Abstract
Because of their potentially important role for the distribution of marine benthic organisms, seamounts have been the subject of focused interest on the part of marine biologists particularly during the past decade. One of the largest seamounts sampled so far is the Great Meteor Seamount (GMS), which is located in the North Atlantic. Although some of the most detailed investigations have been carried out on this particular seamount, it is still a little-known environment regarding benthic copepod diversity and ecology. Therefore, material from 14 stations collected in 1998 was investigated to address the following aspects: (1) species composition and diversity of the harpacticoid family Paramesochridae at the GMS; (2) faunistic comparison with other localities and Intermedopsyllus; and (3) revision of the worldwide distribution of known Paramesochridae. Of the 28 paramesochrid species determined from the GMS, 26 are new to science. The vast majority were found on the plateau; only two species were detected in the deep-sea stations. Other species found at the GMS are already known from East Atlantic deep-sea areas. In the frame of providing new taxonomical information for future research, Scottopsyllus (Intermedopsyllus) antoniae sp. nov. from the plateau of the GMS is described here. The new species can without any doubt be allocated to Scottopsyllus because of the 1-segmented endopods but 3-segmented exopods in P2–P3, the “paramecium”-like shape of P2–P3 endopods, and the 1-segmented exopods in the antenna and the mandible. The new species differs from its congeners mainly by retention of setae on the antennule, the reduced size of furcal seta VI, the fact that the proximal maxillar endite bears only 1 seta, and the loss of the maxillar endopod.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although it has been estimated that there may be as many as 100,000–200,000 seamounts spread throughout the world’s oceans (Wessel 2007), these undersea features are still little-known environments regarding their biodiversity and ecology. This is partly because of the complex conditions associated with seamounts, such as large depth ranges, cryptic topography, hard substrata, fast currents, and geographic isolation, which make them difficult to sample (Rogers 1994). However, at the same time, these specific conditions render seamounts unique habitats for deep-sea and shallow-water organisms (Rogers 1994; Richer de Forges et al. 2000).
The first scattered investigations on seamounts took place in the late nineteenth and early twentieth centuries (Brewin 2007), but only during recent decades have deep-water sampling gear and underwater vehicles allowed detailed sampling of the deep sea. Fundamental questions on species composition, diversity, community structure, and possible endemism at seamounts were first addressed by Hubbs (1959).
Because of their potentially important role for the distribution of marine benthic organisms (Hubbs 1959; Gad and Schminke 2004), there has been focused interest on seamounts by marine biologists during the past decade (e.g., Thistle 1998; Richer de Forges et al. 2000; George and Schminke 2002; Mohn and Beckmann 2002; Bartsch 2003; George 2004a, b; Mironov and Krylova 2006; Samadi et al. 2006). The most detailed investigations on biodiversity, composition and distribution of the seamount benthic macrofauna and meiofauna have been carried out in the North Atlantic, particularly at the Great Meteor Seamount (Emschermann 1971; Grasshoff 1972, 1973, 1977; Bartsch 1973a, b, 1983, 2001a, b, 2003, 2004, 2008; Hartmann-Schröder 1979; George and Schminke 2002; George 2004a, b, 2006; Gad 2004a, b, 2008; Gad and Schminke 2004; Piepenburg and Müller 2004; Mironov and Krylova 2006). The Great Meteor Seamount (GMS) is one of the largest seamounts in the North Atlantic, rising from a depth of 4,200 m up to 275 m below sea level (Ulrich 1971). It is located in the subtropical Northeast Atlantic, west of the Canary Islands and south of the Azores. The distance to the African coast is about 1,600 km. Due to its shape and the large summit plateau, the GMS resembles a giant table mountain and is therefore characterized as a guyot. The plateau is covered by coarse biogenic sediment composed of fragments of mollusc shells and corals (Gad and Schminke 2004). Estimates of the age of the GMS range from 10 m.y. (Wendt et al. 1976) to 35 m.y. (Hinz 1969) and more than 50 m.y. (Grevemeyer 1994) to 82-86 m.y. (Verhoef 1984).
The present study is the result of the expedition M42/3 with the German RV “Meteor” to the Great Meteor Seamount in 1998, as part of the interdisciplinary SEAMEC (Seamount Ecology) project. A first detailed examination of the meiofauna material collected during M42/3 yielded a large number of Harpacticoida (Copepoda), including specimens of the family Paramesochridae Lang 1944 (George and Schminke 2002). They were one of the most abundant taxa found on the plateau of the GMS and presumably represent an important constituent of the harpacticoid fauna. To date, the Paramesochridae contain 125 species belonging to 13 genera and 4 subgenera (Bodin 1997; Boxshall and Halsey 2004; Wells 2007). Members of this family show a wide geographical distribution and are typically small, interstitial animals that inhabit intertidal and shallow-water sandy sediments. Only a few species have been recorded at depths greater than 300 m (Lang 1936; Drzycimski 1967; Becker 1972; Becker et al. 1979; Veit-Köhler 2004, 2005; Veit-Köhler and Drewes 2009; Vasconcelos et al. 2009). This leads to the essential question of how species of a typically interstitial taxon could settle on the Great Meteor Seamount, considering the large distance to the nearest coast and the assumption that fine-grained clayish bottoms in the deep sea may preclude dispersal of interstitial meiofauna adapted to coarse sand (Westheide 1991; Gad and Schminke 2004).
To increase our knowledge about harpacticoid species biodiversity, this study focuses on the Paramesochridae from the Great Meteor Seamount. The investigation includes: (1) a qualitative analysis of the species composition and diversity at the GMS, including distribution patterns on the seamount; (2) a faunistic comparison of the paramesochrid taxa of the GMS with associations from other localities; (3) a summarizing review of the geographical and bathymetric distribution of paramesochrid species; and (4) a description of Scottopsllus (Intermedopsyllus) antoniae sp. nov. from GMS.
Material and methods
Qualitative samples were collected from the GMS during expedition M42/3 of the German RV “Meteor” in 1998 (Pfannkuche et al. 2000). The GMS covers a total area of 1,465 km2 and rises from a depth of approximately 4,200 m to a minimum of 275 m below the water surface. Its summit is represented by a plateau with a maximum length of 54 km, a maximum width of 31 km, and a surface area of about 1,200 km2 (Ulrich 1971; Hinz 1969) The volcanic bedrock of the plateau is covered with a 150- to 400-m-thick cap of biogenic carbonates constituting the seabed of the summit (Piepenburg and Müller 2004). During expedition M42/3, 26 stations were sampled (Table 1), of which 14 stations contained specimens of Paramesochridae (Table 2). Two of them were located in the deep sea at the northeastern slope, while the rest were located on the plateau (Table 1, Fig. 1). The material was collected with a multicorer (MUC, after Barnett et al. 1984), epibenthic sledge (EBS, after Brandt and Barthelnt 1995) and giant boxcorer (GKG, after Hessler and Jumars 1974; see George and Schminke 2002).
Sample treatment is described by George and Schminke (2002). For determination of the paramesochrid species, the specimens were transferred on slides using glycerine as embedding medium (Pfannkuche and Thiel 1988). Sorting was done in the laboratory at Senckenberg am Meer, Department DZMB (German Centre of Marine Biodiversity Research) in Wilhelmshaven, Germany.
Before dissection, the holotype of Sc. (I.) antoniae sp. nov. was drawn from the dorsal and lateral side. Detailed drawings of the lateral and ventral view of the abdomen were also made. Dissected parts of the holotype and the paratypes were placed in glycerin drops, mounted on several slides. All drawings were made of female holotype except the maxillule (Fig. 3 from paratype 1). The paratype parts were drawn without dissection. Drawings were made with the aid of a drawing tube on a Leica differential interference contrast microscope (DMR with UCA condenser, IC prism and doubler ×1.25 and ×1.6). All specimens were deposited in the collection of the Senckenberg Forschungsinstitut und Naturmuseum Frankfurt am Main (SMF), Germany.
Abbreviations used in the text: A1 = antennula; A2 = antenna; aes = aesthetasc; benp = baseoendopod; Cphth = cephalothorax; enp = endopod; exp = exopod; enp-1 (2,3) = proximal (middle, distal) segment of endopod; exp-1 (2,3) = proximal (middle, distal) segment of exopod; FR = furcal ramus/rami; GF = genital field; GMS = Great Meteor Seamount; Md = mandibula; Mxl = maxillula; Mx = maxilla; Mxp = maxilliped; P1–P6 = first to sixth swimming leg.
Results
Taxonomy
Harpacticoida Sars 1903
Paramesochridae Lang 1944
Subfam. Paramesochrinae Huys 1987
Scottopsyllus (Intermedopsyllus) Kunz 1962 sensu Kunz 1981
Scottopsyllus (I.) antoniae sp. nov.
Type material
Holotype:
Female, dissected and mounted on 10 slides (Coll. No. SMF 33623). Paratype 1: non-dissected female, mounted on 1 slide (Coll. No. SMF 33624). Paratype 2: non-dissected male, mounted on 1 slide (Coll. No. SMF 33625). Additional material: 4 females and 6 males from station 516 (Coll. No. SMF 33626), 5 females and 3 males from station 451 (Coll. No. SMF 33627), 1 female and 2 males from station 455 (Coll. No. SMF 33628), all preserved in glycerine slides.
Type locality
Northeast Atlantic, Great Meteor Seamount, 29°49.3′N, 28°37.1′W, southwest plateau, station 516, 325 m depth (Fig. 1).
Etymology
The species name is given in dedication to the first author’s grandmother, Antonie Behrends, and his newborn niece, Antonia Plum.
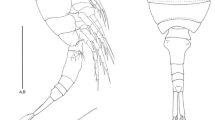
Description of the female
Body (Fig. 2a, b) elongate, cylindrical and slightly depressed dorsoventrally. Body length (including FR) 426 μm. Last abdominal segment and telson tapering posteriorly. Surface of the body covered with small rounded depressions resembling the surface of a golf ball (Fig. 6b). Rostrum nearly indiscernible. First pedigerous somite completely fused to dorsal cephalic shield forming the cphth. Cphth and thoracic somites bearing P2–P4 with sensilla laterally and dorsally. Last thoracic body somite (P5 bearing somite) without sensilla. First abdominal somite completely fused with last thoracic body somite, forming the genital double somite, with 6 sensilla and 2 pores dorsally, 1 sensillum and 3 pores on each lateral side. Following abdominal somite dorsally with 2 sensilla and 1 lateral pore. Penultimate body somite without sensilla, carrying a fine, well developed pseudoperculum. Telson short, tapering posteriorly. FR (Fig. 6a–c) short, about twice as long as wide, with 6 setae: I absent; II long, inserted dorsolaterally in proximal half; III slightly shorter than II, bipinnate in distal part, inserted subdistally; IV slightly longer than III, terminally; V longest seta, terminally; VI shortest seta, inserted terminally at inner side of ramus; VII longer than setae II and III, inserted dorsally on distal half of ramus, slightly displaced inwards. Length/width ratio: 2:1.
A1 (Fig. 3a)
8-segmented; armature formula: I–1, II–9, III–7, IV–3 + aes, V–1, VI–2, VII–3, VIII–6 + aes. Both aes basally fused with 1 seta. First two segments largest, following segments decreasing in size.
A2 (Fig. 3b)
Basis short and strong. Enp 2-segmented; enp-1 with 1 bare and slender abexopodal seta; enp-2 with 2 bare setae laterally and a group of 6 strong geniculate setae apically. Exp 1- segmented with 1 bare lateral seta and 2 apical setae (1 seta broken).
Md (Fig. 4a)
Cutting edge lost during dissection. Md palp biramous, with basis, enp and exp. Basis with 1 unipinnate seta. Enp 1-segmented with 1 lateral seta and 4 apical setae; all setae bare. Exp fused to basis, very small and cone-shaped, with 2 apical bare setae, 1 of which short and thin.
Mxl (Fig. 4b)
Arthrite of praecoxa with 6 strong, stout spines terminally and 1 strong seta at the distal inner corner. Coxal endite with 1 slender naked seta apically. Basis with 5 apical setae and 1 slender naked seta laterally, representing enp. Exp very small, 1-segmented, with 2 bare apical setae.
Mx (Fig. 4c)
Syncoxa with 2 endites, proximal endite bearing 1 seta; distal endite with 2 setae; allobasis drawn out to form 1 strong unipinnate claw bearing 2 additional setae, one of which unipinnate. Enp represented by 4 setae.
Mxp (Fig. 4d) subchelate, comprising syncoxa, basis and 1-segmented enp. Syncoxa short and bare, without any ornamentation. Basis slightly elongate and swollen, with short row of strong spinules. Enp 1-segmented, with claw-like seta accompanied by 1 small outer seta and 2 apical setae.
P1 (Fig. 5a, Table 3)
Coxa without setae. Basis with 1 inner seta, outer seta absent. Enp and exp 2-segmented. Exp-1 almost twice as long as exp-2, with 1 outer seta; exp-2 with 2 outer and 2 apical setae; both segments with outer row of strong spinules. Enp-1 without seta, but with outer row of long spinules. Enp-2 with 1 long, apical seta and 1 shorter, outer seta, both setae bare and geniculate.
P2–P3 (Fig. 5b, c)
Basis with 1 outer, bare seta. Enp 1-segmented, with long spinules along the margin and without seta. Exp 3-segmented, longer than enp, exp-2 shortest segment. Exp-1, exp-2 and exp-3 with stout outer spine and outer row of strong spinules; exp-3 with 2 apical setae, the inner of which unipinnate.
P4 (Fig. 5d)
Basis without outer seta. Enp as in P2 and P3 but smaller. Exp longer than enp. Exp 2-segmented due to fusion of exp-1 and exp-2. Exp-1 with 2 strong outer spines and outer row of strong spinules, exp-2 with 1 strong outer spine and 1 apical, bare seta; apically with row of strong spinules.
P5 (Fig. 6d) benps fused to single, broad and large lamelliform plate. Outer basal seta on short setophore. Each endopodal part of P5 with 1 seta. Exps distinct, not fused, and very small, with 3 slender, bare setae apically.
GF and P6 (Fig. 6e)
Gonopore not covered, almost triangular in ventral view. P6 represented by 2 medially fused plates, each bearing two minute spinules placed on small protrusions.
Male differs from female as follows
A1 (Fig. 7b)
6-segmented, chirocer. Segment II with 1 bipinnate seta, other setae bare; segment V rounded and bulbous; segment VI sharpened. Armature formula: I–1, II–9, III–7 + aes, IV–1, V–10 + aes, VI–11.
P5 (Fig. 7a) strongly resembling that of female, but without such a pronounced endopodal plate and without endopodal seta
P6 (Fig. 7a)
Represented by medially fused plates furnished each with 1 outer and 2 inner bare setae.
Paramesochridae of the Great Meteor Seamount
Paramesochridae were one of the most abundant harpacticoid taxa at the GMS, making up 20.4% of total harpacticoid number of individuals (George and Schminke 2002). Of the 1,249 collected specimens, 623 individuals (49.9%) were copepodids, and 626 (50.1%) adults. More than half of the adults (626 specimens or 65.0%) were found on the southern part of the plateau. The highest absolute individual number N was recorded at station 551 (N = 175, i.e., 28.1%), followed by station 516 (N = 140, 11.2%) and station 451 (N = 68, 5.4%). Only one specimen was found at stations 506 and 517, respectively (Table 3). Median value Z for all stations: Z N = 23.
The obtained adults were assigned to six genera (Apodopsyllus Kunz 1962; Diarthrodella Klie 1949; Kliopsyllus Kunz 1962; Leptopsyllus T. Scott 1894; Paramesochra T. Scott 1892 and Scottopsyllus Kunz 1962) and 28 species (Table 2). The number of species within the stations ranges from S = 1 (stations 506, 517) up to S = 16 (stations 451, 551; Table 3) (i.e., the latter two stations contain 57.1% of the total number of paramesochrid species at the GMS). Stations 451 and 551 show the highest species richness (S). However, for a more objective impression, S should be standardized, and in our case, S can be related to the number of specimens, N, representing a simple measure of N/S (cf. Rose et al. 2005). The N/S ratio (Table 3) indicates how many individuals are needed to encounter a new species (cf. George 2004a). Not taking into account stations with <5 specimens (i.e., st. 505, 506, 517), the highest evenness is observed at station 565 (N/S = 1.5), followed by st. 515 (N/S = 1.6), st. 552 (N/S = 3.3), and st. 521 (N/S = 3.4). The above mentioned stations 451 and 551 show much higher N/S ratios (4.3 and 10.9, respectively).
Paramesochra and Kliopsyllus were the most species-rich genera on the GMS plateau (Table 2) with 11 species for both genera, together comprising 78.6% of all paramesochrid species found. Two species have been recognized within Leptopsyllus, two within Scottopsyllus, one species within Apodopsyllus and one within Diarthrodella. Most of the paramesochrid species at the GMS (S = 26, i.e., 92.9%) have not yet been reported from anywhere else. Only Scottopsyllus (I.) intermedius T. and A. Scott 1895 and Kliopsyllus schminkei Veit Köhler and Drewes 2009 were already known to science.
Bathymetric and geographical distribution of paramesochrid species from the GMS
Most of the 28 species (S = 25 or 89.3%) were found exclusively on the plateau (292–511 m) of the GMS (Fig. 8), while only one species, Kliopsyllus schminkei, was confined to the adjacent deep sea. This species has hitherto been reported from three abyssal plains of the southeast Atlantic Ocean, namely the Guinea, the Angola and the Cape Basins (Veit Köhler and Drewes 2009; Gheerardyn and Veit-Köhler 2009). The records of K. schmikei from the GMS are from a similar depth, namely from the deep-sea stations 505 (4005 m) and 506 (3009 m).
Kliopsyllus sp. 3 and Kliopsyllus sp. 10 have been found at Seine seamount (Büntzow, personal communication), which is also located in the Northeast Atlantic Ocean, approximately 750 nm north-east of the GMS. Another species, Kliopsyllus sp. 7, found between 322 and 476 m depth at the GMS, was also found in the Guinea Basin during the DIVA-2 expedition (2005) (Gheerardyn and Veit-Köhler 2009).
Scottopsyllus (I.) intermedius and Kliopsyllus sp. 10 were found in the deep sea as well as on top of the seamount (Fig. 8). Sc. (I.) intermedius actually occurs in coastal waters of the East Atlantic, the North Sea, the Black Sea and the White Sea (Kornev and Chertoprud 2008). Its geographical distribution in the East Atlantic ranges from the Firth of Forth (Scotland) in the north to the Lüderitz Bay of Namibia in the south. This species could be characterized as a typical interstitial species of shallow-water habitats. Findings at the GMS were almost restricted to the shallow plateau, but one specimen was found at the deep-sea station 505 at 4,005 m depth. This is the first record of a Scottopsyllus species from the deep Atlantic Ocean.
The species Scottopsyllus (I.) antoniae sp. nov. described here is new to science, but was also recorded during the OASIS expedition (2003) (http://www1:uni-hamburg.de/OASIS/) on Sedlo Seamount (Büntzow, personal communication), approximately 630 nautical miles (nm) north of the GMS.
Review of the worldwide geographic and bathymetric distribution of Paramesochridae
Members of the harpacticoid family Paramesochridae have been reported from various parts of the world’s oceans. They are typically small, interstitial animals that mostly inhabit sandy beaches as well as intertidal and shallow-water sandy sediments. Nevertheless, some species have also been recorded repeatedly in deep-sea sediments (e.g., Becker et al. 1979, Thistle 1982; Veit-Köhler 2004, 2005; Rose et al. 2005; Baguley et al. 2006) and were recently also discovered on the Seine and Sedlo seamounts in the Northeast Atlantic (Büntzow, personal communication) and on the Anaximenes seamount in the eastern Mediterranean Sea (George, personal observation).
Table 4 presents a list of all paramesochrid species described so far, with notes on their geographic and bathymetric distribution. Including the newly described species Scottopsyllus (I.) antoniae sp. nov., 126 paramesochrid species with 15 subspecies, belonging to 13 genera and 4 subgenera, have been described thus far.
The majority of Paramesochridae (35 species or 29%) have been found in the Atlantic Ocean, but there are also many records from the Pacific Ocean (26 species or 21%), the Indian Ocean (26 species, 21%), the Mediterranean Sea (8 species or 7%), and even from Antarctic waters (Kliopsyllus andeep Veit-Köhler 2004; Scottopsyllus (Sc.) praecipuus Veit-Köhler 2000) or inland seas like the Black Sea and the Baltic Sea (e.g., Kliopsyllus holsaticus Klie 1929) (Fig. 9).
The bathymetric distribution of Paramesochridae ranges from the littoral down to abyssal depths (see Table 4 and Fig. 10). The vast majority of them occur in (sub-)littoral zones with records generally ranging from beaches down to coastal waters of 146 m depth [Scottopsyllus (Wellsopsyllus) gigas Wells 1965]. Only nine species have been recorded at depths deeper than 300 m, including Scottopsyllus (I.) antoniae sp. nov. (325 m), Kliopsyllus minor Vasconcelos et al. 2009. (492 m), Leptopsyllus elongatus Drzycimski 1967 (515 m), Leptopsyllus (Paraleptopsyllus) articus Lang 1936 (1,750 m), Scottopsyllus (Wellsopsyllus) abyssalis Becker et al. 1979 (2,000 m), Leptopsyllus abyssalis Becker 1972 (3,820 m), Kliopsyllus andeep Veit-Köhler 2004 (4,541 m), Kliopsyllus diva Veit-Köhler 2005 (5,389 m), and Kliopsyllus schminkei Veit-Köhler and Drewes 2009 (5,389 m). Figure 10 shows the bathymetric distribution patterns based on the available record data and indicates that 77% of all species have been found in shallow waters. Only 7% of the species have been recorded at depths below 200 m.
The most species-rich genera of the Paramesochridae are Kliopsyllus (38 species) and Apodopsyllus (25 species) followed by Paramesochra (14 species) and Scottopsyllus (13 species) (Table 4). As mentioned above, the genera Kliopsyllus and Paramesochra are found to be the most diverse paramesochrid taxa at the GMS. The majority of the described species of Kliopsyllus and Paramesochra have been reported from coastal waters, but recent studies extended the depth range of Kliopsyllus down to >4,000 m (Veit-Köhler 2004, 2005; Veit-Köhler and Drewes 2009). Geographically, most species are restricted to regional areas, but certain species can be considered as “cosmopolitans”. For example, Kliopsyllus holsaticus has been recorded from the Northeast Atlantic, the North Sea, the Baltic, and the Black Sea. The reports of Kliopsyllus andeep by Veit-Köhler 2004 have expanded the distribution range of Kliopsyllus to Antarctic waters. Species of Paramesochra show a similar broad distribution range with records from the North Sea and the Northeast Atlantic (Paramesochra dubia T. Scott 1892; Paramesochra borealis Geddes 1981), the Baltic Sea, Hawaii (Paramesochra acutata hawaiiensis Kunz 1981), the Mediterranean Sea (Paramesochra brevifurca Galhano 1970), the Indian Ocean (Paramesochra denticulata Rao and Ganapati 1969), Galapagos Islands (Paramesochra helgolandica galapagoensis Mielke 1984a, b), Panama (Paramesochra kunzi Mielke 1984a, b) and Perth in western Australia (Paramesochra longicaudata Nicholls 1945).
The genus Scottopsyllus, in turn, encompasses 13 species (and 1 subspecies), including the Scottopsyllus (I.) antoniae sp. nov. (cf. Veit-Köhler 2000) described here. Most species were found in coastal waters of northern Europe, but there are also records from King George island [Sc. (Sc.) praecipuus Veit-Köhler 2000], the Lüderitz Bay, Namibia [Sc. (I.) intermedius], the Peru Trench [Sc. (W.) abyssalis], the Galapagos Islands [Sc. (Sc.) langi Mielke 1984a, b] and recently the Great Meteor Seamount [Sc. (I.) antoniae sp. nov., this study].
Discussion
Taxonomy
Scottopsyllus (Intermedopsyllus) antoniae sp. nov. is attributed to the genus Scottopsyllus Kunz 1962 mainly because of the 1-segmented, “paramecium”-shaped endopods of P2 and P3. Besides these conspicuous features, the 1-segmented exp of the antenna, the 1-segmented exp of the mandible and the 3-segmented exopods of P2 and P3 characterize the new species as a member of Scottopsyllus.
In 1962, Kunz established the genera Scottopsyllus and Intermedopsyllus as part of a revision of the Paramesochridae (Kunz 1962). Later on, he relegated these genera to subgeneric status, placing them together with another new subgenus Sc. (Wellsopsyllus) in the genus Scottopsyllus (Kunz 1981). The three subgenera can be distinguished mainly by the segmentation of the endopods and exopods of P4. Sc. (Wellsopsyllus) exhibits a 3-segmented exp and a 1-segmented enp in P4, while in Sc. (Scottopsyllus) the P4 exp is 3-segmented and the enp 2-segmented. Sc. (Intermedopsyllus), in turn, has a 2-segmented exp in P4 due to a fusion of the 2 proximal segments (and a 1-segmented enp). The new species Scottopsyllus (Intermedopsyllus) antoniae sp. nov. is placed in the subgenus Sc. (Intermedopsyllus), as it has this derived character in the exp of P4.
Within Sc. (Intermedopsyllus), the new species can be distinguished from its congeners by virtue of the following characteristics:
Female A1
Sc. (I.) antoniae sp. nov. bears 1 seta on segment 1, 9 setae on segment 2 and 7 setae on segment 3. According to the original descriptions, the three other species of Sc. (Intermedopsyllus) lack a seta on the first segment and bear less setae in the following two segments [4 setae at both segments 2 and 3 in Sc. (I.) intermedius, 5 and 6 setae, respectively in Sc. (I.) minutus Nicholls 1939, 4 and 5 setae, respectively in Sc. (I.) smirnovi Kunz 1992].
Mandibular palp
Sc. (I.) antoniae shows a 1-segmented enp with 5 setae and a 1-segmented, small exp with 2 setae. In contrast, the palpus of Sc. (I.) intermedius possesses a 2-segmented enp with 8 setae in total and an exp with 4 setae, whereas that of Sc. (I.) smirnovi has a 1-segmented enp with 3 setae, while the exp is lacking. Furthermore, the basis lacks the terminal setae in Sc. (I.) smirnovi. The md palpus of Sc. (I.) minutus was not described by Nicholls (1939).
Maxilla
The mx of Sc. (I.) antoniae resembles that of Sc. (I.) minutus. Both species have 2 endites on the syncoxa but can be distinguished by the number of setae. The proximal endite in Sc. (I.) antoniae sp. nov. exhibits only 1 seta, while that of Sc. (I.) minutus bears 2 setae. Sc. (I.) intermedius (after Kunz 1992) possess 3 endites on the syncoxa, while in Sc. (I.) smirnovi the two proximal of the three endites are each represented by a single seta. Furthermore, in Sc. (I.) antoniae sp. nov. the maxillar enp is absent and represented by 4 setae, while it is present but fused to the allobasis in Sc. (I.) minutus and Sc. (I.) intermedius. In Sc. (I.) smirnovi the enp seems to be separated and assembled with 3 setae. Additionally, Sc. (I.) antoniae sp. nov. and Sc. (I.) intermedius share the well developed claw of the allobasis, but in the latter the claw is not accompanied by additional setae.
P2 and P3 exp
The most outstanding feature of Sc. (I.) antoniae sp. nov. is the third seta on the terminal segment of P2 and P3 exp. This seta has never been described in any other species of the genus Scottopsyllus (Intermedopsyllus).
Furca
Sc. (I.) antoniae sp. nov. lacks furcal seta I, retaining only six furcal setae. Also, Sc. (I.) minutus and Sc. (I.) smirnovi lost furcal seta I. However, these species additionally lost seta IV, which is well developed in Sc. (I.) antoniae sp. nov.. The only species retaining all seven furcal setae is Sc. (I.) intermedius.
Ecological remarks
Paramesochridae of the Great Meteor Seamount - distribution and endemism
Due to sedimentological and topographical conditions, the plateau of GMS has been sampled quite pragmatically, using gear that was able to provide sampling material in these circumstances (cf. Martínez Arbizu and Schminke 2000; George and Schminke 2002). Consequently, the sampling was not standardized, and quantitative analysis is therefore impossible. However, regarding the distribution of Paramesochridae at GMS, no homogeneous distribution pattern can be detected. As shown in Table 2, even stations of the same plateau area may differ remarkably with respect to their number of individuals. For instance, eastern stations 551 and 552 were both sampled with the epibenthic sledge (EBS, cf. Table 1). However, station 552 yielded N = 23, whereas neighboring station 551 provided N = 175 (Table 2). Interestingly, samples taken with the box corer (BC) produced almost the same number of specimens (stations 451, 455, 467, 492, cf. Table 2), ranging between 48 and 68 specimens, with the exception of stations 505 (deep sea: N = 3) and station 516 (western plateau: N = 140), and a mean value of Z = 55 individuals. This might be an indication for a non-patchy distribution, although a real quantitative sampling is needed to address that question. In contrast, the EBS demonstrates a large variation in specimen numbers, from 1 to 175, with a mean value of Z = 17 individuals. Evidently, this is due to the specific gear characteristics, as it is designed to catch bigger animals than Paramesochridae (Brandt and Barthelnt 1995).
The high degree of scientifically new species at GMS (>90%) was not surprising, as in many other recently extensive investigated marine environments most of the sampled harpacticoid species are considered to be new to science (e.g., George and Schminke 2002; George 2004a, b, 2005; Rose et al. 2005; Baguley et al. 2006; Gheerardyn and Veit-Köhler 2009). Contrary to Emschermann (1971), who suggested that the deep sea might act as a kind of “faunal reservoir” for the GMS and that its faunal assemblages may mainly consist of deep-sea “resistant” species, George and Schminke (2002) came to the conclusion that the GMS plateau represents an isolated area with only occasional bathymetric and geographic exchange. They base their assumption on the large number of new species found on GMS plateau. Mironov and Krylova (2006) also mention a high degree of endemism (91%) on the GMS for meiofauna organisms. As shown by the results presented here, this hypothesis is supported with respect to Paramesochridae. Nevertheless, many isolated islands and seamounts show a lower endemism than less isolated islands and seamounts (Mironov and Krylova 2006). A differentiation has yet to be made between the taxa or major groups under study, a fact that is often not taken into account in such a comparison. Although a high number of new species does not necessarily mean a high degree of endemism, it certainly may be an indication for a significant to high isolation of the corresponding habitat. With respect to the GMS and to Harpacticoida, it means that the very high percentage of unknown species on the plateau supports the hypothesis of the seamount’s role as a “trapping stone” (Hubbs 1959) for at least that taxon. Quite opposite to that hypothesis, studies on other meiobenthic taxa and on megabenthic and macrobenthic groups from GMS suggest that most species are widespread elements of the North Atlantic fauna, indicating that the GMS area is not isolated. Bartsch (2003) criticizes the hypothesis of George and Schminke (2002), stating that marine mites (Halacarida), as well as several other macrobenthic and megabenthic taxa (including fish) certainly do present a widespread distribution, even though found solely on GMS so far. However, Bartsch’s (2003) argumentation is inconsistent in our opinion. Especially with regard to the meiobenthic Halacarida, she turns from species to genus level, which is meaningless in connection with endemic species. The paramesochrid genera from GMS are widespread in the world’s oceans (Table 4, Fig. 9), but it is a species that provides valuable information on endemism. Bartsch (2003, p. 114 and Table 1) shows that 17 of the 24 halacarid species (>70%) were new to science and exclusively found at GMS. When Bartsch (2003, p. 113) states that “Certainly more species will be found in future investigations”, it is not less speculative than the contrary assumption of the species possibly being endemic ones.
Regarding macrofauna and megafauna, a direct comparison with meiobenthic data, as realized by several authors, is questionable. Percentages of endemism for macrobenthic and megabenthic species on the GMS (Piepenburg and Müller 2004) conform with values from studies of different seamounts (e.g., Rogers 1994; Richer de Forges et al. 2000; Gillet and Dauvin 2003; Ávila and Malaquias 2003) that presented much lower exclusive macrobenthic and megabenthic species than reported by George and Schminke (2002) for meiobenthic Harpacticoida. However, this is not surprising as the lifecycle of macrobenthic and megabenthic organisms includes life stages living in the water column, which is an important prerequisite for wide geographic dispersal. Nevertheless, results obtained by investigations on megafauna and macrofauna must not be extended to meiofauna, which does not meet the mentioned prerequisite. The antithesis presented by Bartsch (2003), and apparently supported by results obtained for macrofauna and megafauna, shows impressively that seamounts do not correspond to generalizing assumptions that attempt to assign single functions to them. A seamount should not be regarded as solely a staging post supporting geographical dispersal of benthic organisms, or only an isolated object retaining “trapped” organisms. The same seamount may play several roles at the same time, depending, among other things, on the taxon referred to. This assessment is not as trivial as one might believe, as demonstrated impressively by McClain (2007) in his criticism of seamount research.
In a study on the typical deep-sea family Argestidae on the GMS, George (2004a) presumed that the relatively high percentage of closely related species on the plateau was due to radiation in that area rather than a successive colonization from other localities. Contrary to the Argestidae, Paramesochridae are considered to be shallow-water rather than deep-sea organisms. In this context, it is remarkable that the paramesochrid community at GMS seems to be dominated by species of the taxa Kliopsyllus and Paramesochra. Species of these genera are typically found in coastal waters. Thus, the assumption by George (2004a, b) of an elevation of the argestid fauna over millions of years together with the growing seamount itself appears unlikely for Paramesochridae. This is supported, for example, by Scottopsyllus (Intermedopsyllus) antoniae sp. nov., which was also sampled on the summits of Sedlo and Seine seamounts. The same applies to Sc. (I.) intermedius, which has only been recorded in intertidal habitats so far (Table 4). Consequently, one might conclude that most of the Paramesochridae at GMS possibly originate from geographically adjacent regions rather than from the surrounding deep sea. It is plausible that “Scenario I: geographical immigration” as described by George (2004a, b, p. 262) applies to the Paramesochridae of the GMS, i.e., most Paramesochridae originate from shallow-water localities and reach the GMS by overcoming the vast deep-sea areas. However, we still have no information or even plausible ideas to explain how meiobenthic organisms surmount both the depth and deep-sea sediments, both of which are considered to be significant barriers for certain interstitial meiofauna species (Gerlach 1977; Westheide 1991; Gad and Schminke 2004). A bathymetrical migration obviously does occur, as demonstrated by the presence of Scottopsyllus (I.) intermedius and Kliopsyllus sp. 10 on both the plateau and in the deep sea (cf. George and Schminke 2002 and George 2004a, b for other examples). Their presence in the deep sea may point to a much higher range of depth and sediment tolerance than presumed so far (but supporting Emschermann’s 1971 hypothesis of deep-sea “resistant” species), and this may also be true for other Paramesochridae found exclusively on the plateau thus far.
Bathymetrical and geographical distribution of the genus Scottopsyllus
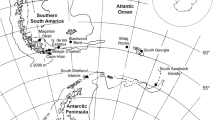
The reports of most paramesochrid species from only shallow waters so far may be the result of scientific history rather than real geographical distribution. Especially along European shorelines, many samples have been taken from littoral zones since the end of the nineteenth century. Consequently, species distribution ranges are concentrated in European waters and also in other regions, mainly confined to littoral zones. During the last decades, new paramesochrid taxa have increasingly been reported in different oceans and depths around the world, indicating a worldwide geographical and a broad bathymetrical distribution for this family. Scottopsyllus may serve as an example to illustrate paramesochrid distribution patterns. Figure 11 shows the geographical distribution of all known Scottopsyllus species (according to Veit-Köhler 2000) and includes the Sc. (I.) antoniae sp. nov. described here. None of the Scottopsyllus species reported in the Atlantic has been found at depths greater than 400 m. Gheerardyn and Veit-Köhler (2009) found two new species of the genus Scottopsyllus at depths between 2,274 m and 5,194 m, but they were restricted to the Scotia Sea (Antarctica). Scottopsyllus (W.) abyssalis was found at 2,000 m depth, but has only been reported in the Peru Trench (Pacific Ocean). In this context, it is remarkable that the finding of Scottopsyllus (I.) intermedius at a GMS deep-sea station is the first record of Scottopsyllus in the Atlantic deep sea. This species occurs in shallow waters from the Firth of Forth (Scotland) to Lüderitz Bay in Namibia, but has never been recorded in the East Atlantic deep sea before.
Geographic distribution patterns of the genus Scottopsyllus. Capital letters (A–L) indicate geographic areas where the known species were recorded. The small table assigns the species to the corresponding area(s). For detailed information cf. Table 4
The study by Gheerardyn and Veit-Köhler (2009) already indicated that ranges of certain species might span thousands of kilometres across the East Atlantic (or also the northern European seas in the present study). The large distribution ranges of certain paramesochrid species found at the GMS are comparable to the distribution patterns of deep-sea Paramesochridae in the Southeast Atlantic and Antarctic abyssal plains (Gheerardyn and Veit-Köhler 2009). These findings support what Giere (1993) called the “meiofauna paradoxon”: small-sized marine animals (even from shallow-water areas), lacking planktonic life stages and strictly bound to marine sediments, show a widespread, often cosmopolitan distribution even at species level. Our findings provide further evidence that there are no real barriers that might hinder the dispersal of copepods in the Atlantic (Gheerardyn and Veit-Köhler 2009). Vermeij (2004) mentioned that the deep sea in itself is not an obstruction to dispersal for seamount organisms. He pointed out that, even for shallow-water organisms, deep-sea basins and sediments offer at most a porous barrier. Several factors, such as suspension and rafting with floating material (Gerlach 1977), plate tectonics and continental drift (Rao 1972; Sterrer 1973; Westheide 1977), or long generation times (Gheerardyn and Veit-Köhler 2009) are proposed in relevant literature as explanations for the large dispersal ranges of meiofauna organisms. Connections via currents between seamounts may also increase the dispersal potential. Regarding the GMS and following Emschermann (1971), a drift of meiofauna organisms and their larvae across large distances is unlikely due to the discontinuous peripheral currents of the Canary Current. To date, such hypotheses are still speculative and seem to be inadequate to explain distribution patterns of small benthic organisms because most of these hypotheses seek an explanation for dispersal near the ocean’s surface.
References
Apostolov A (1972) Catalogue des Copèpodes Harpacticoides marins de la Mer Noira. Zool Anz 188(3/4):202–254
Apostolov A (1973a) Sur divers Harpacticoides (Copèpodes) de la Mer Noire. Zool Anz 190(1/2):88–110
Apostolov A (1973b) Harpacticoides des eaux saumâtres et des étanges côtiers. Zool Anz 191(3/4):281–294
Apostolov A, Marinov T (1988) Copepoda, Harpacticoida. Fauna Bulgarica, 18
Arlt G (1983) Taxonomy and ecology of some harpacticoids (Crustacea, Copepoda) in the Baltic Sea and Kattegat. Zool Jb Syst Ökol Geogr Tiere 110:45–85
Ávila SP, Malaquias MAE (2003) Biogeographical relationships of the molluscan fauna of the Ormonde Seamount (Gorringe Bank, Northeast Atlantic Ocean). J Moll Stud 69:145–150
Baguley JG, Montagna PA, Lee W, Hyde LJ, Rowe GT (2006) Spatial and bathymetric trends in Harpacticoida (Copepoda) community structure in the northern Gulf of Mexico deep-sea. J Exp Mar Biol Ecol 330:327–341
Barnett PRO, Watson J, Connelly D (1984) A multiple corer for taking virtually undisturbed samples from shelf, bathyal and abyssal sediments. Ocean Acta 7:399–408
Bartsch I (1973a) Halacaridae (Acari) von der Josephinebank und der Großen Meteorbank aus dem östlichen Nordatlantik. I. Die Halacaridae aus den Schleppnetzproben. Meteor Forsch-Erg D 15:51–78
Bartsch I (1973b) Halacaridae (Acari) von der Josephinebank und der Großen Meteorbank aus dem östlichen Nordatlantik. II. Die Halacaridae aus den Bodengreiferproben. Meteor Forsch-Erg D 15:13–20
Bartsch I (1983) Ophiuroidae (Echinodermata) from the north-eastern Atlantic deep sea. Meteor Forsch-Erg D 36:37–46
Bartsch I (2001a) A new halacarid genus (Acari: Halacaridae: Halacarinae) from the Great Meteor Seamount, Eastern North Atlantic. Spec Div 6:117–125
Bartsch I (2001b) Agauopsis (Arachnida: Acari: Halacaridae) from the Northeastern Atlantic, description of two species, A. minor (Trouessart) and A. valida sp. nov. Mittt Hamburgt Zool Mus Inst 98:63–75
Bartsch I (2003) Lohmannellinae (Halacaridae: Acari) from the Great Meteor Seamount (Northeastern Atlantic). Description of a new species and reflections on the origin of the seamount fauna. Mitt Hamburg Zool Mus Inst 100:101–117
Bartsch I (2004) Geographical and ecological distribution of marine halacarid genera and species (Acari: Halacridae). Exp Appl Acarol 34:37–58
Bartsch I (2008) Notes on ophiuroids from the Great Meteor Seamount (Northeastern Atlantic). Spixiana 31:233–239
Brandt A, Barthelnt D (1995) An improved supra- and epibenthic sledge for catching Peracarida (Crustacea, Malacostraca). Ophelia 43:15–23
Becker KH (1972) Eidonomie und Taxonomie abyssaler Harpacticoidea. PhD Thesis, Christian Albrechts Universität, Kiel
Becker KH, Noodt W, Schriever G (1979) Eidonomie und Taxonomie abyssaler Harpacticoidea (Crustacea, Copepoda) Teil II. Paramesochridae, Cylindropsyllidae und Cletodidae. Meteor Forsch-Erg 31:1–37
Bocquet C, Bozic B (1955) Idyanthopsis psammophila, gen. et sp. n., Tisbidae des sables de Roscoff. Arch Zool Exp Gen 93(1):1–9
Bodin P (1979) Copepodes Harpacticoides marins des environs de La Rochelle. 5 - Espèces nouvelles ou incertaines. Vie Milieu 27(3-A):311–357
Bodin P (1997) Catalogue of the new marine Harpacticoid Copepods. Doc Trav Inst R Sci Nat Belg 89:1–304
Bodin P, Jackson D (1987) A new species of Leptopsyllus (Copepoda: Harpacticoida: Paramesochridae) from northern Brittany and the west coast of Ireland with a key to the genus. Proc R Irish Acad 87B(6):93–99
Bozic B (1955) Copépodes Harpacticoides des sables des environs de Roscoff. Description de quelques formes nouvelles. Arch Zool Exp Gen 92(1):1–12
Bozic B (1964) Tisbisoma spinisetum, n. gen., n. sp. Copépode Harpacticoide de la Réunion. Bull Soc Zool Fr 89(2–3):219–225
Boxshall GA, Halsey SH (2004) An introduction to copepod diversity. Ray Society, London
Brewin PE (2007) A history of seamount research. In: Stocks KI, Menezes G (eds) Seamounts: ecology, fisheries & conservation. Blackwell, Oxford
Chappuis PA (1954) Harpacticoides psammiques récoltés par C. Delamare Deboutteville en Méditerranée. Vie Milieu 4(2):254–276
Cottarelli V (1971) Paramesochridae (Copepoda, Harpacticoida) di acque interstiziali littorali italiane. Riv Idrobiol 10(1–2):19–32
Cottarelli V, Almatura S (1986) Una nuova specie di Apodopsyllus (Crustacea, Copepoda, Harpacticoida) die acque interstiziali litorali delle Filippine: Apodopsyllus biarticulatus n. sp. Boll Mus Civ St Nat Verona 12:299–305
Cottarelli V, Baldari F (1987) Rossopsyllus obscurus n. sp. (Copepoda, Harpacticoida) from Macquarie Island, South Pacific Ocean. Crustaceana 53(2):181–187
Cottarelli V, Forniz C (1994) Ricerche zoologiche della nave oceanografica “minerva” (C.N.R.) sulle isole circumsarde. XXIII. Meiopsyllus marinae: a new genus and a new species of Paramesochridae from the meiobenthos of Asinara and S. Pietro Islands (Sardinia). Ann Mus Civ Stor Nat “G Doria” 90:577–589
Coull BC, Hogue EW (1978) Revision of Apodopsyllus (Copepoda, Harpacticoida), including two new species and a redescription. Trans Am Microsc Soc 97(2):149–159
Drzycimski I (1967) Zwei neue Harpacticoida (Copepoda) aus dem westnorwegischen Küstengebiet. Sarsia 30:75–82
Emschermann P (1971) Loxomespilon perezi - ein Entoproctenfund im Mittelatlantik. Überlegungen zur Benthosbesiedlung der Großen Meteorbank. Mar Biol 9:51–62
Gad G (2004a) Diversity and assumed origin of the Epsilonematidae (Nematoda) of the plateau of the Great Meteor Seamount. Arch Fish Mar Res 51(1–3):30–42
Gad G (2004b) The Loricifera fauna of the plateau of the Great Meteor Seamount. Arch Fish Mar Res 51(1–3):9–29
Gad G (2008) Colonization and speciation on semounts, evidence from Draconematidae (Nematoda) of the Great Meteor Seamount. Mar Biodiv. doi:10.1007/s12526-009-0007-6
Gad G, Schminke HK (2004) How important are seamounts for the dispersal of interstitial meiofauna? Arch Fish Mar Res 51(1–3):43–54
Galhano MH (1970) Contribuicao para o conhecimento da fauna intersticial em Portugal. Publ Inst Zool “A Nobre”, Porto 110:1–207
Geddes DC (1981) On two interstitial marine harpacticoids (Crustacea: Copepoda) from northern Norway. Sarsia 66(1):19–24
George KH (2004a) Description of two new species of Bodinia, a new genus incertae sedis in Argestidae Por, 1986 (Copepoda, Harpacticoida), with refelections on argestid colonization of the Great Meteor Seamount plateau. Org Divers Evol 4:241–264
George KH (2004b) Meteorina magnifica gen. et sp. nov., a new Idyanthidae (Copepoda, Harpacticoida) from the plateau of the Great Meteor Seamount (Eastern North Atlantic). Meiofauna Mar 13:95–112
George KH (2005) Sublittoral and bathyal Harpacticoida (Crustacea: Copepoda) of the Magellan Region. Composition, distribution and species diversity of selected major taxa. Sci Mar 69(Suppl. 2):147–158
George KH (2006) New Ancorabolinae Sars, 1909 (Copepoda: Harpacticoida: Ancorabolidae) of the Atlantic Ocean. Description of Pseudechinopsyllus sindemarkae gen. et sp. nov. and Dorsiceratus ursulae sp. nov. from the Great Meteor Seamount, and redescription of D. octocornis Drzycimski, 1967, and D. triarticulatus Coull, 1973 (part.). Meiofauna Mar 15:123–156
George KH, Schminke HK (2002) Harpacticoida (Crustacea, Copepoda) of the Great Meteor Seamount, with first conclusions as to the origin of the plateau fauna. Mar Biol 144:887–895
Gerlach SA (1977) Means of meiofauna dispersal. In: Sterrer W, Ax P (eds) The meiofauna species in time and space. Mikrofauna Meeresboden 61:89–103
Gheerardyn H, Veit-Köhler G (2009) Diversity and large-scale biogeography of Paramesochridae (Copepoda, Harpacticoida) in South Atlantic Abyssal Plains and the deep Southern Ocean. Deep-Sea Res. doi:10.1016/j.dsr.2009.05.002
Giere O (1993) Meiobenthology - the microscopic fauna in aquatic sediments. Springer, Berlin
Gillet P, Dauvin JC (2003) Polychaetes from the Irving, Meteor and Plato seamounts, North Atlantic ocean: origin and geographic relationships. J Mar Biol Assoc UK 83:49–53
Gómez S (2002) Some additions to the Mexican fauna: the family Paramesochridae (Copepoda: Harpacticoida) J Crustacean Biol 22:627–641
Grasshoff M (1972) Die Gorgonarien des östlichen Nordatlantik und des Mittelmeeres. I. Die Familie Ellisellidae (Cnidaria, Anthozoa). Meteor Forsch-Erg D 10:73–87
Grasshoff M (1973) Die Gorgonarien des östlichen Nordatlantik und des Mittelmeeres. II. Die Gattung Acanthogorgia (Cnidaria: Anthozoa). Auswertung der “Atlantischen Kuppenfahrten 1967”von F.S. “Meteor”. Meteor Forsch-Erg D 13:1–10
Grasshoff M (1977) Die Gorgonarien des östlichen Nordatlantik und des Mittelmeeres. III. Die Familie Paramuriceidae (Cnidaria, Anthozoa). Meteor Forsch-Erg D 27:5–76
Grevemeyer I (1994) Der Atlantis-Meteor Komplex. Berichte aus dem Zentrum für Meeres- und Klimaforschung. Reihe C, Inst Geoph, 5
Hartmann-Schröder G (1979) Die Polychaeten der “Atlantischen Kuppenfahrt” von F.S. “Meteor” (Fahrt 9 c, 1967). 1. Proben aus Schleppgeräten. Meteor Forsch-Erg D 31:63–90
Hessler RR, Jumars PA (1974) Abyssal community analysis from replicate box corers in the central North Pacific. Deep-Sea Res 21:185–209
Hinz W (1969) The Great Meteor Seamount. Results of seismic reflection measurements with a pneumatic sound source, and their geological interpretation. Meteor Forsch-Erg Reihe C, 2, 63–77 pp
Hubbs CL (1959) Initial discoveries of fish faunas on seamounts and offshore banks in the eastern Pacific. Pac Sci 13:311–316
Huys R (1987) Paramesochra T.Scott, 1892 (Copepoda, Harpacticoida): a revised key, including a new species from the SW Dutch coast and some remarks on the phylogeny of the Paramesochridae. Hydrobiologia 144:193–210
Huys R (1988) A redescription of the presumed associated Caligopsyllus primus Kunz, 1975 (Harpacticoida, Pramesochridae) with emphasis on its phylogenetic affinity with Apodopsyllus Kunz, 1962. Hydrobiologia 162:3–19
Huys R (1996) Biuncus nom. nov., a replacement name for Singularia Huys, 1995 (Copepoda: Harpacticoida: Paramesochridae). J Nat Hist Lond 30:1261
Klie W (1929) Die Copepoda Harpacticoida der südlichen und westlichen Ostsee mit besonderer Berücksichtigung der Sandfauna der Kieler Bucht. Zool Jb 57(3/4):329–386
Klie W (1935) Ostracoden der Familie Cytheridae aus Sand undSchell von Helgoland. Kieler Meeresforsch1:49–72
Klie W (1949) Harpacticoida (Cop.) aus dem Bereich von Helgoland und der Kieler Bucht. I. Kieler Meeresforsch 6:90–128
Kornev PN, Chertoprud ES (2008) Veslonogie rakoobraznye otryada Harpacticoida Belogo morya: morfologiya, sistematika, ekologiya. [Copepod crustaceans of the order Harpacticoida of the White Sea: morphology, systematics, ecology]. Tovarishchestvo Nauchnikh Izdanii KMK, Moscow
Krishnaswamy S (1951) Three new species of sand-dwelling copepods from the Madras coast. Ann Mag Nat Hist 12(4):273–280
Krishnaswamy S (1957) Studies on the Copepoda of Madras. PhD Thesis, University of Madras
Kunz H (1936) Neue Harpacticoiden (Crustacea Copepoda) von Helgoland (Vorlaufige Mitteilung). Kieler Meeresforsch 15:353
Kunz H (1951) Marine Harpacticoiden aus dem Küstensand von Südwestafrica. Kieler Meeresforsch 8(1):76–81
Kunz H (1954) Beitrag zur Kenntnis der Harpacticoiden der Deutschen Bucht. Kieler Meeresfosch 10(2):224–228
Kunz H (1962) Revision der Paramesochridae (Crustacea Copepoda). Kieler Meeresforsch 18(2):245–257
Kunz H (1974) Zwei neue afrikanische Paramesochridae (Copepoda Harpacticoidea) mit Darstellung eines Bewegungsmechanismus für die Furcaläste. Mikrofauna Meeresboden 36:1–20
Kunz H (1975) Copepoda Harpacticoidea aus dem Litoral des südlichen Afrika. I Teil. Kieler Meeresforsch 31(2):179–212
Kunz H (1981) Beitrag zur Systematik der Paramesochridae (Copepoda, Harpacticoida) mit Beschreibung einiger neuer Arten. Mitt Zool Mus Univ Kiel 1(8):2–33
Kunz H (1983) Harpacticoiden (Crustacea: Copepoda) aus dem Litoral der Azoren. Archipélago 4:117–208
Kunz H (1992) Beitrag zur Kenntnis mariner Copepoda Harpacticoida (Fam. Paramesochridae Lang) mit Beschreibung zweier neuer Arten und einer neuen Unterart. Crustaceana 62(1):85–97
Lang K (1936) Copepoda Harpacticoida. Swedish Antarctic Expedition 1901–1903, Further Zoological Results 3:1–68
Lang K (1948) Monographie der Harpacticiden. Håkan Ohlssons Boktryckeri, Lund, Sweden
Lang K (1965) Copepoda Harpacticoida from the Californian Pacific coast. K svenska vatensk Akad Handl 10(2):1–565
Letova VN (1982) Harpacticoida 8Crustacea, Copepoda) from the mud-sandy littoral of the east Murman. In: Marine Invertebrates of coastal bioceonosses of the Arctic Ocean and the Pacific Ocean, Issled. Fauny Morei, OA Skarlato edit, 29(37):46–75
Marinov T (1971) Harpacticoids of the Bulgarian Black Sea coast. Proc Inst Oceanogr Fish Varna 11:43–87
Marinov T (1977) Harpacticoida from the Eastern Central Atlantic Coast. Proc Inst Fish 15:83–98
Martínez Arbizu & Schminke (2000) Results of the DIVA-1 expedition of “Meteor”. Org Divers Evol 5(1):1–238
Masry D (1970) Ecological study of some sandy neaches along the Israeli Mediterranean coast, with a description of the interstitial Harpacticoida (Crustacea, Copepoda). Cah Biol Mar 11(3):229–258
McClain CR (2007) Seamounts: identity crisis or split personality? J Biogeo 34:2001–2008
Mielke W (1975) Systematik der Copepoda eines Sandstrandes der Nordseeinsel Sylt. Mikrofauna Meeresboden 52:1–134
Mielke W (1984a) Einige Paramesochridae (Copepoda) von Panama. Spixiana 7:217–243
Mielke W (1984b) Interstitielle Fauna von Galapagos, XXXI, Paramesochridae. Microfauna Mar 1:63–147
Mielke W (1985a) Zwei neue Kliopsyllus-Arten (Copepoda) aus Chile. Stud Neotrop Fauna Environ 20:97–105
Mielke W (1985b) Diarthrodella chilensis sp. n. und Rossopsyllus kerguelenensis quellonensis subsp. n. (Copepoda, Paramesochridae) von Chile. Zool Scr 14(1):45–53
Mielke W (1987) Interstitielle Copepoda von Nord- und Süd-Chile. Microfauna Mar 3:309–361
Mielke W (1988) Apodopsyllus cubensis n. sp., a new interstitial copepod (Paramesochridae) from Cuba. Stygologia 4(2):155–165
Mielke W (1994) A new interstitial copepod species related to the «Leptomesochra complex» (Copepoda, Ameiridae) from Chile. Microfauna Mar 9:251–259
Mironov AN, Krylova EM (2006) Origin of the fauna of the Meteor Seamounts, north-eastern Atlantic. In: Biogeography of the North Atlantic seamounts. KMK, Moscow
Mitwally H, Montagna PA (2001) Egyptian interstitial Copepoda Harpacticoida with the description of two new species and one new subspecies. Crustaceana 74:513–545
Mohn C, Beckmann A (2002) Numerical studies on flow amplification at an isolated shelf break bank, with application to Porcupine Bank. Cont Shelf Res 22:1325–1338
Monard A (1935) Ětude sur la faune des Harpacticoides marins de Roscoff. Trav Stat Biol Roscoff 13:1–88
Nicholls AG (1935) Copepods from the interstitial fauna of a sandy beach. J Mar Biol Assoc UK 20(2):379–406
Nicholls AG (1939) Some new sand-dwelling copepods. J Mar Biol Assoc UK 23(2):327–341
Nicholls AG (1945) Marine Copepoda from western Australia. V. A new species of Paramesochra, with an account of a new harpacticoid family, the Remaneidae, and its affinities. J R Soc West Aust 29:91–105
Noodt W (1958) Die Copepoda Harpacticoidae des Brandungsstrandes von Teneriffa (Kanarische Inseln). Akad Wiss Lit Mainz Abh Math Natur Kl 2:51–116
Noodt W (1964) Copepoda Harpactiocidea aus dem Litoral des Roten Meeres. Kieler Meeresforsch 20:128–154
Pennak RW (1942) Harpacticoid copepods from some intertidal beaches near Woods Hole, Massachusetts. Trans Am Microsc Soc 61(3):274–285
Pesta O (1959) Harpacticoiden (Crust. Copepoda) aus submarinen Höhlen und den benachbarten Litoralbezirken am Kap von Sorrent (Neapel). Publ Staz Zool Napoli 30(supp):95–177
Petkovski TK (1955) IV Beitrag zur Kenntnis der Copepoden. Acta Mus Maced Sci Nat 3(2–25):71–104
Pfannkuche O, Thiel H (1988) Sample prossessing. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithonian Institution Press, Washington, pp 134–145
Pfannkuche O, Müller TJ, Nellen W, Wefer G (eds) (2000) Meteorberichte 00-1 Ostatlantik 1998. Cruise No. 42, 16 June–26 October 1998. Leitstelle METEOR, Institut für Meereskunde der Universität Hamburg, Hamburg
Piepenburg D, Müller B (2004) Distribution of epibenthic communities on the Great Meteor Seamount (North-east Atlantic) mirrors pelagic processes. Arch Fish Mar Res 51(1–3):55–70
Por FD (1964) Les Harpacticoides (Copepoda Crustacea) des fonds meubles du Skagerak. Cah Biol Mar 5(3):233–270
Rao CG (1972) On the geographical distribution of interstitial fauna of marine beach sand. Proc Indian Natl Sci Acad 38B:164–178
Rao CG, Ganapati PN (1969) On some interstitial copepods from the beach sands of Waltair coast. Proc Ind Acad Sci 70:262–286
Richer de Forges BR, Koslow JA, Poore CGB (2000) Diversity and endemism of the benthic seamount fauna in the southwest Pacific. Nature 405:944–947
Rogers AD (1994) The biology of seamounts. Adv Mar Biol 30:305–350
Rose A, Seifried S, Willen E, George KH, Veit-Köhler G, Bröhldick K, Drewes J, Moura G, Martínez Arbizu P, Schminke HK (2005) A method for comparing within-core alpha diversity values from repeated multicorer samplings, shown for abyssal Harpacticoida (Crustacea: Copepoda) from the Angola Basin. Org Divers Evol 5:3–17
Samadi S, Bottan L, Macpherson E, De Forges BR, Boisselier MC (2006) Seamount endemism questioned by the geographic distribution and population genetic structure of marine invertebrates. Mar Biol 149:1463–1475
Scheibel W (1972) Quatitative-ökologische Untersuchungen am uferfernen Mesopsammon in der Kieler Bucht. PhD thesis University of Kiel
Scheibel W (1975) Kliopsyllus longifurcatus n. sp., ein sandbewohnender Harpacticoide (Copepoda) der Kieler Bucht. Crustaceana 29(3):235–240
Scott T (1892) Paramesochra dubia Rep Fish Bd Scotl 10(iii):252
Scott T, Scott A (1895) On new and rare Copepoda from Scotland. Ann Scott Nat Hist 13:28–35
Serban M (1959) Les Copépodes da la Mer Noire - Note préliminaire sur les Harpacticoides de la côte roumaine. Lucr Stat Zool Mar Agigea vol Festival 1:259–302
Serban M (1968) Description de l´espèce Paramesochra pontica Serban (Copepoda Harpacticoida) de la nappe phréatique de la côte d´Agigea, Mer Noire. Cucr Ses Stiint (1–2 noiem. 1966). Stat Zool Mar “Prof I Borcea”, Agigea, Univ Jassy, vol Festival: 203–208
Soyer J (1975) Harpacticoides (Crustacés Copépodes) de l´archipel de Kerguelen. I. Quelques formes mésospammiques. Bull Mus Natl Hist Nat Paris 168(244):1169–1223
Sterrer W (1973) Plare tectonics as a mechanism for dispersal and speciation in interstitial sand fauna. Neth J Sea Res 7:200–220
Thistle D (1982) Aspects of the natural history of the harpacticoid copepods of San Diego Trough. Biol Oceanogr 1:225–238
Thistle D (1998) Harpacticoid copepod diversity at two physically reworked sites in the deep sea. Deep-Sea Res II 45:13–24
Thompson IC, Scott A (1900) Some recent additions to the Copepoda of Liverpool Bay. Trans Biol Soc Liverpool XIV:139–144
Ulrich J (1971) Zur Topographie und Morphologie der Großen Meteorbank. Meteor Forsch-Erg 6:48–68
Vasconcelos DM, Veit-Köhler G, Drewes J, Santos PJ Parreira dos (2009) First record of Kliopsyllus Kunz, 1962 (Copepoda Harpacticoida) from Northeastern Brazil with description of a deep-sea species: Kliopsyllus minor n. sp. Zootaxa 2096:327–337
Veit-Köhler G (2000) Habitat preferences and sexual dimorphism in species of Scottopsyllus (Cpepoda, Harpacticoida) with the description of Scottopsyllus (S.) praecipus sp.n. from the Antarctic. Vie Milieu 50:1–17
Veit-Köhler G (2004) Kliopsyllus andeep sp. n. (Copepoda: Harpacticoida) from the Antarctic deep sea - a copepod closely related to certain shallow-water species. Deep-Sea Res II 51:1629–1641
Veit-Köhler G (2005) First deep-sea record of the genus Kliopsyllus Kunz, 1962 (Copepoda: Harpacticoida) with the description of Kliopsyllus diva sp.n. - the most abundant member of Paramesochridae at two different sites of the Angola Basin. Org Divers Evol 5:29–41
Veit-Köhler G, Drewes J (2009) Kliopsyllus schminkei sp. n. (Copepoda, Harpacticoida, Pramesochridae) - a new copepod from the south-east Atlantic deep sea (Angola Basin). Zootaxa 2096:313–326
Verhoef J (1984) A geophysical study of the Atlantis-Meteor Seamount comples. Geol Ultraiectina 38:1–151
Vermeij G (2004) Island life: a view from the sea. In: Lomolino MV, Haney LR (eds) Frontiers of biogeography: new directions in geography of nature. Sinauer, Sunderland, pp 239–254
Wells JBJ (1961) Interstitial copepods from the Isles of Scilly. Crustaceana 2(4):262–274
Wells JBJ (1963a) Copepoda from the littoral region of the estuary of the River Exe (Devon, England). Crustaceana 5(1):10–26
Wells JBJ (1963b) On some new and rare Crustacea from Northern Ireland. Ann Mag Nat Hist 13(6):85–96
Wells JBJ (1965) Copepoda (Crustacea) from the meiobenthos of some Scottish marine sub-littoral muds. Proc R Soc Edinburgh 69(I–1):1–33
Wells JBJ (1967) The littoral Copepoda (Crustacea) of Inhaca Island, Mozambique. Trans R Soc Edinburgh 67(7):189–358
Wells JBJ (1971) The Harpacticoida (Crustacea: Copepoda) of two new beaches in south-east India. J Nat Hist 5:507–520
Wells JBJ (2007) An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Magnolia Press, Auckland
Wells JBJ, Rao GC (1987) Littoral Harpacticoida (Crustacea: Copepoda) from Andaman and Nicobar Islands. Mem Zool Surv India 16(4):1–385
Wells JBJ, Kunz H, Rao GC (1975) A review of the mechanisms for movement of the caudal furca in the family Paramesochridae (Copepoda Harpacticoida), with a description of a new species of Kliopsyllus Kunz. Mikrofauna Meeresboden 53:1–16
Wendt I, Kreuzer H, Muller P, von Rad U, Raschka H (1976) K-Ar age of basalts from the Great Meteor and Josephine seamounts (eastern Atlantic). Deep-Sea Res 23:849–862
Wessel P (2007) Seamount characteristics. In: Seamounts: Ecology, Fisheries & Conservation. Blackwell
Westheide W (1977) The geographical distribution of interstitial polychaetes. In: Sterrer W & Ax P (ed) The meiofauna species in time and space. Mikrofauna Meeresboden 61:287–302
Westheide W (1991) The meiofauna of the galapagos - a review. In: James MJ (ed) Galapagos marine invertebrates (taxaonomy, biogeography and evolution in Darwin´s islands). Plenum, New York, pp 37–69
Wilson CB (1932) The copepods of the Woods Hole region, Massachusetts. Bulletin of the United States National Museum 158
Acknowledgements
Special thanks are due to J.B.J. Wells (Wellington, New Zealand) and P. Bodin (Belgium) who provided a checklist for the harpacticoid copepods. We would also like to thank our colleagues Dr. G. Veit-Köhler and M. Büntzow (Wilhelmshaven, Germany) who provided their paramesochrid material from the Seine and Sedlo Seamounts and the deep-sea basins of the Southeast Atlantic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plum, C., George, KH. The paramesochrid fauna of the Great Meteor Seamount (Northeast Atlantic) including the description of a new species of Scottopsyllus (Intermedopsyllus) Kunz (Copepoda: Harpacticoida: Paramesochridae). Mar Biodiv 39, 265–289 (2009). https://doi.org/10.1007/s12526-009-0022-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-009-0022-7