ocean after the 1970s, probably by shipping, even if a more precise origin and path of introduction cannot be traced. The high abundances observed in this species, and its regular observation in intertidal rocky communities, suggest that this species is currently established in the study area. Eulalia clavigera is a relatively large predator, feeding mostly on mussels and barnacles (Emson 1977; Rodrigo et al. 2015), even if some studies regard it chiefly as a scavenger of predation remains left by other species (Michel 1970; Morton 2011); the settlement of a large population of this species is therefore expected to largely change trophic interactions within the native assemblage. For this reason, the demography and effect of the introduced E. clavigera on native species deserve close monitoring.
References
Alós C (2004) Familia Phyllodocidae Örsted, 1843. In: Vieitez JM, Alós C, Parapar J, Besteiro C, Moreira J, Nüñez J, Laborda J, San Martín G (eds) Fauna Iberica 25, Annelida Polychaeta I. Museo Nacional de Ciencias Naturales. CSIC, Madrid, pp 105–209
Audouin JV, Milne-Edwards H (1834) Recherches pour servir a l’histoire naturelle du littoral de la France, ou, Recueil de mémoires sur l’anatomie, la physiologie, la classification et les moeurs des animaux des nos côtes: ouvrage accompagné de planches faites d'après nature. volumen 2, Annélides, première partie. Crochard, Paris, 290 pp, 18 pls
Barroso R, Klautau M, Solé-Cava AM, Paiva PC (2010) Eurythoe complanata (Polychaeta: Amphinomidae), the ‘cosmopolitan’ fireworm, consists of at least three cryptic species. Mar Biol 157:69–80
Bertocci I, Badalamenti F, Lo Brutto S, Mikac B, Pipitone C, Schimmenti E, Vega Fernández T, Musco L (2017) Reducing the data-deficiency of threatened European habitats: spatial variation of sabellariid worm reefs and associated fauna in the Sicily Channel, Mediterranean Sea. Mar Environ Res 130:325–337
Blakeslee AM, Byers JE, Lesser MP (2008) Solving cryptogenic histories using host and parasite molecular genetics: the resolution of Littorina littorea’s north American origin. Mol Ecol 17:3684–3696
Blank M, Laine AO, Jürss K, Bastrop R (2008) Molecular identification key based on PCR/RFLP for three polychaete sibling species of the genus Marenzelleria, and the species’ current distribution in the Baltic Sea. Helgoland Mar Res 62:129–141
Bolton JJ, Andreakis N, Anderson RJ (2011) Molecular evidence for three separate cryptic introduction of the red seaweed Asparagopsis (Bonnemaisoniales, Rhodophyta) in South Africa. Afr J Mar Sci 33:263–271
Bonse S, Schmidt H, Eibye-Jacobsen D, Westheide W (1996) Eulalia viridis (Polychaeta: Phyllodocidae) is a complex of two species in northern Europe: results from biochemical and morphological analysis. Cah Biol Mar 37:33–48
Borja A, Galparsoro I, Solaun O, Muxika I, Tello EM, Uriarte A, Valencia V (2005) The European water framework directive and the DPSIR, a methodological approach to assess the risk of failing to achieve good ecological status. Estuar Coast Shelf Sci 66:84–96
Carlton JT (1989) Man’s role in changing the face of the ocean: biological invasions and implications for conservation of near-shore environments. Conserv Biol 3:265–273
Carlton JT (1996) Biological invasions and cryptogenic species. Ecology 77:1653–1655
Carr CM, Hardy SM, Brown TM, Macdonald TA, Hebert PDN (2011) A tri-oceanic perspective: DNA barcoding reveals geographic structure and cryptic diversity in Canadian polychaetes. Plos One 6:e22232
Carrera-Parra LF, Salazar-Vallejo SI (2011) Redescriptions of Eunice filamentosa and E. denticulata and description of E. tovarae n. sp. (Polychaeta: Eunicidae), highlighted with morphological and molecular data. Zootaxa 2880:51–64
Çinar ME (2013) Alien polychaete species worldwide: current status and their impacts. J Mar Biol Assoc UK 93:1257–1278
Çinar ME, Altun C (2007) A preliminary study on the population characteristics of the lessepsian species Pseudonereis anomala (Polychaeta: Nereididae) in Iskenderun Bay (Levantine Sea, eastern Mediterranean). Turk J Zool 31:403–410
Crooks JA (2002) Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97:153–166
D’Alessandro M, Romeo T, Castriota L, Cosentino A, Perzia P, Martins R (2016) New records of Lumbrineridae (Annelida: Polychaeta) in the Mediterranean biogeographic province, with an updated taxonomic key. Ital J Zool 83:233–243
Ehlers E (1901) Die Polychaeten des magellanischen und chilenischen Strandes: Ein faunisticher Versuch. Fetschrift zur Feier des 150-jährigen Bestehens der Königlichen Gesellschaft der Wissenschaften zu Göttingen. Weidmannsche Buchhandlung, Berlin, 232 pp
Emson RH (1977) The feeding and consequent role of Eulalia viridis (O.F. Müller) (Polychaeta) in intertidal communities. J Mar Biol Assoc UK 57:93–96
Evans RG (1949) The intertidal ecology of rocky shores in south Pembrokeshire. J Ecol 37:120–139
Faulwetter S, Vasileiadou A, Papageorgiou N, Arvanitidis C (2008) Description of a new species of Streptosyllis (Polychaeta: Syllidae) from the Mediterranean and Canary Islands with a re-description of Streptosyllis arenae and comments on the taxonomy of Streptosyllis and some morphologically similar genera. Zootaxa 1847:1–18
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 4:783–791
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Giangrande A (2003) Biodiversity, conservation and the ‘taxonomic impediment’. Aquat Conserv 13:451–459
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid Symp Ser 41:95–98
Holloway MG, Keough MJ (2002) Effects of an introduced polychaete, Sabella spallanzanii, on the development of epifaunal assemblages. Mar Ecol Prog Ser 236:137–154
Hulme PE (2009) Trade, transport and trouble: managing invasive species pathways in an era of globalization. J Appl Ecol 46:10–18
Jeschke JM, Bacher S, Blackburn TM, Dick JTA, Essl F, Evans T, Gaertner M, Hulme PE, Kühn I, Mrugała A, Pergl J, Pyšek P, Rabitsch W, Ricciardi A, Richardson DM, Sendek A, Vilà M, Winter M, Kumschick S (2014) Defining the impact of non-native species. Conserv Biol 28:1188–1194
Kato T, Pleijel F, Mawatari SF (2001) Eulalia gemina (Phyllodocidae: Polychaeta), a new species form Shirahama. Jpn Proc Biol Soc Wash 114:381–388
Kensler CB (1967) Desiccation resistance of intertidal crevice species as a factor in their zonation. J Anim Ecol 36:391–406
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kinberg JGH (1866) Annulata nova (Nephthydea, Phyllodocea, Alciopea, Hesionida, Glycerea, Goniadea, Syllidea, Ariciea, Spiodea, Aonidea, Cirratulida, Opheliacea). Kongelige Vetenskaps-Akademiens Förhandlingar 22:239–258
Kinberg JGH (1910) Konglia Svenska Fregatten Eugenies Resa imkring jorden under befall af C.A. Virgin åren 1851–1853. Vetenskapliga Iakttagelser På H. Maj: T. Konnung Oscar den Förstes befallning utgifna. Kongelige Svenska Vetenskaps-Akademien. Zoologie, 3. Annulata 2:33–78
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:947–2948
Leppäkoski E, Gollasch S, Olenin S (2002) Invasive aquatic species of Europe. Distribution, impacts and management. Kluwer Academic Publishers, Dordrecht, p 583
Lobo J, Teixeira MAL, Borges LMS, Ferreira MSG, Hollatz C, Gomes PT, Sousa R, Ravara A, Costa MH, Costa FO (2016) Starting a DNA barcode reference library for shallow water polychaetes from the southern European Atlantic coast. Mol Ecol Res 16:298–313
Lodge DM, Williams S, McIsaac HJ, Hayes KR, Leung B, Reichard S, Mack RN, Moyle PB, Smith M, Andow DA, Carlton JT, McMichael A (2006) Biological invasions: recommendations for U.S. policy and management. Ecol Appl 16:2035–2054
Marchini A, Galil BS, Occhipinti-Ambrogi A (2015) Recommendations on standardizing lists of marine alien species: lessons from the Mediterranean Sea. Mar Pollut Bull 101:267–273
Michel C (1970) Role physiologique de la trompe chez quatre annelides polychètes appartenant aux genres: Eulalia, Phyllodoce, Glycera et Notomastus. Cah Biol Mar 11:209–228
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Fr Ecol Environ 6:485–492
Morgado EH, Amaral ACZ (1984) Anelídeos poliquetos asociados ao briozário Schizoporella unicornis (Johnston), 4. Phyllodocidae e Hesionidae. Rev Bras Zool 2:49–54
Morton B (2011) Predator-prey-scavenging interactions between Nucella lapillus, Carcinus maenas and Eulalia viridis all exploiting Mytilus galloprovincialis on a rocky shore recovering from tributyl-tin (TBT) pollution. J Nat Hist 45:2397–2417
Nicol EAT (1935) The ecology of a salt-marsh. J Mar Biol Assoc UK 20:203–261
Núñez J, Brito MC, Docoito JR (2005) Anélidos poliquetos de Canarias: Catálogo de especies, distribución y hábitats. Vieraea 33:297–321
Núñez J, Riera R, Brito MC (2010) Nuevos registros de poliquetos macrofaunales en las islas Salvajes. Vieraea 38:55–62
Nygren A (2014) Cryptic polychaete diversity: a review. Zool Scripta 43:172–183
Nygren A, Pleijel F (2011) From one to ten in a single stroke—resolving the European Eumida sanguinea (Phyllodocidae, Annelida) species complex. Mol Phylogenet Evol 58:132–141
Occhipinti Ambrogi A, Savini D (2003) Biological invasions as a component of global change in stressed marine ecosystems. Mar Pollut Bull 46:542–551
Ojaveer H, Galil BS, Minchin D, Olenin S, Amorim A, Canning-Clode J, Chainho P, Copp GH, Gollasch S, Jelmertj A, Lehtiniemi M, McKenzie C, Mikuš J, Miossec L, Occhipinti-Ambrogi A, Pećarević M, Pederson J, Quilez-Badia G, Wijsman JWM, Zenetos A (2013) Ten recommendations for advancing the assessment and management of non-indigenous species in marine ecosystems. Mar Policy 44:160–165
Orensanz JM, Schwindt E, Pastorino G, Bortolus A, Casas G, Darrigran G, Elías R, López Gappa JJ, Obenat S, Pascual M, Penchaszadeh P, Piriz ML, Scarabino F, Spivak D, Vallarino EA (2002) No longer the pristine confines of the world ocean: a survey of exotic marine species in the southwestern Atlantic. Biol Invasions 4:115–143
Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O’Connell C, Wong E, Russel L, Zern J, Aquino T, Tsomondo T (2001) Economic and environmental threats of alien plant, animal, and microbe invasions. Agric Ecosyst Environ 84:1–20
Pollard DA, Hutchings PA (1990a) A review of exotic marine organisms introduced to the Australian region. I. Fishes. Asian Fish Sci 3:205–221
Pollard DA, Hutchings PA (1990b) A review of exotic marine organisms introduced to the Australian region. II. Invertebrates. Asian Fish Sci 3:223–250
Puillandre N, Lambert A, Brouillet S, Achaz G (2012) ABGD, automatic barcode gap discovery for primary species delimitation. Mol Ecol 21:1864–1877
Rilov G, Crooks JA (2009) Biological invasions in marine ecosystems. Ecological, management, and geographical perspectives. Springer Verlag, Berlin
Rodrigo AP, Costa MH, Alves de Matos AP, Carrapiço F, Costa PM (2015) A study on the digestive physiology of a marine polychaete (Eulalia viridis) through microanatomical changes of epithelia during the digestive cycle. Microsc Microanal 21:91–101
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schimmenti E, Musco L, Lo Brutto S, Mikac B, Nygren A, Badalamenti F (2016) Mediterranean record of Eulalia ornata (Annelida: Phyllodocidae) corroborating its fidelity link with the Sabellaria alveolata reef habitat. Mediterr Mar Sci 17:359–370
Schwindt E, López Gappa J, Raffo MP, Tatián M, Bortolus A, Orensanz JM, Alonso G, Diez ME, Doti B, Genzano G, Lagger C, Lovrich G, Piriz ML, Méndez MM, Savoya V, Sueiro MC (2014) Marine fouling invasions in ports of Patagonia (Argentina) with implications for legislation and monitoring programs. Mar Environ Res 99:60–68
Simboura N, Zenetos A (2005) Increasing polychaete diversity as a consequence of increasing research effort in Greek waters: new records and exotic species. Mediterr Mar Sci 6:75–88
Sun Y, Wong E, Keppel E, Williamson JE, Kupriyanova EK (2017) A global invader or a complex of regionally distributed species? Clarifying the status of an invasive calcareous tubeworm Hydroides dianthus (Verrill, 1873) (Polychaeta: Serpulidae) using DNA barcoding. Mar Biol 164:28
Tovar-Hernández MA, Yáñez-Rivera B, Bortolini-Rosales JL (2011) Reproduction of the invasive fan worm Branchiomma bairdi (Polychaeta: Sabellidae). Mar Biol Res 7:710–718
Vilà M, Basnou C, Pyšek P, Joseffson M, Genovesi P, Gollasch S, Nentwig W, Olenin S, Roques A, RoyD HPE, DAISIE partners (2010) How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Fr Ecol Environ 8:135–144
Wittenberg R, Cock MJW (2001) Invasive alien species: a toolkit of best prevention and management practices. CAB International, Wallingford 228 pp
Acknowledgements
We would like to thank Fabio Bulleri, Marco Casu and Chiara Ravaglioli for their help in sampling the Mediterranean specimens of Eulalia cf. clavigera; Michele Barbieri and Federica Squarcia for their support in the molecular laboratory work. Ana Parma and the late Lobo Orensanz enthusiastically shared their home and experience, and the latter also participated in the collecting trips in Puerto Madryn. Lastly, we are grateful to two anonymous reviewers that greatly contributed to the improvement of the manuscript.
Funding
The EC-funded project no. 295,213, RECOMPRA, FP7-PEOPLE-2011-IRSES funded the sampling of E. clavigera in Puerto Madryn by TVF and FB.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Field study
Permits and approval of field or observational studies have been obtained by the authors.
Additional information
Communicated by K. Kocot
Rights and permissions
About this article
Cite this article
Langeneck, J., Diez, M.E., Nygren, A. et al. Worming its way into Patagonia: an integrative approach reveals the cryptic invasion by Eulalia clavigera (Annelida: Phyllodocidae). Mar Biodiv 49, 851–861 (2019). https://doi.org/10.1007/s12526-018-0864-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-018-0864-y