Abstract
Shivalik region is one of the agri-intensive regions in Punjab, India, wherein groundwater quality is a major human health concern. In this study, a total of 57 groundwater samples were collected from the Rupnagar district of this region (one sample per 36 km2) to evaluate its quality, the role of hydrogeochemical processes in its contamination, and further their potential human health hazards. The results indicate that the major water chemistry is governed by carbonate weathering followed by silicate weathering. The Fe, Mg, Mn, Se, and HCO3- concentrations exceeded the BIS drinking water standards in 86, 51, 11, 9, and 79% of the samples, respectively. Piper and Durov plots indicated the dominance of Ca-HCO3- water types, followed by Ca-Mg-Cl- and Ca-Cl-. Furthermore, multivariate analyses indicated the geogenic origin for Fe, Mg, Mn, Se, SO42-, and anthropogenic sources (agrochemicals, cement factories, and fly ash) for NO3-, Cu, and Cr. The estimated carcinogenic risk of As and Cr falls under the very low (10-6) to low (10-5) risks category. Furthermore, the cumulative risk of non-carcinogenic contaminants (F-, U, NO3-) (HI-0.93) is at an alarming level and also close to the boundary line of USEPA limits (HI-1). There is an urgent need to undertake suitable policy measures for sustainability of groundwater quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deterioration of groundwater quality is one of the major concerns of the twenty-first century due to its huge demand for drinking and irrigation purposes (UN water report 2015; Dhawan 2017). The rapid industrialization and increased mining activities have immensely contributed to it (Li et al. 2018, 2019a). The United Nations has set targets under Sustainable Development Goals (SDG) to provide safe and affordable drinking water for all. Goal 6 of SDG focuses on to improve water quality by reducing pollution, eliminating dumping, and minimizing the release of hazardous chemicals by 2030 (UNDP 2020).
The sustainability of groundwater quality is one of the major challenges in the current scenario (Li et al. 2021). The adverse health impacts of contaminated water on humans have been widely reported in many parts of the world (Chowdhury et al. 2016; Ji et al. 2020; He et al. 2020; Palansooriya et al. 2020; Wu et al. 2019, 2020a; Li et al. 2019b). The long-term usage of contaminated water also adversely affects the sustainability of soil and agriculture (Hou et al. 2020). The hydrogeochemical processes occurring in aquifers (dissolution, evaporation, mixing and ion exchange) also influence groundwater quality (Rajesh et al. 2012; Chung et al. 2020).
The developing countries are highly dependent on groundwater for domestic, agricultural irrigation, and industrial activities. Furthermore, groundwater mismanagement in these countries has made the situation more critical in terms of quality and quantity. The geogenic contamination of groundwater with heavy metals/metalloids (like As, U, Se, Fe, Mn) has been reported as one of the major causes of quality deterioration worldwide (Coyte et al. 2019; Martins et al. 2019a, 2019b; Solangi et al. 2019; Houria et al. 2020; Martins et al. 2020; Shaji et al. 2020; Villalba et al. 2020; Rahman et al. 2021). Anthropogenic activities, mainly fertilizers, have also been reported as major causes of groundwater contamination in many countries (Houria et al. 2020; Kansoh et al. 2020).
Nearly, 70% of groundwater has been extracted for agricultural use. As per National Ground Water Association report (2020), India, China, the USA, Pakistan, Mexico, Iran, and Saudi Arabia collectively extract nearly 75% of the global annual groundwater extraction. India, one of the major agrarian countries of the world, extracts the highest amount of groundwater (National Ground Water Association 2020) and faces a serious crisis for groundwater sustainability in terms of quality and quantity. Groundwater fulfills nearly 50% irrigational need of India, which is much higher than the global average (40%) (Dhawan 2017). The Indian state Punjab is known as the heartland of agricultural activities and has been the nation’s Green Revolution leader.
The groundwater of this state is facing a serious crisis of contamination (Hundal and Khurana 2013; Mittal et al. 2014; Kumar et al. 2018; Pacheco et al. 2018a & b; Sharma et al. 2019; Kumar et al. 2020a, 2020b; Sharma et al. 2020a, 2020b). Both geogenic and agricultural sources have reported as the sources of anions [nitrate (NO3)-, sulfate (SO42-), fluoride (F-), phosphate (PO43-)] and heavy metals [(arsenic (As), uranium (U), lead (Pb)] in groundwater of Punjab (Sharma and Singh 2016; Ahada and Suthar 2018, 2019; Kaur et al. 2021; Kumar et al. 2020a, 2020b; Mittal et al. 2020). The elevated levels of these contaminants in groundwater are reported in several parts of Punjab (Shrivastava 2015; Ahada and Suthar 2018, 2019; Sharma et al. 2020a, 2020b), although some parts are still undiscovered and need serious attention. The agri-intensive Shivalik region of Sutlej basin, Punjab, (accounts 11% of the state Punjab area) is one of such region (Yadav et al. 2015). The groundwater of this region is extensively used for drinking and irrigational purposes. Although some scientists have evaluated basic groundwater quality studies in this region (Singh et al. 2011a & b; Sharma et al. 2017, 2018, 2019), detailed investigations concerning the hydrogeochemical process, source apportionment, drinking and agricultural suitability and potential health risk aspects are still lacking. The present study was focused on the Rupnagar district, wherein the increasing demand for groundwater resources for drinking and irrigation has overstressed its quality.
The main objectives of the present study were to investigate (1) hydrochemical characterization of groundwater, (2) evaluation of groundwater quality for drinking and irrigation purposes with the help of Water quality index (WQI) and Heavy metal pollution index (HPI), (3) sources of major groundwater contaminants by using multivariate analyses, and (4) degree of health risks potential for groundwater contaminants. The findings of this study have provided an insightful view of the current status of groundwater quality. This can help the policymakers implement appropriate strategies and preventive measures to manage groundwater quality and sustainability.
Material and method
Structure of the study
The groundwater samples were collected and analyzed for physicochemical parameters, anions, major cations, and heavy metals/trace metals. The suitability of groundwater for drinking purpose was assessed using the water quality index (WQI) and heavy metals pollution index (HPI). The hydrochemistry of groundwater was also evaluated by Gibbs, Piper, and Durov diagrams. Further, source analysis of groundwater contaminants was estimated using different statistical measures like PCA, HCA, and correlation analysis. The health risks of groundwater contaminants have also been predicted through carcinogenic and non-carcinogenic health risks assessment. The flowchart of the entire scheme/methodology is depicted in Fig. 1.
Description of study area
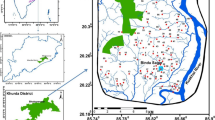
Rupnagar district (formally known as Ropar) is situated in the eastern part of the Punjab State and lies in coordinates between 76° 19′ 00″–76° 45′ 00″ N and 30° 44′ 00″–31° 25′ 00″ E. The study area falls within the Shivalik range of Sutlej basin and occupying 1440 km2 in area (Fig. 2). State Himachal Pradesh geographically surrounds the study area in the north and northeast, Ludhiana, SBS Nagar, and Hoshiarpur districts in the west, Fatehgarh Sahib area in the southern part, and district Mohali in the southeastern part. The Rupnagar district comprises four tehsils, namely Rupnagar, Nangal, Anandpur Sahib, and Chamkaur Sahib. The main occupation of the study area is agriculture, and major crops grown are wheat, paddy, maize, sorghum (jowar), pearl millet (bajra), cotton, sugarcane, and oilseeds (Thakur et al. 2016). The study area faces extreme summer and extreme winter climate. The average annual rainfall is recorded 776 mm, and nearly 78% is received during the monsoon period. The annual temperature fluctuates between 2 and 45 °C.
Geological setting and hydrogeology
The study area’s geomorphology comprises four distinct geological formations, which include Shiwalik Hills, alluvial plain, alluvial fan, and Intermontane valley. These geographical units run parallel to each other. Indo-Gangetic alluvial plain separates Shiwalik Hills range from Himalayan ranges. Charnockite and sandy, silty alluvium are detected as the major rocks types of the central and western part of the Rupnagar district area. The study area’s soil texture is loam to silty clay loam majorly, except some parts of River Sutlej and chaos (seasonal canals). The geological features of the study area are depicted in Map (Fig. 3 and Fig. SM1).
Land use and land cover maps of Rupnagar district area, [Source: District survey report Rupnagar 2016, LISS III Data Multispectral (15th March 2016)]
The sand and boulder have good groundwater potential due to their high porosity and permeability. On the other side, clay and silts are considered for poor groundwater potential due to their lesser permeability. Thus, sands and boulders relatively make good aquifers (Thakur et al. 2016). The aquifer system of the study area distributes into two parts such as southern (Quaternary alluvial deposits) and northern parts (Shivalik formation, Intermontane Valleys and Kandi/Sirowal) (CGWB 2017). Unconfined to confined aquifer types exist in this study area. Groundwater occurs under the phreatic condition for the majority of the study area. At the northern part of the district area (intermontane valley), groundwater flow is towards the south and southeastern direction (Singh et al. 2011a). The depth of groundwater was observed 50 to 459 meters in this study area. The existing gross groundwater draft is 45,735 ham (hectare-meter) for all uses, whereas net annual groundwater availability is 41,947 ham. Approximately, 64232 tubewells are operated by farmers for irrigation purpose in the Rupnagar district. The groundwater discharges 731–1500 liters/min. Mostly 3 out of 5 blocks of the study area come under an overexploited region. Out of 5 blocks, 3 blocks (Chamkaur Sahib, Nurpur Bedi and Morinda) have exceeded the available recharge (> 100%) of the groundwater development stage. As per CGWB, the area suitable for artificial recharge in the district is 100 km2, a sub-surface storage potential of 77 MCM (Million Cubic Meters). Around 102 MCM volume water would be required to recharge for this study area (CGWB 2017). The overall geography is summarized in Table 1.
Collection of groundwater sample and chemical analysis
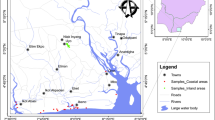
In this study, grid-wise sampling was performed to better represent the study area and ensure the collection of at least one sample from each grid (6 × 6 km2). The groundwater samples were collected from 57 different locations in 1L polypropylene bottles (pre-cleaned and washed with HNO3 and DI water) during October 2018 after 3–5 min of flushing the groundwater (hand pumps, tube wells) of varying depths range 100–350 ft. Global positioning system (GPS Model: etrex 30x) was used to record sampling site’s geographical coordinates and mapped using Google Earth Pro and ArcGIS 10.8 GIS software (Fig. 1). The detailed description of all 57 sampling locations with their geographical coordinates is provided in Table SM2.
The physicochemical characteristics (pH, TDS, EC, salinity, ORP and DO) of groundwater samples were immediately measured in situ by using HANA multi-parameter (Model HI9829). The samples acidified with ultrapure HNO3 (pH < 2) immediately after collection in the field were used for major cations (Na, Mg, K, Ca) and heavy metals/trace elements analysis, while un-acidified samples were used for the estimation of major anions (F-, Cl-, NO3-, PO43-, SO42-) and other basic parameters (hardness, and alkalinity). All samples were properly labelled, shifted immediately to the laboratory in ice box, and then kept in the refrigerator at 4 oC for further analysis. Total hardness (as CaCO3), alkalinity, bicarbonate (HCO3-), and chloride (Cl-) were estimated by using APHA (2012) titration method. The concentration of F-, SO42-, NO3-, and PO43- were estimated using SPADNS, barium chloride turbimetric (IS 2003), UV-visible spectrophotometer and stannous chloride method, respectively. The major cations and heavy metals/trace elements were estimated using flame photometer (Model Systronics 2180) and inductively coupled plasma mass spectrometry (ICP-MS; Thermo Scientific Model iCAPQc), respectively.
Quality control and quality assurance
Duplicates samples and replicated analyses of certified reference materials (CRMs; GBM303-4, MRGeo08) were performed for quality assurance (QA) and quality control (QC). Analytical grades of all the chemicals (company - Lobachemie and Sigma-Aldrich) and Millipore ultrapure water were used to prepare reagents and standards. Standardization of ICP-MS was performed by using a successive dilution of ICP multi-element standard solution (Lobachemie UN NO-3264). The precision was within ± 5% for most of the elemental analysis. Uranium concentration was crosschecked by LED fluorometer model LF-2a (Manufacturer: Quantalase Enterprises Private Limited, Indore, India), based upon the fluorescence principle of U complexes in the aqueous sample (Rathore 2013).
Water quality index
Water quality index (WQI) is a multiparametric water quality measure, which evaluate the groundwater suitability for drinking and domestic purposes. The WQI is calculated by using the method proposed by Ramakrishnaiah et al. (2009). In this drinking quality measure, weights assigned to each parameter according to their relative influence on water quality are presented in Table 2 (Ramakrishnaiah et al. 2009). In the first step of WQI, relative weights (Wi) are determined as per Eq. (1) (value is tabulated in Table 2).
where ‘wi’ is the weights of each parameter, ‘Wi’ is relative weights, and n indicates the number of parameters.
In the 2nd step, the quality rating scale qi is calculated for each parameter by using Eq. (2):
where qi is the quality rating scale for each parameter, Ci is the concentration of ith chemical parameter of water sample (mg/l), Si is permissible standard for BIS (2012) ith parameter of water as depicted in Table 2.
In the third step, subindex (SIi - subindex of ith parameter) is calculated as per Eq. (3) for each parameter.
In the last step, WQI is calculated as following Eq. (4):
Heavy metal pollution index
The cumulative influence of heavy metals/trace elements contamination of groundwater was determined by using the heavy metal pollution index (HPI) (Hossain and Patra 2020). The most influencing heavy metals/trace metals in groundwater were considered for the calculation of HPI as per the context of this study area (Table 2). The HPI was calculated following Eqs. (5) to (7). Subindex (Qi) is determined in the first step as follows:
where, Ci is the ith metal concentration in a sample and, Mi and Li are the maximum permissible limit (MPL) and lower desirable limit (LDL) of ith parameter, respectively. The MPL and LDL values were represented in Table 2. After calculation of subindex (Qi), HPI is calculated from Eq. (6), as follows:
where, Wi - the unit weight of ith metal, Qi - quality rating/sub-index value of ith metal
Unit weight of ith metal is calculated as follows:
Groundwater quality for irrigation purposes
The irrigational quality of groundwater was estimated by using different indices (Eqs. (8) to (12)) such as EC classification (Wilcox 1955), sodium adsorption ratio (SAR; Richards 1954), percent sodium (%Na; Wilcox 1955), permeability index (PI; Doneen 1964), residual sodium carbonate (RSC; Richards 1954), and corrosivity ratio (Wang et al. 2020; Zhou et al. 2020).
The concentrations of all used variables in irrigational indices from Eqs. (8) to (12) are expressed in meq L-1. The SAR and corrosivity ratio are unitless indices and denoted as a factor.
Data analysis and mapping
The descriptive statistics of groundwater data were calculated by using Excel Microsoft Office (version 2010). Piper and Durov diagrams were plotted by using Geochemist’s Workbench software (version 12). Pearson’s correlation matrix (PCM), principal component analysis (PCA), and hierarchical cluster analysis (HCA) were performed by using SPSS software (version 20). Pearson’s correlation analysis was performed to evaluate the relationship between groundwater quality parameters. PCA and HCA were performed to examine the contamination sources of groundwater (Wu et al. 2020b). The eigenvalue value-based varimax rotation method was performed for PCA extraction. Prior to PCA and HCA, the data was pretreated and autoscaled with log-transformation. The GIS software ArcGIS (version 10.8) was used to map of drinking water quality indices in the study area. WQI, HPI, and the health hazard potential of carcinogenic and non-carcinogenic contaminants are mapped over the study area by using Kriging technique.
Potential health risks of groundwater contaminants
Human beings are exposed to groundwater, mainly through oral ingestion. Potential health risks of groundwater contaminants on residents are estimated quantitatively by carcinogenic and non-carcinogenic health risk assessment as per USEPA recommended equations Eqs. (13) to (16) (USEPA 1989). The present study assessed the health risks potential of carcinogenic contaminants for Cr, As, and Pb and non-carcinogenic contaminants for Fe, Cu, Zn, U, F-, and NO3- in term of cancer risk (CR) and hazard quotient (HQ), respectively. The ADI (average daily intake) value of water was considered mainly through ingestion mode (Eq. 13).
where, C concentration of contaminants, IR ingestion rate (2 L water/day), EF exposure frequency (365 days/year), ED exposure duration (30 years), BW body weight (70 kg), and AT average time (25,550 days for carcinogenic risk and 10,950 days for non-carcinogenic risk). These values were considered in the context of the current study area (USEPA 2005; Kumar et al. 2020a, 2020b; Mittal et al. 2020).
USEPA determines the maximum permissible limits of daily oral exposure of toxic chemicals/substance/elements for human. The ADI and RfD are represented in mg/kg-day. SF stands to cancer slope factor and represented in (mg/kg-day)-1. The values of RfD and SF were taken from USEPA IRIS (2020), Wongsasuluk et al. (2014), Anim-Gyampo et al. (2019), Kumar et al. (2020a, 2020b). The RfD values for carcinogenic contaminants As, Cr, and Pb are 3 × 10-4, 3 × 10-3, and 3.5 × 10-4, respectively, while SF values are 1.5, 0.5, and 8.5 × 10-3, respectively. The RfD value of non-carcinogenic contaminants such as Fe, Cu, Zn, U, F-, and NO3- are 0.7, 0.04, 0.3, 6 × 10-4, 3 × 10-4, 0.06, and 1.6, respectively. Hazard index (HI) is a cumulative influence of non-carcinogenic contaminants on human health through groundwater consumption as determined by USEPA (1989) Eq. (16).
where HQi is a non-cancer hazard quotient (HQ) for the ith contaminants
Results and discussion
Hydrochemical characteristics of groundwater
The results of chemical analysis of groundwater and their comparisons with BIS limit (2012) are summarized in Table 3. The majority of groundwater samples were slightly alkaline (mean pH 7.4) and fall within the BIS maximum allowable limit (pH 8.5) for drinking water. The alkaline nature of groundwater of this region has also been reported in previous studies of Singh et al. (2011a), Singh et al. (2014), and Sharma et al. (2019). The EC ranged from 246 to 2308 μS cm-1 over study area. The TDS ranged from 172 to 1615 mg L-1 (mean value 515 mg L-1) and was higher than the BIS acceptable limit in nearly 42% of samples. The groundwater of Rupnagar Nagar district has been classified as hard (121–180 mg L-1) to very-hard water (hardness > 180 mg L-1) (Kaur et al. 2017). The mean hardness was recoded 203 mg L-1, and 42% of samples surpassed the BIS acceptable limit (200 mg L-1). The elevated concentration of total hardness indicates the intense leaching of lime containing agricultural chemicals and detergents to the district's groundwater table (Sharma et al. 2019). The alkalinity level exceeded the BIS drinking water acceptable limit (200 mg L-1) in nearly 80% of the study area. The dominancy of bicarbonate in groundwater is mainly influencing alkalinity level for this region.
The mean concentration of anions followed in the order of HCO3- (304 mg L-1) > SO42- (62.5 mg L-1) > Cl- (38.2 mg L-1) > NO3-(13.2 mg L-1) > F- (0.78 mg L-1) > PO43- (0.18 mg L-1). Except for HCO3-, nearly 95% of groundwater samples were within the BIS/WHO acceptable limits of anions. Thakur et al. (2016) have also reported a similar range of anions concentration for this district. The order of mean concentrations (mg L-1) of major cations was Na (44.2) > Mg (35) > Ca (22.5) > K (14). The concentration of Mg exceeded the BIS limit (30 mg L-1) in 51% of sites among those cations. Thakur et al. (2016) have also made a similar observation of Mg (average Mg 36.4 mg L-1) and Ca level (average Ca 47.4 mg L-1) in this district. The high level of Mg and Ca reflects their major contribution to total hardness.
Assessment of heavy metals/trace elements concentrations
The mean concentration (μg L-1) of heavy metals in groundwater varied in the order of Fe (615) > Zn (133) > Mn (31.4) > Cu (16.4) > U (6.25) > Se (3.23) > Cr (1.87) > Co (1.76) >As (1.48) > Pb (0.19) > Cd (0.07) (Table 3). The Fe content is predominant in groundwater with nearly 86% sites exceeded BIS acceptable limit (300 mg L-1). Sharma et al. (2017) have also reported that Fe and Mn concentrations exceeded the BIS limit in 92% and 9% samples, respectively. Sharma et al. (2018) further reported elevated concentration of Fe in pre- (mean 860 μg L-1) and post-monsoon (mean 2660 μg L-1) at 11 sites of Rupnagar district. The concentration of Se exceeded the BIS/WHO limit (10 μg L-1) at 9% of sites. The concentration range of Se was BDL - 22.4 μg L-1 in this district. The results are consistent with previous studies of Bajaj et al. (2011) and Dhillon and Dhillon (2016). The concentration of Cr (range 0.21–11.94 μg L-1), Pb (BDL-5.73 μg L-1), As (0.07–10.8 μg L-1) Cd (BDL-0.94 μg L-1), and U (0.18–51 μg L-1) were less than their respective BIS/WHO limits in most of the samples. Sharma et al. (2017) reported groundwater As concentration in the range of 2.31–15.9 μg L-1 at 11 sites of the district. Recently, Tiwari et al. (2020) also reported a similar level of As (6.64 μg L-1) and Cr (1.81 μg L-1) in the Rupnagar district.
Drinking water quality assessment based on WQI and HPI
WQI and HPI are the two very important measures to assess drinking water quality. The WQI ranges 1–50, 51–100, 101–200, 201–300, and > 300 are categorized as excellent, good water, poor water, very poor water, and unsuitable water (Ramakrishnaiah et al. 2009). As shown in the spatial distribution map of WQI, most of the study area is under the ‘good water’ category (51–100) (Fig. 4(a)). Nearly similar range of WQI (51–100) was also reported by Singh et al. (2011a) and Chaudhry and Sachdeva (2020) for this study area. The HPI represents the cumulative influence of heavy metals on the overall quality of water. The HPI ranges represent 1–25 for excellent, 26–50 for good, 51–75 for poor, and 76–100 for very poor and > 100 for unsuitable water types (Vetrimurugan et al. 2017). The entire study area can be nearly classified as excellent HPI category (1–25) (Fig. 4(b)), indicating heavy metal contamination in groundwater is not a major concern for this study area. However, Fe concentration in groundwater of the district was mostly high, but, it was not so high to affect its overall quality. Presently, trace amounts of As, Cr, and U in groundwater are not a threat, but need to be monitored regularly for the future.
Irrigation quality of groundwater
The EC classification depicted that nearly all groundwater samples were under good (< 750 μS cm-1) to permissible category (< 2000 μS cm-1), and only 2% of the samples fall under the doubtful category (Table 4). The SAR classification revealed that all the groundwater samples were in excellent category (< 10) for irrigation, indicating no alkali hazard to the crops. The SAR values also suggest no adverse impacts through exchangeable sodium in the entire study area (Thakur et al. 2016). According to Wilcox classification (Na%), most groundwater samples (95%) were found safe for irrigation purposes. The PI value was above 25% permeability at nearly all sites, indicating groundwater suitability for irrigational usages. However, 65% of sites under class II quality (25–75% permeability) reflect that most groundwater samples are marginally suitable for agriculture purposes. Based on the RSC classification of groundwater, 54%, 28%, and 18% of samples fallen under good, doubtful, and unsuitable categories, respectively. The corrosivity ratio of 88% groundwater samples was less than 1, reflecting safe transportation through pipes. However, nearly 12% of groundwater samples were not safe (corrosivity ratio > 1) for transportation purposes through pipes.
Groundwater hydrochemistry
Gibbs, Piper, and Durov diagrams were used to understand the groundwater hydrochemistry (Figs. 5 and 6; Sahoo et al. 2019). The results of these diagrams are well corroborated to each other. Gibbs diagram (Gibbs 1970) (Fig. 5) illustrates that rock-water interaction is the main factor influencing major ions chemistry. Carbonate, silicate, weathered lime, dolomitic limestone, Charnockite, sandy, and silty alluvium are reported as the major rocks types of this region (Singh et al. 2014; Thakur et al. 2016). Weathering of these rocks releases chemical ions species in groundwater. The study of Thakur et al. (2016) also suggests the role of carbonate weathering in groundwater chemistry for this district. Rupnagar district is a hilly region with the dominance of carbonate rocks (dolomitic limestone and weathered lime). Weathering of carbonate rocks during the rainy season may cause groundwater’s alkaline character (Singh et al. 2011b). Weathering of carbonate minerals is responsible for an elevated level of HCO3- ions in groundwater (above than BIS limit) of the study area (Rao et al. 2017).
Further, both Piper and Durov diagrams also give information about the geochemical evolution and origin of groundwater with classifications of different water types (He and Li 2020). The Piper plot demonstrated that most groundwater samples fell under Ca-HCO3- field, followed by Ca-Mg-Cl- and Ca-Cl- types (Fig. 6(a)). Similar findings for this study area were also reported by Thakur et al. (2016). Further, most samples are located under Mg2+ and HCO3- field in Piper plot, thus recognized as the major cation and anion, respectively. After HCO3-, SO42- are recognized as the 2nd abundant ions in groundwater. Durov diagram also shows similar findings as observed Mg2+ and HCO3- are major cation and anions, respectively (Fig. 6(b)). Durov diagram also reveals that most groundwater samples have a TDS less than 1000 mg L-1 in which HCO3- was the main anion. Both Piper and Durov plots indicate the dominance of Ca, Mg, and HCO3- in groundwater. The Ca/Mg ratio of the majority of the samples was < 1. This indicates the contribution of calcite weathering to the existence of Ca and Mg in groundwater. Furthermore, the molar ratio of Na/Cl was > 1, indicates dominancy of silicate weathering (Singh et al. 2011b). The molar ratio of Ca/SO42- > 1 in 52% samples and Ca/SO42- < 1 in 48% samples indicate dissolution of dolomite and precipitation of calcite, respectively. Hence, these minerals are the major sources of Ca in the study area (Singh et al. 2014). These findings suggest that major ions dissolution has existed in this region’s groundwater through the natural geogenic process (Thakur et al. 2016). The detailed impacts of natural lithology and the influence of anthropogenic practices have been assessed by multivariate analysis in the next section for the major existing contaminants in groundwater.
Source analysis of heavy metals/trace element and anions
The sources apportionment pattern of groundwater contaminants was analyzed using multivariate analyses (PCA, HCA and PCM). The PCA components were extracted based on eigenvalue > 1 (Li et al. 2019a). In the PCA analysis, four principal components (PC) were extracted for groundwater characteristics with 50% of the total variance (Table SM3). Subsequently, cluster (dendrogram) and PCM analysis (Table 5) were performed for the reliability of PCA and more specific detection of sources/origins along with their association for groundwater contaminants (Fig. 7).
The PC1 shows high loading of Na, Mg, Cl-, F-, HCO3-, PO43-, and SO42- with 17.45% variance and their occurrence in the same cluster, suggests their common origin and co-existence. The PC1 components showed partial to moderate correlation between them (r = 0.13 to 0.48). The major sources of all PC1 components are suggested to be geogenic. The positive association of SO42- with EC, TDS, and Cl- (r = 0.24 to 0.48) indicates that geogenic activities such as weathering of gypsum and silicate bearing rocks may release SO42- to groundwater (Sharma et al. 2017). The dissolution of F− bearing minerals in an alkaline environment (biotites, fluorite, granite, mica, topaz) is probable source for the existence of F- in groundwater (Singh et al. 2011b; Rasool et al. 2018; Li et al. 2014, 2019b). The interactions of underground rocks like schists, quartzites, and granites with water in an alkali environment are major sources suggested being the season for Na enrichment in groundwater (Singh et al. 2014). The ratio of SO42-/Cl- > 1 indicates fertilizers and effluents are additional sources of SO42- in this region (Lee and Song 2007; Egbi et al. 2020).
The PC2 shows high loading of As, Cr, Co, Cu, and K (variance - 13%), clustered together. The moderate positive correlation between these elements (r = 0.31 to 0.62) indicates a similar origin. The anthropogenic activities such as excessive use of agrochemicals, cement factories, coal fly ash from the thermal power plant are likely the major sources of these heavy metals/trace elements in groundwater (Sharma et al. 2019). The study area has plants of agrochemicals, cement factories, coal, and thermal power.
The presence of PC3 (Ca, Mn, and Zn) with 10% variance is mainly attributed as their natural origins. These elements also clustered together and show partial to the moderate association (r = 0.14 to 0.66). The strong association between Mn and Zn (r = 0.66) indicates a common source. The geogenic processes such as weathering of manganese-bearing rocks in deeper layers of earth and partial influence of anthropogenic sources (leaching of fly ash released by a thermal power plant) may be the major source of Mn in groundwater (Sharma et al. 2017). The gypsum dissolution and precipitation of calcite are suggested to the major sources of Ca (Singh et al. 2014). The alkaline and calcareous nature of soils can be attributed to a low concentration of Zn in groundwater for this study area (Sharma et al. 2018).
The PC4 has a high loading of Fe and Se (variance 9.3%) with partial to moderate correlation (r = 0.18 to 0.48), which indicates their common origin and existence. Selenium sources are strongly regarded as geogenic, and no anthropogenic sources are suggested (Bajaj et al. 2011). The rainwater dilution and movement of Se-bearing rocks and sediments in the Shivalik Hills region are possible sources for Se in groundwater for this region (Dhillon and Dhillon 2014, 2016). Dhillon and Dhillon (2014) have reported Se content in rock samples of lower (1864–2754 μg kg-1) and upper Siwalik sub-Himalayan ranges (11–847 μg kg-1). The majority of soils in this district are alkaline, promoting Se-species’ mobility (Eiche et al. 2015). The major source of Fe has been reported as Fe-bearing minerals like ferruginous quartzite, pyrites, and geotheite (Singh et al. 2011b; Sharma et al. 2018) for this region. Cd and Pb’s location in the same cluster indicates their concurrent existence and may release through coal fly ash, as suggested by Sharma et al. (2019).
Health risk potential of groundwater contaminants
The health risks of drinking groundwater (carcinogenic and non-carcinogenic) on Rupnagar district residents have been presented in Table 6. The mean ADI value of contaminants via ingestion of groundwater was in order of NO3- > F- > Fe > Zn > Cu > U > Cr > As > Pb. The mean carcinogenic risk potential of groundwater contaminants followed in the order of Cr >As > Pb. The majority of the area is under very low (10-6) to low (10-5) carcinogenic risks potential of As and Cr (Fig. 8). Sharma et al. (2017) have also reported the probability of 438 cancer cases/lakh in the district due to As ingestion through drinking water in a study of 11 sites of this study area, which is significantly higher than the USEPA limit (1cases for every 1,000,000). The study further described that local people has also been exposed to low risks of As through rice grain ingestion. The high-risk potential of Cr through groundwater consumption (mean~1E-03) has also been reported by Sharma et al. (2019) at a few Ropar wetland sites. Sharma et al. (2018) has reported Cr presence in wheat grains, rice grains, mustard seeds, and maize grains for this area. The health risk of Pb was not observed at any site in the entire district.
Further, the mean risk potential of non-carcinogenic contaminants (mean HQ) via oral groundwater ingestion was estimated in the order as F- (0.37) > U (~ 0.28) > NO3- (0.23) > Fe (0.025) > Cu (0.011) > Zn (0.01). The non-cancerous contaminants were within the health risk limit (HQ > 1) in the majority of sites of this district (Table 6). The HI value of non-carcinogenic chemicals was < 1 (i.e., 0.93), indicating that cumulative health impacts through drinking of all non-carcinogenic chemicals are alarming, and currently do not pose any significant health risks to residents. Distribution maps (Fig. 8) show the majority of the area is at very low carcinogenic risk. In the case of non-carcinogenic risks, the majority of the area is below USEPA health risks limit (HI-1). However, a high risk of non-carcinogenic chemicals was observed near Nangal block, while carcinogenic risks were observed higher in some areas of Chamkaur Sahib Block.
Comparison of groundwater quality of Shivalik and other regions of Punjab
The groundwater quality of this district is relatively safe compared to many other parts of Punjab. Recently, we examined the groundwater quality in different geographical regions of Punjab such as Sutlej basin (Kumar et al. 2020a, 2020b), Shivalik Hill range (Mittal et al. 2020) and associated parts of Tosham hill region (Kumar et al. 2020a). The U concentration followed a decreasing trend as it passes through the southward to northward part of Punjab. A similar observation was also reported by Shrivastava (2015). Comparison with Tosham hill region that associated with parts of Bathinda and Barnala district (Hundal and Khurana 2013; Bajwa et al. 2017; Kumar et al. 2020a), the Shivalik region is less affected by other toxic contaminants As, U, F-, and NO3- (Thakur et al. 2016; Sharma et al. 2019; Mittal et al. 2020). The other studies in Shivalik region (Hoshiarpur, Kapurthala, Jalandhar, Rupnagar, and SBS Nagar districts) have also been reported concentration of F-, SO42-, As, Fe, Cu, Zn, Cr, Pb, and U in groundwater is within the BIS/WHO drinking water limit (Singh et al. 2011b; Krishan et al. 2014; Lapworth et al. 2017). The similar inference was made from in Shivalik region of state Himachal Pradesh (Dev and Bali 2019) and Uttarakhand (Kumar et al. 2017). However, industrial and agricultural activities in a few locations may have altered groundwater quality in some areas of Shivalik region (Singh et al. 2010; Bhutiani et al. 2017). Moreover, a higher Se level has been reported in the northern Shivalik hill region than other Punjab parts due to Se enriched rocks in the district (Bajaj et al. 2011; Dhillon and Dhillon 2016).
Conclusions
The groundwater of the Shivalik region in Sutlej basin is alkaline in nature and water type Ca-HCO3- exists predominantly. The level of Fe, Mn, and Se in groundwater exceeded the BIS standard on 86, 11, and 9% sites, respectively. The groundwater quality is found to be suitable for both drinking and agricultural purposes. The carcinogenic contaminants As and Cr may pose low category risks (1 in 1,00,000 person) to the residents. The cumulative exposure of non-carcinogenic contaminants (HI-0.93) is alarming and is at the boundary line of USEPA limits (HI-1). Both natural and anthropogenic sources contribute anions and heavy metals/trace elements in groundwater. Multivariate analyses indicate that Fe, Mg, Mn Se, and SO42- are of geogenic origin majorly and anthropogenic sources contribute NO3-, Cu, and Cr to groundwater. The overall assessment suggests the groundwater quality in the Shivalik region is relatively safe, as compared with other parts of Punjab. However, existence of trace amount of As, Cr, and U in groundwater is like an alarm for future.
Thus, continuous groundwater quality monitoring along with proper management of groundwater with strict regulations is essential for maintaining groundwater sustainability for Shivalik region. The findings of the current study can be used to fulfill the United Nations target of sustainable development goals 2030 agenda (SDG 2030). In future work, source analysis of major toxic contaminants of groundwater should be well established.
Data availability
All data set used in the current manuscript is original. The corresponding author has all raw data of the manuscript and related experiments; it can be provided as a reasonable request.
Code availability
Not applicable.
References
Ahada CP, Suthar S (2018) Groundwater nitrate contamination and associated human health risk assessment in southern districts of Punjab, India. Environ Sci Pollut Res 25(25):25336–25347. https://doi.org/10.1007/s11356-018-2581-2
Ahada CP, Suthar S (2019) Assessment of human health risk associated with high groundwater fluoride intake in southern districts of Punjab, India. Expo Health 11(4):267–275. https://doi.org/10.1007/s12403-017-0268-4
Anim-Gyampo M, Anornu GK, Appiah-Adjei EK, Agodzo SK (2019) Quality and health risk assessment of shallow groundwater aquifers within the Atankwidi basin of Ghana. Groundw Sustain Dev 9:100217. https://doi.org/10.1016/j.gsd.2019.100217
APHA (2012) Standard methods for the examination of water and waste, 22nd edn. American Public Health Association, Washington, pp 4–72
Bajaj M, Eiche E, Neumann T, Winter J, Gallert C (2011) Hazardous concentrations of selenium in soil and groundwater in North-West India. J Hazard Mater 189(3):640–646. https://doi.org/10.1016/j.jhazmat.2011.01.086
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2017) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci 10(1):13–19. https://doi.org/10.1016/j.jrras.2015.01.002
Bhutiani R, Kulkarni DB, Khanna DR, Gautam A (2017) Geochemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar, India. Energy Ecol Environ 2(2):155–167. https://doi.org/10.1007/s40974-016-0019-6
BIS (2012) Indian Standards Specifications for Drinking Water.IS: 10500. New Delhi: Bureau of Indian Standards
CGWB (Central Ground Water Board) (2017) Report on Aquifer Mapping and Management Plan Rupnagar District, Punjab. <http://cgwb.gov.in/AQM/NAQUIM_REPORT/Punjab/Ropar.pdf> Accessed 30 August 2020
Chaudhry AK, Sachdeva P (2020) Groundwater quality and non-carcinogenic health risk assessment of nitrate in the semi-arid region of Punjab, India. J Water Health 18(6):1073–1083. https://doi.org/10.2166/wh.2020.121
Chowdhury S, Mazumder MJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569:476–488. https://doi.org/10.1016/j.scitotenv.2016.06.166
Chung SY, Rajendran R, Senapathi V, Sekar S, Ranganathan PC, Oh YY, Elzain HE (2020) Processes and characteristics of hydrogeochemical variations between unconfined and confined aquifer systems: a case study of the Nakdong River Basin in Busan City, Korea. Environ Sci Pollut Res 27:10087–10102. 1-16. https://doi.org/10.1007/s11356-019-07451-6
Coyte RM, Singh A, Furst KE, Mitc WA, Vengosh A (2019) Co-occurrence of geogenic and anthropogenic contaminants in groundwater from Rajasthan, India. Sci Total Environ 688:1216–1227. https://doi.org/10.1016/j.scitotenv.2019.06.334
Dev R, Bali M (2019) Evaluation of groundwater quality and its suitability for drinking and agricultural use in district Kangra of Himachal Pradesh, India. J Saudi Soc Agric Sci 18(4):462–468. https://doi.org/10.1016/j.jssas.2018.03.002
Dhawan V (2017) Water and Agriculture in India. <https://www.oav.de/fileadmin/user_upload/5_Publikationen/5_Studien/170118_Study_Water_Agriculture_India.pdf> Accessed on 15 August 2020
Dhillon KS, Dhillon SK (2014) Development and mapping of seleniferous soils in northwestern India. Chemosphere 99:56–63. https://doi.org/10.1016/j.chemosphere.2013.09.072
Dhillon KS, Dhillon SK (2016) Selenium in groundwater and its contribution towards daily dietary Se intake under different hydrogeological zones of Punjab, India. J Hydrol 533:615–626. https://doi.org/10.1016/j.jhydrol.2015.12.016
District survey report Rupnagar (2016) https://cdn.s3waas.gov.in/s3e2c0be24560d78c5e599c2a9c9d0bbd2/uploads/2020/10/2020102231.pdf <Accessed on 15 February 2021>
Doneen LD 1964 Notes on water quality in Agriculture Published as a Water Science and Engineering Paper 4001. Department of Water Science and Engineering, University of California.
Egbi CD, Anornu GK, Ganyaglo SY, Appiah-Adjei EK, Li SL, Dampare SB (2020) Nitrate contamination of groundwater in the Lower Volta River Basin of Ghana: sources and related human health risks. Ecotoxicol Environ Saf 191:110227. https://doi.org/10.1016/j.ecoenv.2020.110227
Eiche E, Bardelli F, Nothstein AK, Charlet L, Göttlicher J, Steininger R, Sadana US (2015) Selenium distribution and speciation in plant parts of wheat (Triticum aestivum) and Indian mustard (Brassica juncea) from a seleniferous area of Punjab, India. Sci Total Environ 505:952–961. https://doi.org/10.1016/j.scitotenv.2014.10.080
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:795–840. https://doi.org/10.1126/science.170.3962.1088
He S, Li P (2020) A MATLAB based graphical user interface (GUI) for quickly producing widely used hydrogeochemical diagrams. Geochemistry 80(4):125550. https://doi.org/10.1016/j.chemer.2019.125550
He X, Li P, Wu J, Wei M, Ren X, Wang D (2020) Poor groundwater quality and high potential health risks in the Datong Basin, northern China: research from published data. Environ Geochem Health 43:791–812. https://doi.org/10.1007/s10653-020-00520-7
Hossain M, Patra PK (2020) Contamination zoning and health risk assessment of trace elements in groundwater through geostatistical modeling. Ecotoxicol Environ Saf 189:110038. https://doi.org/10.1016/j.ecoenv.2019.110038
Hou D, O'Connor D, Igalavithana AD, Alessi DS, Luo J, Tsang DC, Ok YS (2020) Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat Rev Earth Environ 1:366–381. 16. https://doi.org/10.1038/s43017-020-0061-y
Houria B, Mahdi K, Zohra TF (2020) Hydrochemical characterization of groundwater quality: Merdja plain (Tebessa town, Algeria). Civil Eng J 6(2):318–325. https://doi.org/10.28991/cej-2020-03091473
Hundal HS, Khurana MP (2013) An appraisal of groundwater quality for drinking and irrigation purposes in southern part of Bathinda district of Punjab, northwest India. Environ Earth Sci 70(4):1841–1851. https://doi.org/10.1007/s12665-013-2272-8
IS (2003) Methods of sampling and test (physical and chemical) for water and wastewater. IS: 3025 (Part 24)-1985-Reaffirmed 2003. New Delhi: Bureau of Indian Standards.
Ji Y, Wu J, Wang Y, Elumalai V, Subramani T (2020) Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain, China. Expo Health 12(3):469–485. https://doi.org/10.1007/s12403-020-00357-6
Kansoh R, Abd-El-Mooty M, Abd-El-Baky R (2020) Computing the water budget components for lakes by using meteorological data. Civil Eng J 6(7):1255–1265. https://doi.org/10.28991/cej-2020-03091545
Kaur T, Bhardwaj R, Arora S (2017) Assessment of groundwater quality for drinking and irrigation purposes using hydrochemical studies in Malwa region, southwestern part of Punjab, India. Appl Water Sci 7(6):3301–3316. https://doi.org/10.1007/s13201-016-0476-2
Kaur G, Kumar R, Mittal S, Sahoo PK, Vaid U (2021) Ground/drinking water contaminants and cancer incidence: a case study of rural areas of South West Punjab, India. Hum Ecol Risk Assess 27(1):205–226. https://doi.org/10.1080/10807039.2019.1705145
Krishan G, Lapworth DJ, Rao MS, Kumar CP, Smilovic M, Semwal P (2014) Natural (baseline) groundwater quality in the Bist-Doab catchment, Punjab, India: a pilot study comparing shallow and deep aquifers. Int J Earth Sci Eng 7:16–26
Kumar R, Chauhan A, Rawat L (2017) Physico-chemical analysis of surface and ground water in selected sites of Dehradun, Uttarakhand, India. J Anal Toxicol 6:420. https://doi.org/10.4172/2161-0525.1000420
Kumar R, Vaid U, Mittal S (2018) Water crisis: issues and challenges in Punjab. In: Vijay PS, Shalini Y (eds) Water resources management. Springer, Singapore, pp 93–103. https://doi.org/10.1007/978-981-10-5711-3_7
Kumar R, Mittal S, Sahoo PK, Sahoo SK (2020a) Source apportionment, chemometric pattern recognition and health risk assessment of groundwater from southwestern Punjab, India. Environ Geochem Health 43:1–23. https://doi.org/10.1007/s10653-020-00518-1
Kumar R, Mittal S, Peechat S, Sahoo PK, Sahoo SK (2020b) Quantification of groundwater–agricultural soil quality and associated health risks in the agri-intensive Sutlej River Basin of Punjab, India. Environ Geochem Health 42:4245–4268. 1-24. https://doi.org/10.1007/s10653-020-00636-w
Lapworth DJ, Krishan G, MacDonald AM, Rao MS (2017) Groundwater quality in the alluvial aquifer system of northwest India: new evidence of the extent of anthropogenic and geogenic contamination. Sci Total Environ 599:1433–1444. https://doi.org/10.1016/j.scitotenv.2017.04.223
Lee JY, Song SH (2007) Evaluation of groundwater quality in coastal areas: implications for sustainable agriculture. Environ Geol 52(7):1231–1242. https://doi.org/10.1007/s00254-006-0560-2
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014) Occurrence and hydrogeochemistry of fluoride in shallow alluvial aquifer of Weihe River, China. Environ Earth Sci 71(7):3133–3145. https://doi.org/10.1007/s12665-013-2691-6
Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K (2018) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, northwest China. Mine Water Environ 37(2):222–237. https://doi.org/10.1007/s10230-017-0507-8
Li P, Tian R, Liu R (2019a) Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expo Health 11(2):81–94. https://doi.org/10.1007/s12403-018-0277-y
Li P, He X, Li Y, Xiang G (2019b) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: a case study of Tongchuan, northwest China. Expo Health 11(2):95–107. https://doi.org/10.1007/s12403-018-0278-x
Li P, Karunanidhi D, Subramani T, Srinivasamoorthy K (2021) Sources and consequences of groundwater contamination. Arch Environ Contam Toxicol 80(1):1–10. https://doi.org/10.1007/s00244-020-00805-z
Martins L, Pereira A, Oliveira A, Fernandes A, Sanches Fernandes LF, Pacheco FAL (2019a) An assessment of groundwater contamination risk with radon based on clustering and structural models. Water 11(5):1107. https://doi.org/10.3390/w11051107
Martins L, Pereira A, Oliveira A, Sanches Fernandes LF, Pacheco FA (2019b) A new framework for the management and radiological protection of groundwater resources: the implementation of a portuguese action plan for radon in drinking water and impacts on human health. Water 11(4):760. https://doi.org/10.3390/w11040760
Martins LMO, Pereir AJSC, Oliveira AS, Fernandes LS, Pacheco FAL (2020) A new radon prediction approach for an assessment of radiological potential in drinking water. Sci Total Environ 712:136427. https://doi.org/10.1016/j.scitotenv.2019.136427
Mittal S, Kaur G, Vishwakarma GS (2014) Effects of environmental pesticides on the health of rural communities in the Malwa Region of Punjab, India: a review. Hum Ecol Risk Assess 20(2):366–387. https://doi.org/10.1080/10807039.2013.788972
Mittal S, Kumar R, Sahoo PK, Sahoo SK (2020) Geochemical assessment of groundwater contaminants and associated health risks in the Shivalik region of Punjab, India. Toxin Rev 1-17. https://doi.org/10.1080/10807039.2019.1705145
National Ground Water Association (2020) Facts about global groundwater usage. https://www.ngwa.org/what-is-groundwater/About-groundwater/facts-about-global-groundwater-usage Acceesed on 20 February 2021
Pacheco FAL, MartinsL MO, Quininha M, Oliveira AS, Fernandes LS (2018a) An approach to validate groundwater contamination risk in rural mountainous catchments: the role of lateral groundwater flows. Methods X 5:1447–1455. https://doi.org/10.1016/j.mex.2018.11.002
Pacheco FAL, Martins LMO, Quininha M, Oliveira AS, Fernandes LS (2018b) Modification to the DRASTIC framework to assess groundwater contaminant risk in rural mountainous catchments. J Hydrol 566:175–191. https://doi.org/10.1016/j.jhydrol.2018.09.013
Palansooriya KN, Yang Y, Tsang YF, Sarkar B, Hou D, Cao X, Ok YS (2020) Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: a review. Crit Rev Environ Sci Technol 50(6):549–611. https://doi.org/10.1080/10643389.2019.1629803
Rahman A, Mondal NC, Fauzia F (2021) Arsenic enrichment and its natural background in groundwater at the proximity of active floodplains of Ganga River, northern India. Chemosphere 265:129096. https://doi.org/10.1016/j.chemosphere.2020.129096
Rajesh R, Brindha K, Murugan R, Elango L (2012) Influence of hydrogeochemical processes on temporal changes in groundwater quality in a part of Nalgonda district, Andhra Pradesh, India. Environ Earth Sci 65:1203–1213. https://doi.org/10.1007/s12665-011-1368-2
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. E-J Chem 6(2):523–530. https://doi.org/10.1155/2009/757424
Raman V (1985) Impact of corrosion in the conveyance and distribution of water. J IWWA 15(11):115–121
Rao PN, Rao SA, Rao NS (2017) Geochemical evolution of groundwater in the western delta region of river Godavari, Andhra Pradesh, India. Appl Water Sci 7(2):813–822. https://doi.org/10.1007/s13201-015-0294-y
Rasool A, Farooqi A, Xiao T, Ali W, Noor S, Abiola O, Nasim W (2018) A review of global outlook on fluoride contamination in groundwater with prominence on the Pakistan current situation. Environ Geochem Health 40(4):1265–1281
Rathore DPS (2013) Interpretation and evaluation of the variations in the uranium, major cations and anions content of hydrogeochemical samples with reference to the time interval between sampling and analysis. Explor Res Atom Minerals 23:207–215
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. Soil Sci 78(2):154
Sahoo PK, Dall’Agnol R, Salomão GN, Junior JF, Silva MS, Souza Filho PWM, Powell MA, Angélica, Pontes PR, Costa MF, Siqueira JO (2019) High resolution hydrogeochemical survey and estimation of baseline concentrations of trace elements in surface water of the Itacaiúnas River Basin, southeastern Amazonia: Implication for environmental studies. J Geochem Explor 205:106321
Shaji E, Santosh M, Sarath KV, Prakash P, Deepchand V, Divya BV (2020) Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula. Geosci Front 12:101079. https://doi.org/10.1016/j.gsf.2020.08.015
Sharma N, Singh J (2016) Radiological and chemical risk assessment due to high uranium contents observed in the ground waters of Mansa District (Malwa region) of Punjab state, India: an area of high cancer incidence. Expo Health 8(4):513–525. https://doi.org/10.1007/s12403-016-0215-9
Sharma S, Kaur I, Nagpal AK (2017) Assessment of arsenic content in soil, rice grains and groundwater and associated health risks in human population from Ropar wetland, India, and its vicinity. Environ Sci Pollut Res 24(23):18836–18848. https://doi.org/10.1007/s11356-017-9401-y
Sharma S, Nagpal AK, Kaur I (2018) Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem 255:15–22. https://doi.org/10.1016/j.foodchem.2018.02.037
Sharma S, Nagpal AK, Kaur I (2019) Appraisal of heavy metal contents in groundwater and associated health hazards posed to human population of Ropar wetland, Punjab, India and its environs. Chemosphere 227:179–190. https://doi.org/10.1016/j.chemosphere.2019.04.009
Sharma DA, Keesari T, Rishi MS, Thakur N, Pant D, Sangwan P, Kishore N (2020a) Distribution and correlation of radon and uranium and associated hydrogeochemical processes in alluvial aquifers of northwest India. Environ Sci Pollut Res 27:38901–38915. 1-15. https://doi.org/10.1007/s11356-020-10015-8
Sharma S, Kumar R, Sahoo PK, Mittal S (2020b) Geochemical relationship and translocation mechanism of arsenic in rice plants: a case study from health prone south west Punjab, India. Groundw Sustain Dev 10:100333. https://doi.org/10.1016/j.gsd.2020.100333
Shrivastava BK (2015) Elevated uranium and toxic elements concentration in groundwater in Punjab state of India: extent of the problem and risk due to consumption of unsafe drinking water. Water Qual Expo Health 7(3):407–421. https://doi.org/10.1007/s12403-014-0144-4
Singh S, Singh C, Mukherjee S (2010) Impact of land-use and land-cover change on groundwater quality in the Lower Shiwalik hills: a remote sensing and GIS based approach. Open Geosci 2(2):124–131. https://doi.org/10.2478/v10085-010-0003-x
Singh CK, Shashtri S, Mukherjee S, Kumari R, Avatar R, Singh A, Singh RP (2011a) Application of GWQI to assess effect of land use change on groundwater quality in lower Shiwaliks of Punjab: remote sensing and GIS based approach. Water Resour Manag 25(7):1881–1898. https://doi.org/10.1007/s11269-011-9779-0
Singh CK, Shashtri S, Mukherjee S (2011b) Integrating multivariate statistical analysis with GIS for geochemical assessment of groundwater quality in Shiwaliks of Punjab, India. Environ Earth Sci 62(7):1387–1405. https://doi.org/10.1007/s12665-010-0625-0
Singh CK, Rina K, Singh RP, Mukherjee S (2014) Geochemical characterization and heavy metal contamination of groundwater in Satluj River Basin. Environ Earth Sci 71(1):201–216. https://doi.org/10.1007/s12665-013-2424-x
Solangi GS, Siyal AA, Siyal P (2019) Analysis of Indus Delta groundwater and surface water suitability for domestic and irrigation purposes. Civ Eng J 5(7):1599–1608. https://doi.org/10.28991/cej-2019-03091356
Thakur T, Rishi MS, Naik PK, Sharma P (2016) Elucidating hydrochemical properties of groundwater for drinking and agriculture in parts of Punjab, India. Environ Earth Sci 75(6):467. https://doi.org/10.1007/s12665-016-5306-1
Tiwari R, Aulakh RS, Bedi JS, Gill JPS, Kumar A (2020) Occurrence and spatial distribution of metals and arsenic in groundwater sources of Punjab (India), and their health risk assessment. Toxicol Environ Chem 102:1–16. https://doi.org/10.1080/02772248.2020.1808895
UN water report (2015) The United Nations World Water Development Report 2015. <http://www.unesco.org/new/fileadmin/MULTIMEDIA/HQ/SC/images/WWDR2015Facts_Figures_ENG_web.pdf> Accessed on 15 August 2020
UNDP (2020) United Nations Development Programme, The 2030 Agenda for Sustainable Development. <https://www.undp.org/content/undp/en/home/sustainable-development-goals/goal-6-clean-water-and-sanitation/targets.html> Accessed on 15 February 2021
USEPA (1989) Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A, Interim Final), EPA/540/1-89/002. U.S. EPA, Washington, DC. <https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf> Accessed on 15 August 2020
USEPA (2005) Guidance on selecting age groups for monitoring and assessing childhood exposures to environmental contaminants. Risk Assessment Forum Washington, DC., pp. 50, 397 K, November 2005, 630-P-03-003F
USEPA IRIS (2020) Environmental Protection Agency’s IRIS Assessments. <http://www.epa.gov/iris/> Accessed 15 August 2020
Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM (2017) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Applied Water Science 7(6):3267–3280
Villalba E, Tanjal C, Borzi G, Páez G, Carol E (2020) Geogenic arsenic contamination of wet-meadows associated with a geothermal system in an arid region and its relevance for drinking water. Sci Total Environ 720:137571. https://doi.org/10.1016/j.scitotenv.2020.137571
Wang D, Wu J, Wang Y, Ji Y (2020) Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: analysis, assessment, and management. Expo Health 12(2):307–322. https://doi.org/10.1007/s12403-019-00314-y
WHO (2011) Guidelines for drinking water quality, vol 1, 4th edn. World Health Organization, Geneva
Wilcox LV (1955) Classification and use of irrigation waters. United States Department of Agriculture, Washington
Wongsasuluk P, Chotpantarat S, Siriwong W, Robson M (2014) Heavy metal contamination and human health risk assessment in drinking water from shallow groundwater wells in an agricultural area in Ubon Ratchathani province, Thailand. Environ Geochem Health 36(1):169–182. https://doi.org/10.1007/s10653-013-9537-8
Wu J, Zhou H, He S, Zhang Y (2019) Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ Earth Sci 78(15):446. https://doi.org/10.1007/s12665-019-8471-1
Wu J, Zhang Y, Zhou H (2020a) Groundwater chemistry and groundwater quality index incorporating health risk weighting in Dingbian County, Ordos basin of northwest China. Geochemistry 80(4):125607. https://doi.org/10.1016/j.chemer.2020.125607
Wu J, Li P, Wang D, Ren X, Wei M (2020b) Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum Ecol Risk Assess 26(6):1603–1621. https://doi.org/10.1080/10807039.2019.1594156
Yadav RP, Panwar P, Arya SL, Mishra PK (2015) Revisit of Shivalik region in different states of northwestern India. J Geol Soc India 86(3):351–360. https://doi.org/10.1007/s12594-015-0322-4
Zhou Y, Li P, Xue L, Dong Z, Li D (2020) Solute geochemistry and groundwater quality for drinking and irrigation purposes: a case study in Xinle City, North China. Geochemistry 80(4):125609. https://doi.org/10.1016/j.chemer.2020.125609
Acknowledgements
The authors are grateful to the Board of Research in Nuclear Science (BRNS), Department of Atomic Energy, (DAE-BRNS), Mumbai, for providing financial assistance. The authors are thankful to Central University of Punjab, Bathinda, and DST–FIST support for chemical analysis. The authors express sincere gratitude to local people for memorable cooperation during the time of sampling work.
Funding
The research work was funded by the Board of Research in Nuclear Science (BRNS), Department of Atomic Energy, (DAE-BRNS), Mumbai. [Sanction number: 36(4)/14/49/2014-BRNS/36044].
Author information
Authors and Affiliations
Contributions
Sunil Mittal and Ravishankar Kumar have designed and performed the experimental/research work, as well as written the manuscript. Prafulla Kumar Sahoo and Sunil Kumar Sahoo were involved in the interpretation of data and statistical analysis and also helped to review and edit the manuscript. Raghavendra Prasad Tiwari was involved in data analysis, and manuscript editing works.
Corresponding author
Ethics declarations
Ethics approval
Not applicable to this research work.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Broder J. Merkel
Supplementary Information
ESM 1
(DOCX 2472 kb)
Rights and permissions
About this article
Cite this article
Mittal, S., Sahoo, P.K., Sahoo, S.K. et al. Hydrochemical characteristics and human health risk assessment of groundwater in the Shivalik region of Sutlej basin, Punjab, India. Arab J Geosci 14, 847 (2021). https://doi.org/10.1007/s12517-021-07043-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-07043-0