Abstract
Two new species of Emertonia (Paramesochridae) were collected during cruises of RV “Meteor” to the Great Meteor Seamount (1998), the Angola Basin (2000) and the Guinea Basin (2005) and of RV “L’Atalante” to the Pacific Nodule Province (2004). The widely distributed Emertonia clausi sp. n. was represented by 13 individuals found in depths from 4,005 m to 5,389 m in Atlantic and Pacific abyssal plains. A revision of material from the plateau and the slope of the Great Meteor Seamount revealed that E. clausi sp. n. is one of the rare eurybathic species of Harpacticoida, as it was also found at the plateau and the slope of the seamount. Emertonia ingridae sp. n. was represented by two individuals reported only from the Atlantic Guinea Basin at 5,139 m and 5,167 m water depth. The new species are placed in Emertonia due to their characteristic swimming legs with 1-segmented endopods in P2–P4 with one apical setal element. E. clausi sp. n. and E. ingridae sp. n. are closely related species, as they have many characters in common, but differ in subtleties. Unique features of both, E. clausi sp. n. and E. ingridae sp. n., are the single seta in enp2 P1 and the structure of seta V of the furcal rami, which changes from robust to flexible after approximately 0.4 × the length of the seta. The robust part of the seta carries in E. ingridae sp. n. two spinule pairs, in E. clausi sp. n. three pairs, the distal pair being situated at the point of structure change. The drawn-out parts of the baseoendopods of the female P5 are cleft medially and angled at the distal ends in both species. The most obvious differences between the two species are the relationship of length to width of the furcal rami, the total length of the furcal rami compared to the three last body somites, the articulated/non-articulated base of seta VII at the furcal rami, and the presence/absence of a seta at the syncoxa of the maxilliped. The new species presented here raise the number of valid members of the genus to 39.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the course of the global Census of Marine Life field project CeDAMar (Census of the Diversity of Abyssal Marine Life). many international deep-sea expeditions such as ANDEEP I–III, DIVA-1–3, and NODINAUT were carried out. During these cruises the benthos of abyssal plains of the Southern Ocean, the Atlantic and the Pacific Ocean was investigated. A high sampling effort with the multicorer (Barnett et al. 1984) and other devices revealed several hundred individuals of Paramesochridae, a family of harpacticoid copepods that is rarely found in deep-sea sediments (Gheerardyn and Veit-Köhler 2009).
Paramesochridae mostly have a cylindrical body shape, generally a small rostrum and differing degrees of reduction in segmentation and setation in the endopods of the swimming legs (Boxshall and Halsey 2004). Emertonia Huys, 2009 can be distinguished from the other genera of the subfamily Paramesochrinae Huys, 2009 by their one-segmented endopods of P2–P4 with one apical seta. According to the short working diagnosis by Veit-Köhler (2004), this is the most obvious characteristic of this genus.
At present, Emertonia contains 39 species, including Emertonia clausi sp. n. and Emertonia ingridae sp. n. To date, nine new species that belong to Emertonia have been discovered in the CeDAMar deep-sea material (for data from the Southeast Atlantic and the Southern Ocean, see Gheerardyn and Veit-Köhler 2009). Including the two species presented here, six deep-sea species of Emertonia have been described so far. The other most recently described deep-sea species are Emertonia schminkei (Veit-Köhler and Drewes, 2009) from the Atlantic Guinea, Angola and Cape Basins and Emertonia minor (Vasconcelos, Veit-Köhler, Drewes & Parreira dos Santos, 2009) from the Brazilian deep sea.
Materials and methods
Meiofauna samples that contained specimens of the new species were collected during the SEAMEC expedition in 1998 (Meteor 42.3; Plum and George 2009), the DIVA-1 cruise in 2000 (Meteor 48.1; Gheerardyn and Veit-Köhler 2009), during the DIVA-2 campaign in 2005 (Meteor 63.2; Menzel and George 2012), and the NODINAUT expedition in 2004 (Mahatma 2009). The majority of the sediment samples were taken with the multicorer, one sample was collected with the giant box corer and one with the blade corer.
Specimens of Emertonia clausi sp. n. were found in the Angola Basin, the Guinea Basin, at the Great Meteor Seamount and in the Pacific Nodule Province inside the track. Emertonia ingridae sp. n. was only found in the Guinea Basin (Table 1; Fig. 1).
Sediment samples were preserved with 5 % buffered formalin. In the laboratory, they were washed with tap water through a 40-μm-mesh sieve. To extract meiofauna from the remaining sediment particles, samples were centrifuged with a colloidal silica polymer (H.C. Stark, Levasil 200/40 %, ρ = 1.17) as flotation medium. Kaolin was used to hold back the heavier particles during decantation (McIntyre and Warwick 1984) while the less dense meiofauna organisms are found in the floating matter. After every centrifugation step (three repetitions of 6 min at 4,000 rpm), the floating matter was decanted and rinsed with tap water. Meiofauna was sorted to higher taxon level. Copepods were transferred to a mixture of glycerine and water (1:1). Emertonia specimens were detected with the aid of a Leica MZ 12.5 stereomicroscope and a Leica DMR microscope.
The holotypes of E. clausi sp. n. and E. ingridae sp. n. were scanned with a confocal laser scanning microscope. Specimens were stained with saturated aqueous solution of acid fuchsin with water–water solution (modified after Michels and Büntzow 2010) and viewed on a Leica TCS SP5 (DZMB, Senckenberg am Meer) equipped with a Leica DM 5000 B microscope (640× magnification) and three visible light lasers. Based on the image stacks, maximum intensity projections were created with the Leica LAS software (Leica Microsystems). The final images were assembled and adjusted for contrast and brightness using the software Adobe Photoshop CS4.
Specimens of E. clausi sp. n. and E. ingridae sp. n. selected for description were mounted in glycerine on slides and drawn from the dorsal and lateral view. None of the specimens of E. clausi sp. n. and E. ingridae sp. n. were dissected in order to preserve them and to avoid the risk of damage. All structures were drawn from the undissected specimens, and it is possible that tiny spinules, pores and surface structures may have been overlooked. Nevertheless, the general segmentation and setation of the appendages and mouthparts was clearly visible. Illustrations were made using a Leica DM 2500 microscope equipped with differential interference contrast (DIC) at 400–2,000 × magnification.
Abbreviations used in this text: exp = exopod, enp = endopod, benp = baseoendopod, P1–P6 = swimming legs 1–6, and enp1 = the first segment of the endopod, aes = aesthetasc, A1 = antennule.
Emertonia clausi Pointner & Veit-Köhler, sp. n.
Taxonomy
Paramesochridae Lang, 1944
Emertonia Huys, 2009
Emertonia clausi Pointner & Veit-Köhler, sp. n
Type material
The examined species are registered and deposited in the Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Germany. All specimens of the examined material were collected in the Angola and Guinea Basin. Station numbers indicate “expedition name cruise-number/gear deployment number-core number”.
Male holotype, SMF (37054): not dissected, 1 slide; DIVA-1 station M48.1/346-7-12 (16°17.0′S, 5°27.0′E), 07/27/2000, depth 5,389 m
Female allotype, SMF (37055): not dissected, 1 slide; DIVA-2 station M63.2/58-3 (0°0.00′S, 2°25.0′W), 03/14/2005, depth 5,060 m
Male paratype, SMF (37056) not dissected, 1 slide; DIVA-1 station M48.1/342-1-8 (17°08.0′S, 4°42.0′E), 07/24/2000, depth 5,415 m
Eleven additional individuals were detected; information is found in Table 1.
Type locality
Type locality is station M48.1/346 (16°17′S, 5°27′E) in the Angola Basin.
Description
Male
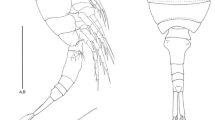
Habitus (Fig. 2) of male shown on images taken with confocal laser scanning microscope.
Body (Fig. 3) Total body length measured from anterior tip of rostrum to posterior margin of telson: Holotype 0.27 mm, including caudal rami: 0.32 mm. Body cylindrical, slightly depressed dorsoventrally, with prosome only slightly wider than urosome. Whole cephalosome covered with small, round depressions resembling surface of golf ball, exemplarily shown in frame (Fig. 3a). Pores and sensilla present in small numbers on whole body. Six sensilla located on second free somite and genital somite, four sensilla on cephalosome, first, third, fourth, sixth and seventh free somite. Two sensilla found on telson and none on eighth free somite. Pores on every somite except penultimate urosomite. Penultimate urosomite with fine pseudoperculum. On ventral side, posterior end of anal somite with one row of spinules (Fig. 4b) and urosomites three and four fringed with one continuous row of minute spinules (Fig. 5a).
Furcal rami (Fig. 4b) Long and cylindrical, 5.67× longer than wide, with proximal and distal pores and six visible elements; seta II slender, seta III as long as seta II but pinnate, seta IV slender, some holes indicate possible pinnation, longer than seta III, seta V slender, with three pairs of spinules in posterior quarter, opposite to each other, at distal pair of spinules constitution of seta changes to rat-tailed, seta VI minute, situated at inner margin of caudal rami and seta VII slender, situated on knob-like elevations on dorsal surface, longer than seta II with one single feather. Seta I not visible.
Antennule (Fig. 6) Seven-segmented, segment I with spinules along inner margin. Segment II and III are partially fused. Segment VII of distinct triangular shape, inner margin with scales. Setal ornamentation:
-
I (1) One short seta
-
II (1) One slender seta
-
III (6) Four slender, naked setae, one of them longer than the others, two stout, pinnate setae
-
IV (6) Six long, slender setae, three naked, three plumose
-
V [5+(1+aes)] Two long, slender and naked setae, one of them fused at base with aesthetasc, three small, naked setae and one longer, plumose seta
-
VI (3) Three identical setae, long, slender and naked
-
VII [6+(1+aes)] Six slender, naked setae, three of them long, the others shorter, one aesthetasc fused at base with slender seta
Antenna (Fig. 7a) Basis with small depression on surface. Endopod two-segmented, enp1 with one long, plumose abexopodal seta and spinules on outer margin. Enp2 dorsal view (Fig. 7b) with small cuticular depressions, one row of three minute spinules, two spinules at outer margin, three longer spinules at inner margin and three grouped subapical naked setae, apical margin (Fig. 7a) with six setae, one pinnate, four geniculate, all of different size. Exopod one-segmented with one plumose seta, two lateral naked spines, one with bifurcate tip, two apical naked spines and one row of three minute spinules.
Mandible (Fig. 7d) Coxa with slender and elongated gnathobasis, small ridge on inner margin, cutting edge with two big and two smaller teeth, one of which bidentate. Basis of mandibular palp (Fig. 7c) with one slender seta, enp1 with two slender setae, enp2 apically with six slender, naked setae fused together at base. Exp smaller than enp, one-segmented with two lateral naked spines and two apical, plumose setae.
Maxillule (Fig. 7e) Praecoxal arthrite with two juxtaposed slender setae, one lateral naked seta. Inner margin of arthrite with six stout setae, three of which with single pinnule. Coxal endite with four long, slender and naked setae. Basis with four slender setae, three of which plumose, one of which longer than the others. Enp one-segmented with three different setae, one small and naked, one stout, plumose and one slender, plumose. Exp one-segmented with two slender setae, one of which plumose.
Maxilla (Fig.7f) Praecoxa and coxa fused to form syncoxa bearing three endites. Proximal endite slightly bilobed with one seta on proximal lobe and two setae on distal lobe, one of which stout, other plumose, middle endite with three slender, plumose setae, one of which smaller, distal endite with three plumose setae, two of which small. Allobasis with two setae, both long, slender and pinnate. Enp two-segmented with two long setae on enp1 and three slender and long setae on enp2.
Maxilliped (Fig. 7g) Syncoxa with two short rows of minute spinules and one long, naked seta. Basis without ornamentation or setae. Enp one-segmented, with five naked setae, two long and geniculate, two small and hairlike, one long and stout.
Swimming legs (Fig. 8) P1–P4 very small. Coxa present. Setal formula (Table 2) given according to Lang (1934).
P1 (Fig. 8a) Basis with one outer and one inner naked seta. Enp slightly longer than exp, two-segmented and enp1 armed with spinules along outer margin, enp2 with one long, naked seta. Exp two-segmented, both segments with one row of spinules on outer margin, exp1 with one outer pinnate spine, exp2 with two plumose outer spines and two plumose, terminal setae.
P2–P4 (Fig. 8b–d) Basis of P2–P4 bearing one outer seta. Basis of P2 with additional, long, inner setules, basis of P3 with one single minute spinule and basis of P4 with spinule row accompanying outer seta. Enp of P2–P4 one-segmented, but with one slight depression at outer side indicating former segmentation, and rows of setules at different sizes along outer margin. Enp P2–P4 with one apical setal element surrounded by crown-like row of spinules. Apical seta of enp P2 and P3 plumose, spine of enp P4 long and stout with spatulate tip. Exp P2–P4 three-segmented, on outer margin of every segment one row of spinules, exp1 P2 with one row of long setules on inner margin. Cuticular depressions present only in exp1 P3 and exp2–3 P4, enp P2 with subapical, large pore. Exp1–2 P2–P4 with one outer knife-like shaped spine. Exp3 P2–P3 with two outer spines, proximal one knife like, apical one knife-like in P2 and rat-tailed in P3, two apical, plumose setae, outer one rat-tailed in both legs, inner one rat-tailed only in P3. Exp3 P4 with one apical and one outer seta, both rat-tailed, inner one longer and slightly pinnate. Intercoxal sclerite exemplarily shown for P4 (Fig. 8d).
P5 (Fig. 5a) Legs fused, small exopod clearly separated from baseoendopod. Benp bears one outer, plumose seta. Surface of benp naked with two pores. Exp with four stout spines, outer three naked, inner one plumose, size of the spines increases inwards, row of minute spinules on inner margin of exp.
P6 (Fig. 5a) Both legs consisted of medially touching plates with three setae. Outer one longest, plumose and wavy, inner two of same size and stout. Surface of each leg covered with one large pore and some small cuticular depressions.
Female
Body (Fig. 9) Total body length measured from anterior tip of rostrum to posterior margin of anal somite (allotype): 0.30 mm, including caudal rami 0.36 mm. Second and third urosomites fused to form genital complex. Whole body covered with sensilla. On ventral side, lateral margin of urosomite three and four covered with short spinule rows. Posterior margin of penultimate somite with two short rows of spinules (Fig. 5b).
Furcal rami (Fig. 4a) Long and cylindrical, 6.42× longer than wide, with proximal and distal pores and six visible elements; seta II long and slender, seta IV with several pinnules, seta VII naked. Setae III, V and VI of same appearance as in male. Row of short spinules along apical ventral rim of furcal rami. Seta I not visible.
Antennule (Fig. 10) Eight-segmented. Segment I with one row of setules on inner margin. Setation of segment VII shown separately in Fig. 10b for reasons of clarity. Setal ornamentation:
-
I (1) One naked seta
-
II (7) Five slender, long and naked, two short and stout setae
-
III (8) Five slender, long setae, four plumose, one short, slender seta, two stout, pinnate setae
-
IV [1+(1+aes)] Two slender, plumose setae, one of which fused at basis with slender aesthetasc
-
V (1) One slender, plumose seta
-
VI (2) Two naked setae, one of which longer than the other
-
VII (3) Three naked, slender setae, one of which much shorter than the others
-
VIII [4+(1+aes)] Five naked, slender setae of different sizes, two of which smaller, one of the longer ones fused at basis with aesthetasc
Mouthparts as in male.
Swimming legs almost as in male. Only difference: seta on enp P4 gradually tapering, without frayed tip (Fig. 8e).
P5 (Fig. 5b) Legs fused, small exopod clearly separated from baseoendopod. Benp with one outer, basal and plumose seta. Surface of benp naked with two big pores. Drawn-out endopodal lobes angled and medially cleft to half way of leg’s length. Each of endopodal lobes armed with two stout, bipinnate setae, inner one shorter than outer one. In most individuals these setae cross each other. Exp with three naked stout setae, size increases inwards. Outer one intersects middle seta. Inner margin of exp with one row of minute spinules, on right side of body not as easy to detect as on left side.
P6 and genital complex (Fig. 5b) Sixth legs represented by small, fused outgrowth, with one plumose seta on each side. Genital complex seen as pore with ridge above it.
Emertonia ingridae Pointner & Veit-Köhler, sp. n.
Taxonomy
Paramesochridae Lang, 1944
Emertonia Huys, 2009
Emertonia ingridae Pointner & Veit-Köhler, sp. n.
Type material
The examined species are registered and deposited in the Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Germany. All specimens of the examined material were collected in the Guinea Basin. Station numbers indicate “expedition name cruise-number/gear deployment number-core number”.
Female holotype, SMF (37057): not dissected, 1 slide; DIVA-2 station M63.2/75-7 (0°50.0′N, 5°35.0′W), 03/19/2005, depth 5,139 m
Male allotype, SMF (37058): not dissected, 1 slide; DIVA-2 station M63.2/100-10 (0°37.2′N, 6°28.1′W), 03/23/2005, depth 5,167 m
Type locality
Type locality is station M63.2/75 (0°50′N, 5°35′W) in the Guinea Basin.
Description
Female
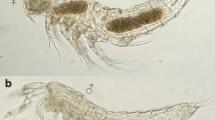
Habitus (Fig. 11) shown from dorsal and ventral view on images taken with a confocal laser scanning microscope.
Body (Fig. 12) Total body length measured from anterior tip of rostrum to posterior margin of telson: Holotype 0.27 mm, including the caudal rami: 0.32 mm. Body cylindrical, slightly depressed dorsoventrally, with prosome only slightly wider than urosome. Whole cephalosome covered with small, round depressions resembling surface of a golf ball, exemplary shown in inset in Fig. 12a. Pores and sensilla present in small numbers on whole body. Four sensilla on cephalosome and genital somite, two sensilla on free somites three and four. Pores on every somite except for penultimate and telson. Penultimate somite with fine pseudoperculum. Ventral side of genital double-somite with one row of small and one row of long spinules, following somite with one row of small spinules (Fig. 13). Posterior margin of urosomites (except for penultimate somite) ventrolaterally with minute spinules (Fig. 14).
Furcal rami (Fig. 13) Long and cylindrical, 4.62× longer than wide, with distal pores and six visible elements; seta II slender, dorsally displaced, seta III slender, longer than II, with small flagellum, situated laterally at posterior end, seta IV slender, longer than III, seta V slender, with two pairs of spinules, bilaterally arranged, at distal pair of spinules constitution of seta changes to rat-tailed, seta VI minute, situated at inner margin of caudal rami, and seta VII slender and nearly as long as seta II. Seta I absent.
Antennule (Fig. 15) Eight-segmented, segment I with spinules along inner margin. Setal ornamentation:
-
I (0)
-
II (5) Three slender naked setae, two small plumose, fir-like setae
-
III (8) Five slender naked setae, one slender seta with one single hair, two small plumose setae
-
IV (1) One slender naked seta
-
V [1+(1+aes)] One plumose seta and one aesthetasc fused at base with long slender, naked seta
-
VI (1) One long slender plumose seta
-
VII (5) Five slender naked setae, two of them small
-
VIII [5+(1+aes)] Three slender naked setae, one naked seta, one small plumose seta and one aesthetasc fused at base with long slender seta
Antenna (Fig. 16a) Basis without cuticular depressions. Endopod two-segmented, enp1 with one long plumose abexopodal seta, enp2 armed with two spinule rows, proximal of which very minute, other one apical and broad, three grouped subapical setae, one small and pinnate, two stout and naked, and one small spinule. Apical margin with eight setae, one pinnate, five geniculate, longest of them with single pair of spinules, one naked and stout, two naked and slender, all of different sizes. Exopod one-segmented with three lateral and two apical naked spines of different sizes.
Emertonia ingridae sp. n., mouthparts: a male antenna (allotype); b female mandibular palpus (holotype); c female maxillule (holotype); d male maxilla (allotype), setae at endopod, allobasis and third endite are shown; e male maxilla (allotype), setae at second and first (bilobed) endite are shown; f female maxilliped (holotype). Scale bar 20 μm
Mandible Gnathobase not illustrated due to its small size and difficulties in observation. Basis of mandibular palp with one seta (Fig. 16b). Enp two-segmented; first segment with two slender setae and one small spine, second segment apically with six slender, naked setae fused together at base. Exp smaller than enp, one-segmented, with two naked, slender setae.
Maxillule (Fig. 16c) Praecoxal arthrite with two juxtaposed slender setae, one lateral naked seta and three small spinules. Inner margin of arthrite with five strong, stout spines. Coxal endite with three slender, naked setae and one plumose seta. Basis with endite armed with six setae, one of which plumose. Enp one-segmented with three slender setae. Exp one-segmented with one row of spinules and two apical setae, one of which plumose.
Maxilla (Fig. 16d, e) Praecoxa and coxa fused to form syncoxa bearing three endites; proximal endite slightly bilobed with one plumose setal element on proximal lobe and two on distal lobe, one of which stout and with only one pinnate, other slender; middle endite with three setae, two naked and one plumose (Fig. 16e); distal endite with three naked setae, two stout and one slender. Allobasis with two setal elements, both slender, one naked, other plumose. Enp presumably one-segmented with five setae, all slender and naked, different sizes (Fig. 16d).
Maxilliped (Fig. 16f) Syncoxa with one row and one cluster of spinules and ornamented with few cuticular depressions. Basis with one row of spinules and without armature. Enp one-segmented, on apical side with three naked long setae and one small seta with only one hair.
Swimming legs (Fig. 17) P1–P4 very small. Coxa present. Seta formula (Table 3) given according to Lang (1934).
P1 (Fig. 17a) Basis with one outer, plumose and one inner naked seta. Enp slightly longer than exp, both rami two-segmented and armed with spinules on outer margin of enp1 only. Enp1 without seta, enp2 with one terminal and naked seta. Exp1 with one outer plumose spine, exp2 with two plumose, outer spines and two plumose terminal setae, one of which spine-like.
P2–P4 (Fig. 17b–d) Basis of P2–P4 bearing one outer seta. Basis of P2 with long, inner setules. Enp of P2–P4 one-segmented with one slight depression on outer side, setules in rows at different positions and shapes: P2 and P4 in distal part of enp, P3 along complete enp. Enp P2–P4 with one apical seta, P2 seta with three hairs emerging from small pores, P3 biplumose and P4 naked, knife-shaped. Exp P2–P4 three-segmented, exp1 P2 and P3 with one inner row of long setules, exp1–3 P2–4 with one outer spine, knife-shaped, and one row of stout spinules. Apical setae of exp3 P2 with notch, distally plumose; exp3 P3 apical setae rat-tailed and plumose. Exp3 P4 with outer spine shorter than terminal seta, being rat-tailed and plumose. Intercoxal sclerite exemplarily shown for P4 (Fig. 17d).
P5 (Fig. 14a) Legs fused, small exopod clearly separated from baseoendopod. Benp with anterior pore and with one outer, basal seta with one hair. Surface of benp naked, drawn-out, endopodal parts extremely angled and deeply cleft medially, with two stout, bipinnate setae on each side, outer one being longer than inner one. Exp with three stout setae, inner one pinnate; smaller, outer seta intersects middle one. Inner margin of exp with one row of short spinules.
P6 and genital complex (Fig. 14a) Sixth leg represented by small, fused outgrowth, with one plumose seta on each side. Genital field with attached spermatophore. (Fig. 14a).
Male
Habitus (Fig. 18) Second and third urosomite separate. Total body length measured from anterior tip of rostrum to posterior margin of anal somite (allotype): 0.27 mm, including caudal rami: 0.31 mm.
Furcal rami (Fig. 13) Long and cylindrical, 4.42× longer than wide, with proximal and distal pores and six visible elements; setae II–VII with same appearance as female. Seta I absent.
Antennule (Fig. 19) Seven-segmented. Segment I with few setules on inner margin. Segment VII of distinct triangular shape. Setal ornamentation:
-
I (0)
-
II (2) One slender seta, one small plumose fir-like seta
-
III (3) Three slender naked setae of different sizes
-
IV (7) Four long, slender setae, two plumose, three short setae, two of which plumose
-
V [6+(1+aes)] Three small spine-like and four naked slender setae, one of which fused basally with one large, slightly damaged aesthetasc
-
VI (0)
-
VII [10+(1+aes)] 11 naked and slender setae of different size, one of which fused with aesthetasc
Mouthparts and swimming legs As in female, sexual dimorphism only in antennule, P5 and P6.
P5 (Fig. 14b) Legs fused, small exopod clearly separated from baseoendopod. Benp bears one outer, basal plumose seta. Surface of benp naked with kind of cuticle pattern, endopodal parts represented by small bumps. Exp with four stout spines, outer two naked, smaller. Inner two spines with distinct dint, innermost distally plumose, other one pinnate. Surface of right exp with one row of short spinules, left exp naked.
P6 (Fig. 14) Each leg consists of one plate with three setae. Outer seta slender and plumose, inner two small, pinnate and stout.
Etymology
These two new species are dedicated to the first author’s parents Claus and Ingrid Pointner, because she wants to thank them for assisting her in all situations in life.
Discussion
Systematics
History of the genus Emertonia
The genus Emertonia was first described by Wilson (1932). Kunz (1962) created the genus Kliopsyllus by uniting species from the genera Paramesochra T. Scott, 1892, Emertonia Wilson, 1932 and Leptopsyllus T. Scott, 1894. Huys (2009) renamed the genus Kliopsyllus to Emertonia for two reasons. In the first place he stated that the generic name Kliopsyllus was only a junior subjective synonym of Emertonia Wilson, 1932. Secondly, the author analysed the remaining syntypes of Emertonia gracilis Wilson, 1932 and stated that they completely matched Kunz’s (1962, 1981) and Apostolov and Marinov’s (1988) identification for the genus Kliopsyllus. Since E. gracilis is the type species by original designation and Kliopsyllus is only a junior synonym for Emertonia, Huys (2009) reintroduced the name Emertonia for this genus.
Based on Wells (2007), the genus Emertonia contains at present 39 species, including the species presented herein. The most recently discovered species are E. schminkei, described as Kliopsyllus schminkei, Emertonia brevicaudata (Kornev & Chertoprud, 2008), described as Kliopsyllus brevicaudatus, E. minor, described as Kliopsyllus minor, Emertonia diva (Veit-Köhler, 2005), described as Kliopsyllus diva, and Emertonia andeep (Veit-Köhler, 2004), described as Kliopsyllus andeep.
Placement of Emertonia clausi sp. n. and differentiation from congeners
Given the current taxonomic situation, we have provisionally placed the two new species in Emertonia as they agree with the characters given by Veit-Köhler (2004): two-segmented exp and enp of P1, three-segmented exp of P2–P4, only two setae in exp3 P4, and one-segmented enps of P2–P4 with one apical seta.
Due to the fact that the two new species are very similar, the following part deals only with E. clausi sp. n., but the mentioned facts also apply to the rare species E. ingridae sp. n.
E. clausi sp. n. is unique within the genus. It can be distinguished from its congeners by the following attributes:
Enp2 P1 bears only one seta.
The texture of seta V of the furcal rami changes after ≈0.4× the length of the seta. The proximal part is robust with four pairs of pinnules. The distal part of the seta is rat-tailed, flexible and slender. At the point of structure change from robust to flexible the seta is very delicate and breaks off easily. Rat-tailed setae are also found in the swimming legs; exp3 of P2 bears one of this kind, exp3 P3 three and exp3 P4 two.
Some spines on exp1–3 of P2–P4 show a special and unique attribute, their shape resembles a knife edge. Setae on exp3 P2 and P3 and male exp P5 are thinned out and distally plumose.
The pair of female P5 are medially fused as in all other species of the genus but the drawn-out parts of the baseoendopod have a unique feature. They are deeply cleft medially and angled at the tip.
The inner margin of segment VII of the male antennule features a special scale-like ornamentation, which is unique and recorded the first time within all species of the genus Emertonia.
Some other characters of E. clausi sp. n. are also present in other species of the genus Emertonia. Seta VII of the furcal rami is articulate in E. clausi sp. n. as in, e.g., E. diva and Emertonia chilensis (Mielke, 1985). This feature is not seen in, e.g., E. schminkei and E. andeep. The antenna of E. clausi sp. n. bears two rare attributes: Enp2 carries three grouped subapical naked setae, as it does in Emertonia perharidiensis (Wells, 1963) and E. schminkei. Most of the other species have at maximum two setae, but E. diva and E. andeep have four. However, it has to be kept in mind that in some of the older descriptions this group of tiny elements may have been overlooked. The second trait is that one lateral spine of the antennary exp has a bifurcate tip, as has an apical spine in, e.g., Emertonia constricta pacifica (Mielke, 1984). There is also a rare feature found at the maxilliped. E. clausi sp. n. bears one seta at the syncoxa just as in, e.g., E. schminkei and E. minor. However, the syncoxa has not been drawn in all of the previous descriptions.
All other species of the genus Emertonia have two setae at the enp2 P1, such as Emertonia longisetosa (Krishnaswamy, 1951) (described as Paramesochra longisetosa) and E. schminkei. The apical seta of enp P4 in male seems to be spatulate and frayed like the same seta of Emertonia californica (Kunz, 1981) whereas the female of both species bear one seta with gradually a tapering tip. The male exp P5 of E. clausi sp. n. bears four setae of different sizes, as do the exps of E. holsatica (Klie, 1929), E. atlantica (Kunz, 1981), and E. furcavaricatus (Kunz, 1974). But only E. holsatica has the same arrangement of these setae, the outermost being the smallest and the size increasing inwards. The two legs of the male P6 are not medially fused as in, e.g., E. idiotes (Wells, 1967). Both legs are completely separated as in E. schminkei. All species, unless otherwise indicated, have originally been described as Kliopsyllus (Huys, 2009).
The combination of unique characters and attributes, that are similar or absent in other species, identifies E. clausi sp. n. as a new species of the genus Emertonia.
Differences from the related species Emertonia ingridae sp. n.
The species Emertonia ingridae sp. n. is very similar to E. clausi sp. n. To date, only two individuals of the very rare Emertonia ingridae sp. n. have been found.
There are four easily detectable characters that distinguish the two new species: the relation of length to width of the furcal rami, the total length of the furcal rami compared to the last three body somites, seta VII is articulated in E. clausi sp. n. but not in E. ingridae sp. n. and the presence/absence of a seta on the syncoxa of the maxilliped. The proportions of E. clausi sp. n. (n = 13) are as follows: furcal rami length to width 6.32 (min: 5.44; max: 7.14), furcal rami to last three somites 1.01 (min: 0.84; max: 1.25). In contrast, the proportions of E. ingridae sp. n. (n = 2) are: furcal rami length to width 4.52 (min: 4.42; max: 4.62), furcal rami to last three somites 0.79 (min: 0.75; max: 0.83) (all numerical values are averaged; see Table 1).
Furcal rami. Seta V of the furcal rami shows in both species the change of structure from stout to flexible, but in E. clausi sp. n. three pairs of minute spinules are present at the apical third of the stout part of the seta. E. ingridae sp. n. bears only two pairs of comparable spinules.
With a closer look at the ventral side of the abdomen it is possible to see the different armature, especially in the male. E. clausi sp. n. presents one continuous row of minute spinules at the posterior margin of urosomites three and four, E. ingridae sp. n. bears stout spinules only at the left and the right side of the body.
Antennule. The male antennule of E. clausi sp. n. presents some differences to that of E. ingridae sp. n. The triangle-shaped segment VII of E. clausi sp. n. features a special scale-like ornamentation at the inner margin, which was not detected in E. ingridae sp. n. Although both new species bear the same number of segments, a total of seven, and setae, altogether 30, the ornamentation and distribution of the setae are different. E. clausi sp. n. develops at every segment at least one seta. In E. ingridae sp. n. the segments I and VI bear no seta. The female antennule is eight-segmented in both species, but with quite different setal ornamentation. E. clausi sp. n. bears altogether 29 setae, E. ingridae sp. n. 28. The aesthetascs are all fused at the basis with one seta, but they develop on different segments. The distal aesthetasc of E. ingridae sp. n. belongs to segment V and is as long as the following three segments. In E. clausi sp. n. it develops from segment IV and is twice as long as the following four segments.
Mouthparts. In the mouthparts, the two species show the following differences: In the antenna, E. ingridae sp. n. bears seven apical setae on the enp, one of which has one pair of pinnules at the apical side of the geniculate part. E. clausi sp. n. only shows six apical setae and no pinnules. The three lateral spines of the antennary exp are naked in E. ingridae sp. n., but in E. clausi sp. n. one is plumose and one shows a bifurcate tip. The two apical setae of the exp of the mandibular palp are naked in E. ingridae sp. n. and plumose in E. clausi sp. n., which additionally bears two lateral spines at the exp. The number of pinnate setal elements on the maxillule and maxilla of E. clausi sp. n. is higher than in E. ingridae sp. n. E. clausi sp. n. carries one seta on the syncoxa of the maxilliped whereas E. ingridae sp. n. neither has this seta nor a pore or hole indicating a lost seta. In E. clausi sp. n. the two apical setae on the enp of the maxilliped are geniculate, while in E. ingridae sp. n. they are not.
Swimming legs. In the same way the swimming legs indicate the close relatedness of the species. In some features they are quite similar but several other attributes are variable. In general, the spinulation of the exps P2–P3 of E. clausi sp. n. appears to be spinier and more pronounced than in E. ingridae sp. n. The apical inner seta of exp3 P2 is in both species long and plumose. The apical outer seta in E. ingridae sp. n. is shaped like the inner one but in E. clausi sp. n. it presents a special rat-tailed structure and is additionally pinnate along its outer margin. Both apical setae of exp3 P3 show the same structure, but E. ingridae sp. n. is much more plumose than E. clausi sp. n. Additionally, the large outer seta is rat-tailed in E. clausi sp. n. but knife-shaped in E. ingridae sp. n. This character also appears in exp3 P4. The apical seta in exp3 P4 is rat-tailed with only tiny pinnules in E. clausi sp. n. and rat-tailed and plumose in E. ingridae sp. n. In E. clausi sp. n., the enps P2–4 carry an apical row of spinules that surround the seta like a crown. This feature is not found in E. ingridae sp. n.
P5. The outer seta of the exp P5 of female and male of E. ingridae sp. n. in most cases clearly intersects the proximate one, the seta at the same position of E. clausi sp. n. is oriented parallel to or touching the close-by seta, but there is no crossing. The outer setae of the benp P5 of E. clausi sp. n. are different to the equivalent setae of E. ingridae sp. n. In E. clausi sp. n. the setae are much longer and more plumose in both sexes.
P6. The inner two setae of the male P6 are also different in the two species. E. clausi sp. n. presents two stout, naked setae, which are half as long as the outer seta. In contrast, E. ingridae sp. n. bears two stout, pinnate setae, measuring only one third of the length of the outer seta.
As shown above, not only the differences in the proportions of the furcal rami and the setal armature of the maxilliped show that the two species are distinct. Every single part of the body presents differences and similarities between the two new species E. clausi sp. n. and E. ingridae sp. n. Due to the combination of these attributes, it is obvious that these are two closely related, but different, species.
Biogeography
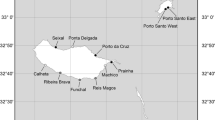
The geographical distributions of Emertonia ingridae sp. n. and Emertonia clausi sp. n. differed greatly (Table 1). The first time E. clausi sp. n. was collected was during the SEAMEC expedition M 42.3 to the Great Meteor Seamount in 1998. During the RV “Meteor” cruises M 48.1 DIVA-1 and M 63.2 DIVA-2 in the Atlantic and the RV “L’Atalante” NODINAUT expedition to the Pacific Nodule Province this new species was not only rediscovered but in the Guinea Basin a very closely related species, E. ingridae sp. n was found. In contrast to E. clausi sp. n. it is a very rare species with a very small range size. It was only found at two stations in the Guinea Basin (Fig. 1), although samples were taken from the deep-sea basins off the African west coast, in the Southern Ocean and the Pacific Ocean.
The 13 individuals of E. clausi sp. n. were sampled in the Guinea Basin, the Angola Basin, in the deep sea surrounding the Great Meteor Seamount (GMS) in the Northeast Atlantic, and in the Northeastern Pacific Nodule Province between the Clarion and the Clipperton Fracture Zone. The single Pacific specimen of E. clausi sp. n. was encountered inside a 26-year-old track, where the nodules were removed during a pilot survey at the eastern area of the French mining claim in 1978 (Mahatma 2009).
Only few Harpacticoida are eurybathic, one of which is the recently described Zosime anneae Koller & George, 2011. It was found at the GMS from the plateau down to the rise. Due to the fact that its distribution ranges over 4,000 m water depth the species is exposed to many changing environmental factors such as pressure, sediment structure, salinity, light conditions, or temperature (Koller and George 2011). A revision of the material collected at the GMS revealed that this also applies for E. clausi sp. n. The species was also found on the plateau and the slope of the GMS, the shallowest station being 292 m deep. Therefore, the distribution of this new species does not seem to be limited to the deep sea. It is exposed to the same environmental conditions as Z. anneae, consequently E. clausi sp. n. is also supposed to be an eurybathic species.
According to Gheerardyn and Veit-Köhler (2009) the possibilities for the distribution of harpacticoid copepods in the deep sea are limitless, even for Paramesochridae. Most members of this family show morphological characteristics such as reduced swimming legs that make it unlikely that they emerge into the water column (Thistle and Sedlacek 2004). The first wide-spread deep-sea harpacticoid species was described by Seifried and Martínez Arbizu (2008). They found Bradya kurtschminkei Seifried & Martínez Arbizu, 2008 in samples from the Porcupine Abyssal Plain in the north Atlantic and in the Guinea Basin, the Angola Basin and the Cape Basin, which seemed to be its southernmost limit of occurrence as it was not present in samples from the Southern Ocean. Conversely, the recently described paramesochrid Wellsopsyllus antarcticus Kottmann & Veit-Köhler, 2013 was only present in the Southern Ocean (Kottmann et al. 2013; Gheerardyn and Veit-Köhler 2009). However, the results of Menzel et al. (2011) additionally reinforced the previous findings of wide distribution ranges in benthic copepods. The authors stated that geological structures like submarine ridges or seamounts have no or only a limited influence on the dispersal of deep-sea Harpacticoida. Contrary to the deep sea, seamounts like the GMS are isolated areas (George and Schminke 2002), but the specimen of Emertonia clausi sp. n. collected from the GMS was found in the surrounding deep sea and on the plateau itself. It is astonishing that E. ingridae sp. n. was not detected at the same locations as the closely related, presumably cosmopolitan species E. clausi sp. n. While the multicorer is the best device for collecting meiofauna from undisturbed sediment cores (Barnett et al. 1984), the blade corer and especially the box corer cannot be regarded as standard for meiofauna sampling (Plum and George 2009). Therefore, rare species such as E. ingridae sp. n. may just not have been collected.
The possibility that two closely related species are so different in their distribution is fascinating but not unusual. Gheerardyn and Veit-Köhler (2009) found species of the genus Emertonia (in their publication still named Kliopsyllus) in four abyssal plains (regions) of the Southeast Atlantic and the Atlantic part of the Southern Ocean. Three species were spread over three regions (E. andeep, E. diva, E. schminkei) and two species in two regions (their Kliopsyllus sp. 1 and 2, now E. clausi sp. n.). However, they reported another four species that were only found in one deep-sea basin (their Kliopsyllus sp. 3–6, sp. 5 now E. ingridae sp. n.). Nevertheless, the distribution of Emertonia ingridae sp. n. should be studied more intensely to clarify its real range size.
According to Veit-Köhler (2005) the small number of to date described deep-sea Emertonia species was not because of the rarity of these organisms but due to the lack of taxonomists working with them. However, the present contribution is based on a survey of all Paramesochridae from the CeDAMar expeditons available to date. Members of the family Paramesochridae are very rare throughout the deep sea and several species have very wide distribution ranges. Therefore, we state that small range sizes may not necessarily reflect the real distribution of species of deep-sea Paramesochridae.
References
Apostolov A, Marinov TM (1988) Copepoda, Harpacticoida, Fauna Bulgarica 18. Aedibus Acad Scient Bulgaricae Sofia 1–384
Barnett PRO, Watson J, Connelly D (1984) A multiple corer for taking virtually undisturbed samples from shelf, bathyal and abyssal sediments. Oceanol Acta 7:399–408
Boxshall GA, Halsey SH (2004) An introduction to copepod diversity. Ray Society, London 166:1–966
George KH, Schminke HK (2002) Harpacticoida (Crustacea, Copepoda) of the Great Meteor Seamount, with first conclusions as to the origin of the plateau fauna. Mar Biol 144:887–895. doi:10.1007/s00227-002-0878-6
Gheerardyn H, Veit-Köhler G (2009) Diversity and large-scale biogeography of Paramesochridae (Copepoda, Harpacticoida) in South Atlantic abyssal plains and the deep Southern Ocean. Deep-Sea Res I 56:1804–1815. doi:10.1016/j.dsr.2009.05.002
Huys R (2009) Unresolved cases of type fixation, synonymy and homonymy in harpacticoid copepod nomenclature (Crustacea: Copepoda). Zootaxa 2183:1–99
Klie W (1929) Die Copepoda Harpacticoida der südlichen und westlichen Ostsee mit besonderer Berücksichtigung der Sandfauna der Kieler Bucht. Zool Jahrb 57:329–386
Koller S, George KH (2011) Description of a new species of Zosime Boeck, 1872 (Copepoda: Harpacticoida: Zosimeidae) from the Great Meteor Seamount, representing one of the few eurybathic Harpacticoida among the distinct plateau and deep-sea assemblages. Meiofauna Marina 19:109–126
Kornev PN, Chertoprud EC (2008) Copepod crustaceans of the order Harpacticoida of the White Sea: Morphology, Systematics, Ecology. Biology Faculty, Moscow State University. Tovarishchestvo Nauchnikh Izdanii KMK, Moscow
Kottmann J, Kihara TC, Glatzel T, Veit-Köhler G (2013) A new species of Wellsopsyllus (Copepoda, Harpacticoida, Paramesochridae) from the deep Southern Ocean and remarks on its biogeography. Helgol Mar Res 67:33–48. doi:10.1007/s10152-012-0302-7
Krishnaswamy S (1951) Three new species of sand-dwelling copepods from the Madras coast. Ann Mag Nat Hist 4:273–280
Kunz H (1962) Revision der Paramesochridae (Crustacea Copepoda). Kieler Meeresforsch 18:245–257
Kunz H (1974) Zwei neue afrikanische Paramesochridae (Copepoda Harpacticoidea) mit Darstellung eines Bewegungsmechanismus für die Furkaläste. Mikrofauna Meeresbod 36:1–20
Kunz H (1981) Beitrag zur Systematik der Paramesochridae (Copepoda, Harpacticoida) mit Beschreibung einiger neuen Arten. Mitt Zool Mus Univ Kiel 1:2–33
Lang K (1934) Marine Harpacticiden von der Campbell-Insel und einigen anderen südlichen Inseln. Acta Univ Lund 30:1–56
Lang K (1944) Monographie der Harpacticiden (Vorläufige Mitteilung). Almqvist & Wiksells Boktryckeri, Uppsala, 39p
Mahatma R (2009) Meiofauna communities of the Pacific Nodule Province: abundance, diversity and community structure. PhD thesis, Carl von Ossietzky Universität, Oldenburg
McIntyre AD, Warwick RM (1984) Meiofauna techniques. In: Holme NA, McIntyre AD (eds.) Methods for the study of marine benthos, 2nd edn. Blackwell, Oxford, pp 217–244
Menzel L, George KH (2012) Copepodid and adult Argestidae Por, 1986 (Copepoda: Harpacticoida) in the southeastern Atlantic deep sea: diversity and community structure at the species level. Mar Biol 159(6):1223–1238. doi:10.1007/s00227-012-1903-z
Menzel L, George KH, Martínez Arbizu P (2011) Submarine ridges do not prevent large-scale dispersal of abyssal fauna: a case study of Mesocletodes (Crustacea, Copepoda, Harpacticoida). Deep-Sea Res I 58:839–864. doi:10.1016/j.dsr.2011.05.008
Michels J, Büntzow M (2010) Assessment of Congo red as a fluorescence marker for the exoskeleton of small crustaceans and the cuticle of polychaetes. J Microsc 238:95–101. doi:10.1111/j.1365-2818.2009.03360.x
Mielke W (1984) Einige Paramesochridae (Copepoda) von Panamá. Spixiana 7:217–243
Mielke W (1985) Interstitielle Copepoda aus dem zentralen Landesteil von Chile: Cylindropsyllidae, Laophontidae, Ancorabolidae. Microfauna Marina 2:181–270
Plum C, George KH (2009) The paramesochrid fauna of the Great Meteor Seamount (Northeast Atlantic) including the description of a new species of Scottopsyllus (Intermedopsyllus) Kunz (Copepoda: Harpacticoida: Paramesochridae). Mar Biodiv 39:265–289. doi:10.1007/s12526-009-0022-7
Scott T (1892) Additions to the fauna of the Firth of Forth. Part IV. Rep Fish Board Scotl, Edinb 10:244–272
Scott T, Scott A (1894) On some new and rare Crustacea from Scotland. Ann Mag Nat Hist 13:137–149
Seifried S, Martínez Arbizu P (2008) A new and exceptional species of Bradya Boeck, 1873 (Copepoda: Harpacticoida: Ectinosomatidae) from the abyssal plain of the Angola Basin and the variability of deep-sea Harpacticoida. Zootaxa 1866:303–322
Thistle D, Sedlacek L (2004) Emergent and non-emergent species of harpacticoid copepods can be recognized morphologically. Mar Ecol Prog Ser 266:195–200
Vasconcelos DM, Veit-Köhler G, Drewes J, Santos PJP (2009) First record of the genus Kliopsyllus Kunz, 1962 (Copepoda Harpacticoida, Paramesochridae) from Northeastern Brazil with description of the deep-sea species Kliopsyllus minor sp. nov. Zootaxa 2096:327–337
Veit-Köhler G (2004) Kliopsyllus andeep sp. n. (Copepoda: Harpacticoida) from the Antarctic deep sea – a copepod closely related to certain shallow-water species. Deep-Sea Res II 51:1629–1641. doi:10.1016/j.dsr2.2004.06.027
Veit-Köhler G (2005) First deep-sea record of the genus Kliopsyllus Kunz, 1962 (Copepoda: Harpacticoida) with the description of Kliopsyllus diva sp. n. - the most abundant member of Paramesochridae at two different sites of the Angola Basin. Org Divers Evol 5:29–41. doi:10.1016/j.ode.2004.10.003
Veit-Köhler G, Drewes J (2009) Kliopsyllus schminkei sp. n. (Copepoda, Harpacticoida, Paramesochridae) – a new copepod from the southeast Atlantic deep sea (Angola Basin). Zootaxa 2096:313–326
Wells JBJ (1963) Copepoda from the littoral region of the estuary of the River Exe (Devon, England). Crustaceana 5:10–26
Wells JBJ (1967) The littoral Copepoda (Crustacea) of Inhaca Island, Mozambique. Trans R Soc Edinb 67:189–358
Wells JBJ (2007) An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Zootaxa 1568:1–872
Wilson CB (1932) The copepods of the Woods Hole region, Massachusetts. Bull US Natl Mus 158:1–635
Acknowledgments
The authors would like to thank Prof. Dr. Pedro Martínez Arbizu, Dr. Kai Horst George, Marco Bruhn (DZMB, Senckenberg am Meer), and Dr. Elke Willen (University of Oldenburg) for sampling the meiofauna during the three RV “Meteor” expeditions. Dr. Sybille Seifried (University of Oldenburg) handled the DIVA-1 material. The technical staff of the DZMB, Annika Hellmann, Jutta Heitfeld and Marco Bruhn, are thanked for sorting the DIVA-2 samples. Dr. Kai Horst George provided Paramesochridae of the Great Meteor Seamount. The NODINAUT material was sampled by Dr. Joëlle Galéron, Dr. Lénaïck Menot (IFREMER) and Prof. Dr. Pedro Martínez Arbizu, copepods were classified by Dr. Radith Mahatma (DZMB, Senckenberg am Meer). Dr. Hendrik Gheerardyn (Royal Belgian Institute of Natural Sciences) determined the working species of Paramesochridae from the CeDAMar expeditions. This is a contribution to CeDAMar (Census of the Diversity of Abyssal Marine Life), a field project of the Census of Marine Life (CoML).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pointner, K., Kihara, T.C., Glatzel, T. et al. Two new closely related deep-sea species of Paramesochridae (Copepoda, Harpacticoida) with extremely differing geographical range sizes. Mar Biodiv 43, 293–319 (2013). https://doi.org/10.1007/s12526-013-0158-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-013-0158-3