Abstract
This study represents an isotopic survey of modern plants developed to establish baseline isotopic values in order to explore prehistoric herd management strategies employed by the South American camelid herders that occupied the southern Andean highlands during the past 3000 years. We present carbon (δ13C) and nitrogen (δ15N) isotopic compositions of natural pastures collected from different plant communities along an altitudinal gradient in the dry Puna of Argentina. Our results show that C3 plants are the most abundant along the whole altitudinal gradient and that C3 and C4 plants exhibit a differential distribution, the latter being less abundant in those sites located above 3900 meters above sea level (masl). At the same time, plants growing at low-altitude sites with low water availability exhibit higher δ15N values than plants growing at high-altitude sites with higher water availability. These results explain the negative correlation found between altitude and South American camelid bone collagen δ13C and δ15N values published in previous studies. This work represents a fundamental step towards the building of an isotopic ecology for the dry Puna area with the ultimate goal to explore herd management strategies employed by human groups in the past. In this sense, modern plant and South American camelid tissue isotopic compositions would provide a frame of reference to interpret isotopic compositions measured on archaeofaunal remains recovered at pastoral sites, with the aim to explore mobility and pastureland use by prehistoric herders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stable isotope analyses are frequently employed as a tool for reconstructing diet and mobility patterns of prehistoric human groups (Ambrose and Krigbaum 2003; Kellner and Schoeninger 2007; Price et al. 2012, among many others) and diet and migration patterns of prehistoric animal populations (Madgwick et al. 2013; Stevens et al. 2013; Thornton et al. 2011, among many others). Particularly, the carbon and nitrogen isotopic compositions of animal and human tissues reflect the isotopic compositions of the foods consumed (Kelly 2000; Tykot 2004). Therefore, interpretations of isotopic data from archaeological contexts depend on an accurate knowledge of the range and variation in isotopic compositions of potentially consumed food stuffs and their position in the food chain (Ambrose 1991). In the past few years, the development of studies that address the baseline variation in the isotopic compositions of soils and plants has been highly encouraged by most users of stable isotope analyses in many disciplines such as ecology or archaeology (Casey and Post 2011; Knudson et al. 2014; Post 2002; Szpak et al. 2013, among others). In fact, many studies highlight the importance of building an isotopic baseline for any study area before addressing trophic reconstructions for the past (Ambrose and DeNiro 1986; Burton et al. 2001; among others).
Recently, several studies have explored the potential of stable isotope analysis to investigate prehistoric herding strategies in different parts of the globe (Balasse et al. 2002; Britton et al. 2008; Finucane et al. 2006; Müldner et al. 2014; Stevens et al. 2013; Towers et al. 2011, among others). In this sense, stable isotope analysis of animal tissues represents an innovative approach to investigate how human groups exploited and managed animals in the past, allowing us to address issues such as mobility and foddering, which usually escape traditional zooarchaeological or genetic techniques applied to archaeofaunal remains (Makarewicz and Tuross 2012).
Particularly in the dry Puna of Argentina, previous works have addressed prehistoric herding and hunting strategies through stable isotope analysis of animal tissues (Fernández and Panarello 1999–2001; Mengoni Goñalons 2007; Samec et al. 2014; Yacobaccio et al. 2010). Since South American camelids (SAC) represent the most important animal species for human groups in this area in both prehistoric and modern times, δ13C and δ15N values have been measured on domesticated and wild SAC remains recovered at different archaeological sites and interpreted using modern stable isotope values of domesticated and wild SAC as a frame of reference (Fernández and Panarello 1999–2001; Samec et al. 2014; Yacobaccio et al. 2010). Nevertheless, these applications require establishing baseline isotopic values from plant material to fully understand the causes of variation in both modern and past SAC δ13C and δ15N values.
Thus, the main purpose of this work is to understand the causes of isotopic variation among plants from the dry Puna of Argentina and to develop appropriate isotopic baselines for archaeofaunal and paleodietary data interpretation. Previous studies have proposed that plant species presence and relative abundances depend mostly on altitude and its effect on temperature, moisture, atmospheric pressure and solar irradiance (Cavagnaro 1988; Llano 2009; Panarello and Fernández 2002; Szpak et al. 2013; Tieszen and Chapman 1992, among others). Therefore, this study explores the variation in plant δ13C and δ15N values and their correlation with altitude through stable isotope analysis of natural pastures currently available for wild and domesticated SAC consumption. Particularly, we aim to explore the distribution of C3 and C4 plant species and their δ13C and δ15N values among different vegetation assemblages along an altitudinal gradient where modern SAC tissues were already sampled (Samec 2012; Yacobaccio et al. 2009). These new results will allow us to discuss δ13C and δ15N values measured on SAC tissues in a new light, with the ultimate goal of building an isotopic ecology for the dry Puna of Argentina to interpret archaeofaunal isotopic values. In this sense, the development of a local survey of the vegetation potentially consumed by the animals managed by the prehistoric herders that inhabited the area has enormous implications in the investigation of prehistoric herding strategies, human dietary reconstructions and palaeoenvironmental reconstructions through stable isotope analyses (Ambrose 1991; Pate 1997; Pate and Anson 2008).
Carbon isotopic composition of plants and herbivorous ungulates
Within terrestrial ecosystems, carbon enters the food web through the uptake of atmospheric CO2 by plants during photosynthesis (O'Leary 1988). Three photosynthetic pathways exist for terrestrial plants: C3, C4, and crassulacean acid metabolism (CAM) (Ehleringer and Cerling 2002; O’Leary 1981, 1988). These photosynthetic pathways exhibit differential discrimination against 13C resulting in clear differences between the δ13C values of C3 and C4 plants (O'Leary 1981; Smith and Epstein 1971). C3 plants, which follow the Calvin-Benson pathway, exhibit δ13C values ranging from −35 to −20 ‰ with an average between −28 and −26 ‰ (Dawson et al. 2002; Tieszen 1991). These plants include trees, shrubs, temperate grasses and legumes (Cerling 1992) and dominate temperate and high-elevation environments such as those found in the southern Andes (Panarello and Fernández 2002). In contrast, C4 plants, which follow the Hatch-Slack pathway, exhibit higher δ13C values ranging from −19 to −6 ‰ with an average between −14 and −12 ‰ (Smith and Epstein 1971; Tieszen 1991). These plants include tropical- and arid-adapted grasses from warmer and saline environments (Smith and Epstein 1971) and several cultivated plants such as maize, a main crop in the southern Andes (Cadwallader et al. 2012). On the other hand, CAM (crassulacean acid metabolism) represents a third photosynthetic option by which plants can fix carbon like C4 plants during the night but also like C3 plants during the day and therefore exhibit δ13C values intermediate to those plant species (O'Leary 1981). CAM plants include cacti, bromeliads and other succulents, which often grow in arid and semi-arid environments (O'Leary 1981; Tieszen 1994).
Environmental conditions, such as temperature, water availability, irradiance and atmospheric pressure, can influence the distribution of C3, C4 and CAM plants and their δ13C values. Thus, these values may vary between plants of the same photosynthetic pathway, functional group and/or species that grow under different conditions (Codron et al. 2005; Ehleringer et al. 1997; Heaton 1999; Tieszen 1991, among others). Several studies have found that the distribution of C3 and C4 plants is influenced by altitudinal variation, according to its effects on temperature, precipitation and irradiance (Cavagnaro 1988; Llano 2009; Panarello and Fernández 2002; Szpak et al. 2013; Tieszen and Chapman 1992; Tieszen et al. 1979). According to these studies, C4 plants are only present at lower elevations due to increased temperature and water stress and practically disappear above a certain threshold (between 3000 and 4000 meters above sea level [masl] depending on the specific area) where these conditions are no longer found (Tieszen et al. 1979). At the same time, several studies have revealed that δ13C values of C3 plants also vary across wide altitudinal gradients in relation to altitude (Körner et al. 1988; Marshall and Zhang 1994; Sparks and Ehleringer 1997; Szpak et al. 2013; Tieszen 1991). For instance, Körner et al. (1991) have found that carbon isotope discrimination among terrestrial C3 herbaceous plants decreases with altitude at several places around the globe, which results in higher δ13C values even for individual species within the C3 plant range. According to these authors, this altitudinal trend in 13C discrimination is caused by both temperature and atmospheric pressure variations (Körner et al. 1991). In that respect, Tieszen and Chapman (1992) have found that the δ13C values measured on C3 plants increase with altitude across an entire altitudinal gradient (between sea level and 4400 masl) in northern Chile, but those species that occurred along the driest portions of the transect presented higher values than those that grew where water stress was less severe. This latter trend is in accordance with those studies that have found that water stress causes stomatal closure resulting in higher δ13C values (Farquhar and Sharkey 1982; Farquhar et al. 1987; Tieszen 1991, among others). Nevertheless, Szpak et al. (2013) carried out a survey in northern Peru and found a positive correlation between altitude (between sea level and 4000 masl) and δ13C values measured on C3 plants regardless of water availability variations. According to these authors, their results highlight the determinant role of altitude in the carbon isotope composition of C3 plants over water availability (Szpak et al. 2013). In any case, these results highlight the complex interaction between altitude and water availability variations along altitudinal gradients in arid and semi-environments (Marshall and Zhang 1994).
All these aspects related to variation in the isotopic composition of terrestrial plants are relevant issues when dealing with the results of isotopic analyses of animal and human tissues. In the case of carbon stable isotopes, the types of plants consumed by any herbivore will be reflected in the δ13C value of its tissues since a constant enrichment in 13C is produced during incorporation resulting in an isotopic offset between a consumer’s tissue and its diet (Schwarcz and Schoeninger 1991). This offset, which is the “isotopic discrimination factor”, can vary between 1 and 5 ‰, although most investigators work with a value of 5 ‰ when dealing with free-ranging herbivore bone collagen (Bocherens et al. 1996; Kelly 2000; Koch et al. 1994; Lee-Thorp et al. 1989). Therefore, several authors have established that the carbon isotope composition of bone collagen in herbivorous ungulates is controlled by (a) the distribution of C3 and C4 plants and its relative abundances, (b) the selective feeding habits particular to the different species, and (c) human intervention in diet in the case of domestic animals (Koch et al. 1994; Stevens et al. 2006). As we have previously reviewed, C3 and C4 distribution is determined by environmental variables such as temperature, precipitation, solar irradiance among others, and thus, herbivore δ13C values will indirectly reflect the manifestation of such variables (Stevens et al. 2006).
Nitrogen isotopic composition of plants and herbivorous ungulates
Within terrestrial ecosystems, nitrogen enters the food web through the absorption of nitrogenous compounds (typically NH4 + and NO3 -) by terrestrial plants directly from the soil or through symbiosis with atmospheric N2-fixing bacteria (Ambrose 1991; Virginia and Delwiche 1982). Non-N2-fixing plants exhibit a wide range of δ15N values depending on soil isotopic composition and growth form but are usually higher than the δ15N values of N2-fixing plants, mostly legumes, which range between −2 and 2 ‰ (Peterson and Fry 1987; Virginia and Delwiche 1982). Overall, the δ15N values of terrestrial plants exhibit a range from −5 to 20 ‰, in which the highest values are found in arid and saline environments; whereas, the lowest ones are found in wetter environments with increased rainfall and elevation (Ambrose 1991; Heaton 1987; Virginia and Delwiche 1982). In this sense, the δ15N values of plants can be influenced by environmental and physiological factors including climate, soil type, water and nitrogen availability, salinity, root depth and age, among others (Amundson et al. 2003; Austin and Vitousek 1998; Evans 2001; Handley and Raven 1992; Heaton 1987; Virginia and Delwiche 1982). These factors can lead to substantial differences in δ15N values (up to 10–12 ‰) between different plant parts and specimens of the same plant species and between different microhabitats located within the same plant community (Handley and Raven 1992; Heaton 1987; Hobbie et al. 2000). Several studies have found a strong negative correlation between rainfall and δ15N values measured on soils (Amundson et al. 2003; Austin and Vitousek 1998) and plants (Hartman and Danin 2010; Heaton 1987; Swap et al. 2004; Szpak et al. 2013). Since nitrogen is assimilated by consumers along the food web with a discrimination factor of 3–5 ‰ at each step (Ambrose 1991; Bocherens and Drucker 2003; DeNiro and Epstein 1981), a negative correlation between rainfall and δ15N values has also been found in herbivore bone collagen (Heaton et al. 1986; Murphy and Bowman 2006; Pate and Anson 2008; Sealy et al. 1987, among others) as well as other animal tissues (Hartman 2011). Initially, some authors have postulated physiological mechanisms to cope with water stress to explain the high δ15N values measured on animal tissues in arid environments (see Ambrose 1991 for details on the models suggested by: Ambrose and DeNiro 1986; Schoeninger and DeNiro 1984; Sealy et al. 1987). Nevertheless, the results of both experimental and field research provide support to reject physiological explanations in favour of those that emphasise diet as the main cause of variation in nitrogen isotope composition, explaining higher δ15N values in animal tissues as a result of higher δ15N values in soils and plants (Ambrose 2000; Hartman 2011; Murphy and Bowman 2006). According to this, variation in the δ15N values within a food web and its correlation with water availability can be related to the relative openness of the nitrogen cycle, as well as the predominant fraction of the soil (organic or inorganic) and the nitrogenous compounds available in it (see Amundson et al. 2003; Austin and Vitousek 1998, for details). At the same time, a previous work has found that plant δ15N values increase when temperature increases (Amundson et al. 2003). In turn, a positive correlation has been observed between mean annual temperature and herbivore bone collagen δ15N values (Stevens et al. 2006). According to all these results, variations in the δ15N values measured on bone collagen extracted from herbivorous animals can be related to spatial differences in local environments and to the influence of variables like temperature and water availability in soil and plant δ15N values (Ambrose 1991; Towers et al. 2011).
However, in spite of the numerous works exploring plant and ungulate δ15N values and their correlation with water availability and temperature, very little is known about the relation between these values and altitude. In this sense, Szpak et al. (2013) found a negative correlation between altitude and plant δ15N values from the coast to the highlands in northern Peru. At the same time, Ambrose (1991) presented a negative correlation between altitude and plant δ15N values in East Africa related to differences in water availability and temperature along an altitudinal gradient, a correlation that was also present in herbivore tissues of the same area. Both these studies show that altitude, and its effect on temperature and water availability, must be taken into account when studying δ15N values variation in both plant and animal tissues.
The study area: the dry Puna of Argentina
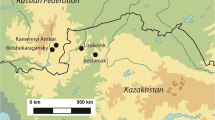
The Puna of Argentina is located between 22° and 27° S and between 3000 and 5000 masl. This region is a highland desert that contains several NE-SW oriented mountain ranges. It is characterised by wide temperature variation between day and night, high solar radiation and low atmospheric pressure due to altitude. The only sources of freshwater are a few rivers and several springs scattered throughout the landscape. Primary productivity is concentrated on stable hydrological systems such as primary basins, high ravines and wetlands. Precipitation occurs during summertime and is governed by the South American monsoon system (Zhou and Lau 1998). This system produces about 80 % of the annual rainfall between December and February (Vuille and Keimig 2004). Precipitation also exhibits a latitudinal gradient which delimits two sub-regions within the Puna of Argentina: the dry Puna, located north of 24° S, with a mean annual precipitation of 300 mm/year; and the salt Puna, located south of 24° S, with a mean annual precipitation that barely reaches 100 mm/year (Bianchi et al. 2005).
Some of the most important plant communities identified in the dry Puna are the following (Cabrera 1976; Ruthsatz and Movia 1975; Braun Wilke et al. 1999) (Fig. 1):
-
1.
Shrub steppe (tolar) is located between 3500 and 3900 masl. It is dominated by species such as Parastrephia lepidophylla and Fabiana densa and exhibits a low proportion of herbs (5 %).
-
2.
Mixed steppe is located between 3900 and 4100 masl. It represents an ecotonal landscape and is composed of both grasses and shrubs.
-
3.
Herbaceous steppe (pajonal) is located between 4100 and 4700 masl. It is dominated by Festuca spp. and other grasses, like Poa spp. and Stipa spp.
-
4.
Wetlands (vegas) are scattered along the landscape between 3500 and 4700 masl. These are restricted patches with high biomass and plant cover through the whole year, composed mainly of hygrophilous grasses such as Deyeuxia spp. and Muhlenbergia spp.
-
5.
Salt flats (Salinas or salares) are also scattered along the landscape between 3400 and 4200 masl. These large salt bodies are surrounded by flat zones colonised by sparse halophytic plants such as Atriplex spp. and Distichlis spp.
Materials and methods
Sampling locations
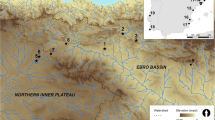
Plant specimens were collected at eight sites distributed along an altitudinal gradient from 3415 to 4245 masl (Fig. 2). These eight sites represent the five different plant communities described above. Salinas Grandes (3415 masl) is located in a vast salt flat characterised by low vegetation density and diversity. Ojo de Agua (3650 masl) is located at a gorge characterised by the presence of shrub steppe vegetation represented by shrubs and some grasses. Lapao is a locality where two different plant communities are present: the shrub steppe (3680 masl), characterised by the presence of shrubs and some herbs, and the wetland (3660 masl), characterised by the presence of grasses and herbs. Pista (3890 masl) is located at a small hill characterised by the presence of shrub steppe vegetation represented by shrubs, grasses and some cacti. Curque (4040 masl) is located at the mixed steppe where both shrubs and highland grasses are represented. Taire is a locality where two different plant communities are present: the mixed steppe (4105 masl), characterised by the presence of both shrubs and grasses, and the wetland (4015 masl), characterised by the presence of grasses and herbs. Laguna Ana represents a salt flat (4110 masl) with low vegetation density and diversity, surrounded by an herbaceous steppe (4135 masl) where only some scattered grasses and shrubs are present. Tuzgle represents a vast wetland (4230 masl), characterised by the presence of sedges, herbs and grasses, surrounded by an herbaceous steppe (4235 masl) that develops on the slope of the Tuzgle volcano in which grasses dominate the vegetation.
Plant material
We employed a quadrat sampling technique (25 m2 squares in all cases, except in the cases of Lapao where a total of 62 m2 were sampled, and Taire and Laguna Ana where 8 m2 were sampled) collecting at least a plant specimen for each species recognised in the field and more than one specimen in the case of the most represented species at each site. In this sense, taxa such as Parastrephia sp. (tola) which are not frequently ingested by SAC but are consistently represented in the vegetation assemblages were also collected. Once plant specimens were collected, they were pressed in brown paper bags and air-dried in the field.
The plants were identified to species in the field when possible, and if not in the laboratory, and then grouped into C3 and C4 photosynthetic pathways according to their δ13C values, and compared to previous results for the area (Fernández et al. 1991; Fernández and Panarello 1999-2001; Panarello and Fernández 2002). However, not all of the plants in this study were identified to species level (see supplementary Table 1).
Sample preparation
At the laboratory, edible plant parts (mostly leaves and stems, see supplementary Table 1) were washed with ultrasonic baths for 45 min and then oven dried at 60 °C for 24 h. Afterwards, the samples were hand ground using an agate mortar and pestle and homogenised to obtain the average isotopic composition for each plant specimen (Cadwallader et al. 2012). We recognise that by selecting the edible parts of the plant specimens and homogenising them, our results may be masking some of the variation in both δ13C and δ15N values between different plant tissues (Badeck et al. 2005; Szpak 2014). Nevertheless, we consider that these variations do not represent a major problem in setting a paleodietary baseline because most of these differences are below 0.5 ‰ and do not exhibit a clear pattern (Szpak et al. 2013).
Mass spectrometry
Measurements of each sample δ13C and δ15N values were made on a CarloErba Elemental Analyser (CHONS) coupled to a Finnigan MAT Delta V continuous-flow isotope ratio mass spectrometer (CF-IRMS) through a Thermo ConFlo IV interface using internal standards. These standards (caffeine: δ13C = −39.33 ‰, δ15N = 7.02 ‰; sugar: δ13C = −11.41 ‰; and collagen: δ13C = −18.18 ‰, δ15N = 6.12 ‰) were calibrated against VPDB and AIR reference standards for carbon (L-SVEC, NBS-19 and NBS-22) and nitrogen (IAEA N1 and IAEA N2) (Coplen et al. 1992, 2006; Craig 1957). Replicates of internal standards showed analytical errors (SD) to be on the order of ±0.2 ‰ for both δ13C and δ15N values.
Results
We analysed a total of 150 plant specimens, which exhibited δ13C values ranging from −31.7 to −12.2 ‰ and δ15N values ranging from −3.1 to 13 ‰ (supplementary Table 1). For C3 plant specimens (n = 131), δ13C values ranged from −31.7 to −21 ‰, with a mean value of −25.4 ‰, and δ15N values ranged from −3.1 to 13 ‰, with a mean value of 3.7 ‰, reflecting in both cases a normal distribution (Shapiro-Wilk test for C3 plants δ13C values W = 0.98, p = 0.10 and δ15N values W = 0.99, p = 0.39). For C4 plant specimens (n = 16), δ13C values ranged from −15.7 to −12.2 ‰, with a mean value of −14.2 ‰, and δ15N values ranged from 1.1 to 8.6 ‰, with a mean value of 4.8 ‰, reflecting in both cases a normal distribution (Shapiro-Wilk test for C4 plants δ13C values W = 0.95, p = 0.56 and δ15N values W = 0.90, p = 0.10). For CAM plant specimens (n = 3), δ13C values ranged from −14.7 to −12.8 ‰, with a mean value of −13.8 ‰, and δ15N values ranged from 4.5 to 6.0 ‰, with a mean value of 5.2 ‰. As can be observed, CAM plant carbon stable isotope composition falls within the C4 range of δ13C values, reflecting the arid conditions of the environment in which they grew (Eickmeier and Bender 1976). Of all the specimens sampled in this study, C4 plant species are mostly grasses (n = 13) while C3 plant species are mostly shrubs (n = 61), but there are also grasses (n = 34) and some herbs (n = 31), as expected in hot dry regions (Koch et al. 1991) (supplementary Table 1).
These results show that C3 plants are by far the most abundant in all localities sampled regardless of altitude (Table 1). In turn, C4 plants are present in five of the eight sampled localities and these five are located along the whole altitudinal gradient sampled, contrary as expected considering previous studies (Panarello and Fernández 2002; Samec 2014; Szpak et al. 2013; Tieszen and Chapman 1992). At Salinas Grandes and Taire mixed steppe sites, 33 % of the plant specimens sampled (n = 3 specimens at both sites) were C4 plants; whereas, at Lapao shrub steppe site, 14 % of the plant specimens sampled (n = 43) were C4 plants (Table 1). Thus, C4 plants are present in all the vegetation communities sampled: shrub steppe, mixed steppe, herbaceous steppe, wetland and salt flat. However, if we compare localities sampled below and above 3900 masl, C4 plants are more abundant below that threshold (C4 plants <3900 masl: n = 12, % = 13.79, including CAM plants: n = 15, % = 17.24, vs. C4 plants >3900 masl: n = 4, % = 6.35), reflecting a differential distribution of these plants along the gradient, as expected from previous results (Panarello and Fernández 2002; Samec 2014; Szpak et al. 2013; Tieszen and Chapman 1992).
δ13C values do not show a correlation with altitude (r = –0.11, p = 0.18), but this is to be expected given that both C3 and C4 plants are being considered. Taking into account only C3 plants, δ13C values also do not show a significant correlation (r = –0.05, p = 0.56), in contrary to results reported in previous investigations, although the altitudinal range surveyed in these studies was wider (~0–4000 masl) than the one considered here (Körner et al. 1991; Szpak et al. 2013) (Fig. 3). On the other hand, δ15N values of both C3 and C4 plants exhibit a significant negative correlation with altitude (r = –0.64, p = 5.6 × 10−19) since values decrease as altitude increases, as expected based on previous investigations (Ambrose 1991; Szpak et al. 2013) (Fig. 4).
Discussion: implications for archaeological research
The study of the variation in plant isotopic compositions is particularly relevant as a baseline for the reconstruction of prehistoric diets of human and animal populations by means of isotopic analyses (Cadwallader et al. 2012; Casey and Post 2011; Szpak et al. 2013). Although most of the wild plants analysed in this study would not have been consumed directly by the human groups that occupied the dry Puna in the past, these results are important as a baseline for the reconstruction of prehistoric animal exploitation strategies and particularly herd management practices (Finucane et al. 2006; Thornton et al. 2011).
As expected from previous results for the area, our results show that C3 plants are predominant in all the plant communities sampled and that the δ13C values of both C3 and C4 plants overlap with those presented in previous works (Fernández et al. 1991; Panarello and Fernández 2002). At the same time, the δ15N values of the sampled plants overlap with previous results from other arid areas, such as East and South Africa (Ambrose 1991; Heaton 1987).
Our results have identified differences in the relative abundances of C3 and C4 plants between the different vegetation communities sampled; these present a distribution that would counteract the effects of atmospheric pressure differences marked by altitude over C3 plant δ13C values and therefore affect the average δ13C signal for each plant community (Tieszen and Chapman 1992). In this sense, C4 plants are proportionately more abundant at low altitude and dry sites in relation to high altitude and moister ones and, thus, the average δ13C value of the natural pastures available along the altitudinal range considered will be higher at low-altitude sites (Szpak et al. 2013). Furthermore, our results do not show a positive correlation between altitude and C3 plant δ13C values as suggested by previous works, although the altitudinal range considered here is particularly narrow in comparison with those studies (~3400–4300 masl vs. ~ 0–4000 masl) (Körner et al. 1991; Szpak et al. 2013). It is important to mention that the differences in water availability between the sampling locations considered here are also a factor that could interfere with the expected pattern for C3 plants, as exposed before (Tieszen and Chapman 1992). On the other hand, our results show that δ15N values correlate with altitude as a response to differences in moisture availability between low- and high-altitude sites, as expected according to previous studies (Ambrose 1991; Szpak et al. 2013).
If we consider the patterns described here, altitude seems to be the most important variable affecting the distribution of C3 and C4 plants, and therefore the average δ13C signal for each plant community, and also their δ15N values. Nevertheless, we consider that altitude by itself does not explain the differential distribution of C3 and C4 plants or the distribution of the δ15N values; instead, water availability and temperature determined by altitude seem to be the causal factors for this pattern. Unfortunately, this is only a working hypothesis that cannot be addressed by this study since there are no measured climate data for some of the localities sampled due to the scattered distribution of climate stations in the Puna of Argentina.
At this point, our results confirm that the higher δ13C and δ15N values measured on SAC from low-altitude sites compared with the lower δ13C and δ15N values measured on SAC from high-altitude sites within the dry Puna of Argentina already published in previous studies can be explained by the consumption of local plants (Fernández and Panarello 1999–2001; Samec 2012, 2014; Yacobaccio et al. 2009). In this sense, previous analyses performed on bone collagen extracted from both wild and domesticated modern SAC resulted in a significant correlation of both δ13C and δ15N values with altitude (Samec 2012, 2014; Samec et al. 2014; Yacobaccio et al. 2009) in accordance with other results within the Andean area (Thornton et al. 2011). In a previous work, Samec (2014) explored the diet of two contemporary llama herds which were fed natural pastures from different plant communities. Both δ13C and δ15N values of the two herds showed significant differences; the herd feeding below 3900 masl presented a mean δ13C value of −17.6 ‰ and a mean δ15N value of 8.3 ‰; whereas, the herd feeding above 3900 masl presented a mean δ13C value of −19.6 ‰ and a mean δ15N value of 5.9 ‰. At the same time, Samec (2014) estimated 95 % confidence intervals for the percentage of C4 and CAM plants included in the diet of both herds using the model of Phillips and Gregg (2001). These resulted in a range between 19 and 27 % in the case of the herd feeding below the 3900 masl threshold and a range between 0 and 9 % in the case of the herd feeding above that threshold, approaching the percentages of C4 and CAM plants present in the plant communities sampled above and below 3900 masl and presented in this study. Thus, previous results measured on modern llama bone collagen now can be easily explained by the natural abundances of C3 and C4 plants and the distribution of their δ15N values along the altitudinal range sampled within this study. These results can be employed to distinguish the use of plant communities placed below 3900 and above 3900 masl as pasturelands by the pastoral groups that occupied this area in the past by comparing these modern standards with the δ13C and δ15N values measured on archaeological bone materials. In the future, we hope to be able to establish the seasonal use of the different plant communities considered here by performing a serial sampling of llama tooth tissues.
Final remarks
The application of stable isotope analyses to the study of human diets in the past has a long history in archaeology (Hastorf 1985; Tykot 2004). Along its development, the need for isotopic baselines for accurate interpretations has become evident to most archaeologists employing these techniques. Nevertheless, most of the attempts to develop isotopic baselines for dietary reconstruction have focused on vertebrate fauna (Katzenberg and Weber 1999; Müldner and Richards 2005; Richards et al. 2000, among many others). This study moves a step forward by providing a better understanding of the baseline isotopic variation in plants from the dry Puna of Argentina, putting the emphasis in the sampling of natural pastures employed by the modern pastoral groups that inhabit the area. These groups employ traditional herding strategies that often involve the use of different pasturelands located in different plant communities at different altitudes during the annual cycle (Yacobaccio 2007). Yacobaccio et al. (1998) have found that the herders of the Susques area within the dry Puna of Argentina employ grass steppes and mixed steppes as winter pastures during the dry season, and shrub steppes and wetlands as summer pastures during the wet season. Keeping this information in mind, the development of an isotopic ecology for the area could provide a new tool to explore questions such as herders’ mobility and pastureland use in the past by means of isotopic analysis of animal tissues. In this sense, the isotopic compositions measured on archaeofaunal remains could be interpreted through the frame of reference composed of modern plant and herbivore isotopic values (Ambrose 1991). In the study area, previous investigations had already employed modern SAC values to interpret carbon and nitrogen isotopic compositions of SAC remains recovered in pastoral sites with remarkable results (see Fernández and Panarello 1999–2001; Yacobaccio et al. 2010). In this sense, the results presented here will provide a full picture on that matter, allowing us to explain variation in the isotopic composition of animal tissues through variation in the isotopic composition of the plants consumed and its relation to environmental variables determined by altitude such as temperature and water availability.
References
Ambrose SH (1991) Effects of diet, climate and physiology on nitrogen isotope abundances in terrestrial foodwebs. J Archaeol Sci 18:293–317
Ambrose SH (2000) Controlled diet and climate experiments on nitrogen isotope ratios in rats. In: Ambrose SH, Katzenberg MA (eds) Biochemical approaches to paleodietary analysis. Kluwer Academic/Plenum Publishers, New York, pp 243–259
Ambrose SH, DeNiro MJ (1986) The isotopic ecology of East African mammals. Oecologia 69:395–406
Ambrose SH, Krigbaum J (2003) Bone chemistry and bioarchaeology. J Anthropol Archaeol 22(3):193–199
Amundson R, Austin AT, Schuur EAG, Yoo K, Matzek V, Kendall C, Uebersax A, Brenner D, Baisden WT (2003) Global patterns of the isotopic composition of soil and plant nitrogen. Glob Biogeochem Cycles 17:1031
Austin AT, Vitousek PM (1998) Nutrient dynamics on a precipitation gradient in Hawaii. Oecologia 113:519–529
Badeck FW, Tcherkez G, Nogués S, Piel C, Ghashghaie J (2005) Post-photosynthetic fractionation of stable carbon isotopes between plant organs—a widespread phenomenon. Rapid Commun Mass Spectrom 19:1381–1391
Balasse M, Ambrose SH, Smith TD, Price D (2002) The seasonal mobility model for prehistoric herders in the south-western Cape of South Africa assessed by isotopic analysis of sheep tooth enamel. J Archaeol Sci 29:917–932
Bianchi AR, Yañez CE, Acuña LR (2005) Bases de datos mensuales de las precipitaciones del Noroeste Argentino. Informe del Proyecto Riesgo Agropecuario. INTA-SAGPYA
Bocherens H, Drucker D (2003) Trophic level isotopic enrichments for carbon and nitrogen in collagen: case studies from recent and ancient terrestrial ecosystems. Int J Osteoarchaeol 13:46–53
Bocherens H, Pacaud G, Petr A, Lazarev PA, Mariotti A (1996) Stable isotope abundances (13C, 15N) in collagen and soft tissues from Pleistocene mammals from Yakutia: implications for the palaeobiology of the mammoth steppe. Palaeogeogr Palaeoclimatol Palaeoecol 126:31–44
Braun Wilke RH, Picchetti LPE, Villafañe BS (1999) Pasturas montanas de Jujuy. UNJu
Britton K, Müldner G, Bell M (2008) Stable isotope evidence for salt-marsh grazing in the Bronze Age Severn Estuary, UK: implications for palaeodietary analysis at coastal sites. J Archaeol Sci 35:2111–2118
Burton RK, Snodgrass JJ, Gifford-Gonzalez D, Guilderson T, Brown T, Koch PL (2001) Holocene changes in the ecology of northern fur seals: insights from stable isotopes and archaeofauna. Oecologia 128:107–115
Cabrera AL (1976) Regiones fitogeográficas argentinas. Enciclopedia Argentina de Agricultura y jardinería, 2da Edición, tomo II. Buenos Aires. Editorial Acme
Cadwallader L, Beresford-Jones DG, Whaley OQ, O'Connell TC (2012) The signs of maize? A reconsideration of what δ13C values say about palaeodiet in the Andean region. Hum Ecol 40:487–509
Casey MM, Post DM (2011) The problem of isotopic baseline: reconstructing the diet and trophic position of fossil animals. Earth Sci Rev 106:131–148
Cavagnaro JB (1988) Distribution of C3 and C4 grasses at different altitudes in a temperate arid region of Argentina. Oecologia 76:273–277
Cerling TE (1992) Development of grasslands and savannas in East Africa during the Neogene. Palaeogeogr Palaeoclimatol Palaeoecol 97:241–247
Codron J, Codron D, Lee-Thorp JA, Sponheimer M, Bond WJ, De Ruiter D, Grant R (2005) Taxonomic, anatomical, and spatio-temporal variations in the stable carbon and nitrogen isotopic compositions of plants from an African savanna. J Archaeol Sci 32:1757–1772
Coplen TB, Krouse HR, Bohlke JK (1992) Reporting of nitrogen-isotope abundances. Pure Appl Chem 64:907–908
Coplen TB, Brand WA, Gehre M, Gröning M, Meijer HAJ, Toman B, Verkouteren RM (2006) New guidelines for δ13C measurements. Anal Chem 78:2439–2441
Craig H (1957) Isotopic standards for carbon and oxygen and correction factors for mass spectrometric analysis of carbon dioxide. Geochim Cosmochim Acta 12:133–137
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Ann Rev Ecol Sys 33:507–559
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Ehleringer JR, Cerling TE (2002) C3 and C4 photosynthesis. In: Munn RE (ed) Encyclopedia of global environmental change. The earth system: biological and ecological dimensions of global environmental change. Wiley, New York, pp 186–190
Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112:285–299
Eickmeier WG, Bender MM (1976) Carbon isotope ratios of crassulacean acid metabolism species in relation to climate and phytosociology. Oecologia 25:341–347
Evans RD (2001) Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci 6:121–126
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33:317–345
Farquhar GD, Hubick KT, Terashima I, Condon AG, Richards RA (1987) Genetic variation in the relationship between photosynthetic CO2 assimilation rate and stomatal conductance to water loss. In: Biggens J (ed.) Progress in photosynthesis research Vol IV. Dordrecht: Martinus Nijhoff Publishers
Fernández J, Panarello HO (1999-2001) Isótopos del carbono en la dieta de herbívoros y carnívoros de los Andes Jujeños. Xama 12–14:71–85
Fernández J, Markgraf V, Panarello HO, Albero M, Angiolini FE, Valencio S, Arriaga M (1991) Late Pleistocene/early Holocene environments and climates, fauna and human occupation in the Argentine Altiplano. Geoarchaeology 6:251–272
Finucane BC, Maita Agurto P, Isbell WH (2006) Human and animal diet at Conchopata, Perú: stable isotope evidence for maize agriculture and animal management practices during the Middle Horizon. J Archaeol Sci 33:1766–1776
Handley LL, Raven JA (1992) The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ 15:965–985
Hartman G (2011) Are elevated δ15N values in herbivores in hot and arid environments caused by diet or animal physiology? Funct Ecol 25(1):122–131
Hartman G, Danin A (2010) Isotopic values of plants in relation to water availability in the eastern Mediterranean region. Oecologia 162:837–852
Hastorf CA (1985) Dietary reconstruction in the Andes. Anthropol Today 6:19–21
Heaton THE (1987) The 15N/14N ratios of plants in South Africa and Namibia: relationship to climate and coastal/saline environments. Oecologia 74:236–246
Heaton THE (1999) Spatial, species and temporal variation in the 13C/12C ratios of C3 plants: implications for palaeodiet studies. J Archaeol Sci 26:637–650
Heaton THE, Vogel JC, von la Chevallerie G, Collett G (1986) Climatic influence on the isotopic composition of bone nitrogen. Nature 322:822–823
Hobbie EA, Macko SA, Williams M (2000) Correlations between foliar δ15N and nitrogen concentrations may indicate plant–mycorrhizal interactions. Oecologia 122:273–283
Katzenberg MA, Weber A (1999) Stable isotope ecology and palaeodiet in the Lake Baikal region of Siberia. J Archaeol Sci 26:651–659
Kellner CM, Schoeninger MJ (2007) A simple carbon isotope model for reconstructing prehistoric human diet. Am J Phys Anthropol 133(4):1112–1127
Kelly JF (2000) Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can J Zool 78:1–27
Knudson KJ, Webb E, White C, Longstaffe FJ (2014) Baseline data for Andean paleomobility research: a radiogenic strontium isotope study of modern Peruvian agricultural soils. Archaeol Anthropol Sci 6(3):205–219
Koch PL, Behrensmeyer AK, Fogel ML (1991) The isotopic ecology of plants and animals in Amboseli National Park. Kenya. Annual Report of the Director Geophysical Laboratory, Washington, DC, pp 163–171
Koch PL, Fogel ML, Tuross N (1994) Tracing the diets of fossil animals using stable isotopes. In: Lajtha K, Michener B (eds) Stable isotopes in ecology and environmental science. Blackwell Scientific Publications, Oxford, pp 63–92
Körner C, Farquhar GD, Roskandics S (1988) A global survey of carbon isotope discrimination in plants from high altitude. Oecologia 74:623–632
Körner C, Farquhar GD, Wong C (1991) Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 88:30–40
Lee-Thorp JA, Sealy JC, van der Merwe NJ (1989) Stable carbon isotope ratio differences between bone collagen and bone apatite and their relationship to diet. J Archaeol Sci 16:585–599
Llano C (2009) Photosynthetic pathways, spatial distribution, isotopic ecology, and implications for pre-Hispanic human diets in central-western Argentina. Int J Osteoarchaeol 19:130–143
Madgwick R, Sykes N, Miller H, Symmons R, Morris J, Lamb A (2013) Fallow deer (Dama dama dama) management in Roman south-east Britain. Archaeol Anthropol Sci 5:111–122
Makarewicz C, Tuross N (2012) Finding fodder and tracking transhumance: isotopic detection of early goat domestication processes in the Near East. Curr Anthropol 53:495–505
Marshall JD, Zhang J (1994) Carbon isotope discrimination and water-use efficiency in native plants of the north-central Rockies. Ecology 75:1887–1895
Mengoni Goñalons GL (2007) Camelid management during Inca times in N. W. Argentina: models and archaeozoological indicators. Anthropozoologica 42:129–141
Müldner G, Richards MP (2005) Fast or feast: reconstructing diet in later medieval England by stable isotope analysis. J Archaeol Sci 32:39–48
Müldner G, Britton K, Ervynck A (2014) Inferring animal husbandry strategies in coastal zones through stable isotope analysis: new evidence from the Flemish coastal plain (Belgium, 1st–15th century AD). J Archaeol Sci 41:322–332
Murphy BP, Bowman D (2006) Kangaroo metabolism does not cause the relationship between bone collagen δ15N and water availability. Funct Ecol 20:1062–1069
O'Leary MH (1981) Carbon isotope fractionation in plants. Phytochemistry 20:553–567
O'Leary MH (1988) Carbon isotopes in photosynthesis. Bioscience 38:325–336
Panarello HO, Fernández JC (2002) Stable carbon isotope measurements on hair from wild animals from altiplanic environments of Jujuy, Argentina. Radiocarbon 44:709–716
Pate FD (1997) Bone chemistry and paleodiet: reconstructing prehistoric subsistence-settlement systems in Australia. J Anthropol Archaeol 16:103–120
Pate FD, Anson TJ (2008) Stable nitrogen isotope values in arid-land kangaroos correlated with mean annual rainfall: potential as a palaeoclimatic indicator. Int J Osteoarchaeol 18(3):317–326
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Price TD, Burton J, Cucina A, Zabala P, Frei R, Tykot RH, Tiesler V (2012) Isotopic studies of human skeletal remains from a sixteenth to seventeenth century AD churchyard in Campeche, Mexico: diet, place of origin, and age. Curr Anthropol 53(4):396–433
Richards MP, Pettitt PB, Trinkaus E, Smith FH, Karavanic I, Paunovic M (2000) Neanderthal diet at Vindija and Neanderthal predation: the evidence from stable isotopes. Proc Natl Acad Sci 97:7663–7666
Ruthsatz B, Movia C (1975) Relevamiento de las estepas andinas del noreste de la provincia de Jujuy. FECYT, Argentina
Samec CT (2012) Variabilidad dietaria en camélidos de la Puna: un modelo actual a partir de la evidencia isotópica. In Kuperszmit et al. (eds.) Entre Pasados y Presentes III. Estudios Contemporáneos en Ciencias Antropológicas. Colección Investigación y Tesis. Editorial MNEMOSYNE. Buenos Aires. pp. 666-683
Samec CT (2014) Ecología isotópica en la Puna Seca Argentina: un marco de referencia para el estudio de las estrategias de pastoreo en el pasado. Cuadernos del Instituto Nacional de Antropología y Pensamiento Latinoamericano, Series Especiales 2(1):61-85
Samec CT, Morales MR, Yacobaccio HD (2014) Exploring human subsistence strategies and environmental change through stable isotopes in the dry Puna of Argentina. Int J Osteoarchaeol 24:134–148
Schoeninger MJ, DeNiro MJ (1984) Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals. Geochim Cosmochim Acta 48:625–639
Schwarcz H, Schoeninger MJ (1991) Stable isotope analyses in human nutritional ecology. Yrbk Phys Anthropol 34:283–321
Sealy JC, Van Der Merwe NJ, Lee Thorp JA, Lanham J (1987) Nitrogen isotope ecology in southern Africa: implications for environmental and dietary tracing. Geochim Cosmochim Acta 51:2707–2717
Smith BN, Epstein S (1971) Two categories of 13C/12C ratios for higher plants. Plant Physiol 47:380–384
Sparks JP, Ehleringer JR (1997) Leaf carbon isotope discrimination and nitrogen content for riparian trees along elevational transects. Oecologia 109:362–367
Stevens RE, Lister AM, Hedges REM (2006) Predicting diet, trophic level and palaeoecology from bone stable isotope analysis: a comparative study of five red deer populations. Oecologia 149:12–21
Stevens RE, Lightfoot E, Hamilton J, Cunliffe BW, Hedges REM (2013) One for the master and one for the dame: stable isotope investigations of Iron Age animal husbandry in the Danebury environs. Archaeol Anthropol Sci 5:95–109
Swap RJ, Aranibar JN, Dowty PR, Gilhooly WP, Macko SA (2004) Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: patterns and implications. Glob Chang Biol 10:350–358
Szpak P (2014) Complexities of nitrogen isotope biogeochemistry in plant-soil systems: implications for the study of ancient agricultural and animal management practices. Front Plant Sci 5:1–19
Szpak P, White CD, Longstaffe FJ, Millaire JF, Vásquez Sánchez VF (2013) Carbon and nitrogen isotopic survey of northern Peruvian plants: baselines for paleodietary and paleoecological studies. PLoS One 8, e53763
Thornton EK, DeFrance SD, Krigbaum JS, Williams PR (2011) Isotopic evidence for middle horizon to 16th century camelid herding in the Osmore Valley, Peru. Int J Osteoarchaeol 21:544–567
Tieszen LL (1991) Natural variations in the carbon isotopes of plants: implications for archaeology, ecology and paleoecology. J Archaeol Sci 18:227–248
Tieszen LL (1994) Stable isotopes on the plains: vegetation analyses and diet determinations. In Owsley DW, Jantz RL (eds.) Skeletal Biology in the Great Plains: A Multidisciplinary View. Smithsonian Press. pp. 261-282
Tieszen LL, Chapman M (1992) Carbon and nitrogen isotopic status of the major marine and terrestrial resources in the Atacama desert of northern Chile. First World Congress on Mummy Studies. Puerto de la Cruz, Islas Canarias, España, pp 409–425
Tieszen LL, Senyimba MM, Imbamba SK, Troughton JH (1979) The distribution of c3 and c4 grasses and carbon isotope discrimination along an altitudinal and moisture gradient in Kenya. Oecologia 37:337–350
Towers J, Jay M, Mainland I, Nehlich O, Montgomery J (2011) A calf for all seasons? The potential of stable isotope analysis to investigate prehistoric husbandry practices. J Archaeol Sci 38:1858–1868
Tykot R (2004) Stable isotopes and diet: you are what you eat. In: Martini M (ed) Physics methods in archaeometry. Società Italiana di Fisica, Bologna, pp 433–444
Virginia RA, Delwiche CC (1982) Natural 15N abundance of presumed N2-fixing and non-N2-fixing plants from selected ecosystems. Oecologia 54:317–325
Vuille M, Keimig F (2004) Interannual variability of summertime convective cloudiness and precipitation in the central Andes derived from ISCCP-B3 data. J Clim 17:3334–3348
Yacobaccio HD (2007) Andean camelid herding in the south Andes: ethoarchaeological models for archaeozoological research. Anthropozoologica 42:143–154
Yacobaccio HD, Madero CM, Malmierca MP (1998) Etnoarqueología de Pastores Surandinos. Grupo Zooarqueología de camélidos, Buenos Aires
Yacobaccio HD, Morales MR, Samec CT (2009) Towards an isotopic ecology of herbivory in the Puna ecosystem: new results and patterns in Lama glama. Int J Osteoarchaeol 19:144–155
Yacobaccio HD, Samec CT, Catá MP (2010) Isótopos estables y zooarqueología de camélidos en contextos pastoriles de la puna (Jujuy, Argentina). In Gutiérrez MA et al. (eds.) Zooarqueología a principios del siglo XXI. Aportes teóricos, metodológicos y casos de estudio. Editorial del Espinillo, Buenos Aires, pp. 77-86
Zhou J, Lau KM (1998) Does a monsoon climate exist over South America? J Clim 11:1020–1040
Acknowledgments
We wish to thank Brenda Oxman, Alicia Cruz, Juan Maryañski, Marcelo Morales and Sabrina Bustos for their help during field trips, and Mariela Borgnia and Francisco Ratto for their collaboration in plant identification. We are grateful to Estela Ducós and Nazareno Piperissa for their invaluable assistance during laboratory procedures. We also wish to thank Guti Tessone, Malena Pirola and Violeta Killian Galván for their insights in discussing the manuscript. We also thank two anonymous reviewers for their careful reading of the manuscript and detailed comments, which greatly improved the quality of our work. This research was supported by grants from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET PIP 0569), the Universidad de Buenos Aires (UBACyT 230BA) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT PICT 2013-0479).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(XLSX 21.4 KB)
Rights and permissions
About this article
Cite this article
Samec, C.T., Yacobaccio, H.D. & Panarello, H.O. Carbon and nitrogen isotope composition of natural pastures in the dry Puna of Argentina: a baseline for the study of prehistoric herd management strategies. Archaeol Anthropol Sci 9, 153–163 (2017). https://doi.org/10.1007/s12520-015-0263-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12520-015-0263-2