Abstract
Water quality of Chilika lagoon was assessed for pre- and post-monsoon seasons. A marked spatial and temporal variability in the water quality was observed in this study. Principal component analysis (PCA) reveals the influence of salinity, anthropogenic factors, as well as vegetation on the water quality. The higher concentration of nitrate (NO3−) and phosphate (PO43−) in certain pockets of the lagoon water was due to the prevalence of agricultural, municipal, and domestic waste. A positive correlation between PO43− and DSi indicates their terrigenous input from freshwater influx. Cyclone Phailin had profound impact on several water quality parameters. pH, dissolved oxygen (DO), and dissolved inorganic carbon (DIC) are governed largely by the presence of macrophytes. The impact of severe cyclone Phailin can be assessed from the very low salinity values persisting in the lagoon even 2.5 months after the cyclone hit Odisha coast. Some toxic heavy metals like Copper (Cu), Lead (Pb), Nickel (Ni), Cobalt (Co), Manganese (Mn), and Iron (Fe) exceeded the Environmental Protection Agency (EPA) standards at many locations in both the seasons. Non-conservative behavior of nutrients indicates well mixed nature of lagoon which is also indicative of good primary productivity. Northern sector (NS) of the lagoon is susceptible to eutrophication being riverine influenced region. Southern sector has more stable ion chemistry as a result of least freshwater discharge, constant supply of saline water, and lesser water renewal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal lagoons are shallow water bodies exhibiting high variability in their physical, chemical, and biological characters. They tend to be unstable environments and the short- and long-term variations that occur in these ecosystems are large in comparison to other saline environments. They receive abundant nutrients from freshwater and support plentiful form of life due to large salinity gradient (Clark et al. 2006; Beltrame et al. 2009). The hydrodynamics of a lagoon is governed by the circulation and mixing of freshwater inputs via river discharge and inflow of saline waters through tidal flows (Lara and Dittmar 1999). Lagoons usually exhibit estuarine characteristics and are regions of high primary productivity (Sylaios and Theocharis 2002; Panigrahi et al. 2009). Salinity is an important factor, which is having direct and indirect control over the nutrient availability and their transformations in the saline aquatic environments. Tropical coastal lagoons are under huge anthropogenic pressures, but have been studied to a lesser extent when compared to temperate lagoons (Balasubramanian et al. 2004; Burford et al. 2008).
In Chilika lagoon, changes in land use pattern and agricultural practices in the catchment basin and adjoining areas has led to addition of enormous amount of nitrogen and phosphorous as residual fertilizers. The discharge of fresh water into the lagoon is quite high during monsoon and post-monsoon while it is negligible or absent during other periods (Mohanty and Panda 2009). The present study focuses on distribution of major nutrients: nitrogen, phosphorus, and dissolved silica besides other water quality parameters since these nutrients play key role in maintaining the trophic state of the lagoon, phytoplankton abundance, growth and metabolism which form the major link in food webs (Satpathy et al. 2010a, b).
Study area
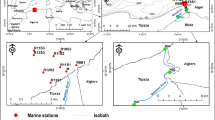
Chilika Lake is the largest tropical lagoon of Asia spread between latitude 19° 28′–19° 54′ N and longitude 85° 05′–85° 38′ E. It is a semi-enclosed, coastal lagoon on the east coast of India in the state of Odisha. Chilika was designated wetland of international importance under Ramsar convention in 1981. It is a biodiversity hotspot and supports many rare, endangered, and vulnerable species besides harboring 1 million birds (Sahu et al. 2014). The lagoon is about 65 km long and varies in breadth from 18 km in north to 5 km in south (Fig. 1). Present studies have estimated the lagoon area to be nearly 704 km2 during summer which spreads to 1020 km2 in monsoon (Gupta et al. 2008). The water depth in the lagoon varies from 0.9 to 2.6 m in the dry season and from 1.8 to 3.7 m in the rainy season. The lagoon is situated on the southern part of the Mahanadi delta-complex. Chilika basin is largely formed by silicate minerals like quartz and feldspar (Zachmann et al. 2009). The lagoon is cut off from the Bay of Bengal by a continuous sandy barrier-spit measuring 60 km in length and 150 m in breadth (Khandelwal et al. 2008). The lagoon is connected to the Bay of Bengal in the east. The barrier spit separates the lagoon from the Bay of Bengal and provides an inlet at its northeast extremity which remains open due to continuous flushing action brought by waves and currents. Changes in the position, shape, and breadth of inlet has occurred from time to time depending on the interplay of available energy from land and sea and also due to the silt brought by the tributaries of Mahanadi Daya and Bhargavi rivers. At present, the lagoon is connected with the Bay of Bengal near Satapada (Sipakuda) by means of an artificial opening made in September 2000.
The main rock types in the Chilika basin are khondalites, charnockites, quartzites, sandstones, conglomerate, and red clays which at places are covered by laterite and alluvium. Physiographically, the region is distinguished into rocky hills at places, rolling plain with lateritic shelves, and terraces in upper reaches of the drainage area. Sandstones, grit, and conglomerates are found in the eastern part of the lagoon (Rai 1985). The rolling plain is characterized by the lateritic residual and transported alluvial soil and deltaic plain is characterized by alluvial soil which is sandy and coarse in texture. Lateritic gravels and ferruginous concretions are common in alluvial soil. Chilika lagoon is part of the Mahanadi delta which has several dead, defunct, and abandoned river channels. These palaeochannels are important sites for palaeobotany, reserves of many important ores, and preserve sedimentary records useful to understand the palaeoclimate conditions (Khandelwal et al. 2008; Misra and Jena 2015).
The catchment area of the lagoon has a tropical climate with an average rainfall of 1238 mm. The local weather condition is mostly controlled by the southwest (SW) and northeast (NE) monsoons and the lagoon experiences the SW monsoon between July and September and the NE monsoon between November and December. December to February is winter which is followed by summer from March to June. The lagoon has several hydrological influences; the most important are its connection to the bay of Bengal in the east and freshwater flow in the north due to the joining of tributaries of Mahanadi river. Many rivers and rivulets join the lagoon through its western catchment. Almost 52 small rivers and streams join the lagoon. The main tributaries of Mahanadi (such as Bhargavi, Daya, and Makara) account for almost 61% (850 m3 s−1) of the total freshwater.
Materials and methods
A total of 26 surface water samples were collected from 4 hydro-ecological (based on the different sectors of the lagoon during pre and post-monsoon seasons (Fig. 1). The lagoon is virtually divided into four sectors based on the hydrological influence, salinity, vegetation, and connection with the sea. The pre-monsoon sampling was conducted in the 1st week of June 2013 and post-monsoon sampling was conducted in January 2014. The post-monsoon sampling was done 2.5 months after the landfall of very severe cyclone Phailin. The water samples were collected in 500 ml clean propylene bottles. The sampling was done on a boat. The surface sample was collected by filling the plastic bottles with water from upper few centimeters. Cleaning of all the plastic bottles was carried out by soaking in 5% nitric acid (HNO3 v/v) for 24 h and then rinsing with doubled-distilled water (DDW). After collection, the water samples were poisoned with HgCl2 for nutrients and dissolved inorganic carbon (DIC) analysis. Another set of samples were collected in 100 ml bottles for dissolved metals analysis. The water samples were filtered through 0.45 μm nylon filters and acidified below pH < 2 by addition of concentrated HNO3. The samples were transferred to the laboratory in the boxes and stored at 4 °C until analysis. All the analysis was completed within 15 days of sampling. In every analysis, appropriate blanks were run as controls. pH, salinity, and EC in the water samples were analyzed on the field with portable multiparameter kit after calibrating with respective buffers for pH and EC. The parameters like DO, DIC, DOC, NO3−, NO2−, PO43−, H4SiO4, SO42−, Cl−, Na+, K+, Ca2+, and Mg2+ were analyzed by standards procedure for surface water.
-
The dissolved oxygen (DO) was measured by Wrinkler’s method
-
HCO3− by titrimetric method
-
Dissolved organic carbon (DOC) was analyzed by TOC analyzer (Shimadzu TOC -5000 analysers)
-
Nitrite (NO2−) by indophenol method
-
Nitrate (NO3−) by cadmium reduction method and phosphate (PO43−) by ascorbic acid method
-
H4SiO4 (dissolved silica) molybdo-silicate method
-
SO42− by turbiditric method
-
Sodium and potassium (Na+ and K+) by Flame photometer (Elico Flame Photometer, CL-378)
-
The heavy metals were analyzed on atomic absorption spectrophotometer (Thermoscientific M series)
Results and discussion
The four regions remained significantly different from one another with respect to many parameters. The salinity variation was significantly different in the two seasons. With respect to different sectors, also salinity varied within the lagoon. Sample numbers 1–4 were collected from the outer channel region, 5–12 from northern sector, 13–19 from the central sector, and 20–26 from southern sector. The values of various parameters have been presented in Table 1.
pH
The lagoon water is alkaline in nature. The mean pH value in the pre-monsoon season was 8.1 (7.5–8.6), whereas it was 8.6 (7.4–10.2) in the post-monsoon period. The spatial distribution of pH was more uniform in pre-monsoon as compared to post-monsoon season (Fig. 2). The highest pH values are observed in the southern sector in pre-monsoon period. High pH values in southern sector are because of greater photosynthetic activity by algae and phytoplankton which remove dissolved CO2 from the surface water and shift the equilibrium toward alkaline (Henneman and Petrucio 2011; Jayakumar et al. 2013). Lowest pH values were observed in the northern sector, due to large freshwater input from the distributaries of Mahanadi river. The average value of pH was higher in the post-monsoon season when compared to the pre-monsoon because of the utilization of CO2 by the photosynthetic activity of the macrophytic flora, thus shifting the equilibrium towards alkaline (Das et al. 2008). The lowering of salinity in the post-monsoon season gave rise to thick mats of higher plants like Potamogeton pectinatus, Najas foveolata, Hydrilla, and many other weeds. The rates of primary production allows a large amount of withdrawals of CO2 and HCO3− resulting in a large rise in pH values, at times > 10. In the post-monsoon season, we observe a small patch of water with very high pH of the order 10.2 in the northern sector which is due to a large number of macrophytes with high photosynthetic activity in this region (Fig. 2).
Salinity
Salinity is one of the most important factors which determine the concentration of other nutrients in a brackish water system. The mean salinity value in pre-monsoon season was 21.44 PSU (1.3–32.8), whereas the post-monsoon mean value was 4.40 PSU (0.2–13.8). Spatial distribution of salinity showed more stratification during the pre-monsoon season as compared to the post-monsoon season (Fig. 3). The salinity value in pre-monsoon period was similar to the previous studies in this lagoon (Nayak and Behera 2004; Panigrahi et al. 2007; Rajawat et al. 2007; Panigrahi et al. 2009; Muduli et al. 2012); however, the salinity values in the post-monsoon period were lower than previous studies (Panigrahi et al. 2007; Panigrahi et al. 2009). The higher and comparatively stable salinity values observed in the southern sector during the pre-monsoon could be a result of the input of saline water from Rushikulya estuary through the enclosed Palur canal throughout the year which maintains an even salinity value (Muduli et al. 2012). Another reason for higher and stable salinity values in the southern sector could be less freshwater discharge in this region. Moderate salinity values observed in the central sector during pre-monsoon is due to good circulation and mixing of both marine and freshwater in this region. This region is well mixed due to the freshwater discharge from small rivulets and streams joining the western catchment of the lagoon and entry of the seawater from the inlets present in the outer channel region.
Spatial distribution of salinity during the post-monsoon depicts very low values in the entire lagoon. During post-monsoon, salinity decreases abruptly over the entire lagoon. The heavy rainfall brought by the southwest and northeast monsoon causes the addition of huge amounts of freshwater into the lagoon which causes substantial dilution lowering down the salinity values considerably (Rajawat et al. 2007). Very low values for salinity ranging from 0.2 to 13.8 PSU in the entire lagoon during the post-monsoon period could also be a result of the large influx of freshwater during the very severe cyclone Phailin which occurred in October 2013. The heavy downpour of about 738 mm during the cyclone may have further lowered the salinity in the post-monsoon season (Sahoo et al. 2014).
Dissolved inorganic carbon
Dissolved inorganic carbon (DIC) mean value during the pre-monsoon period was 170.4 mg/l (132–203.6 mg/l). The average value of DIC during post-monsoon was 140.91 mg/l (103.6–167.7 mg/l). In aquatic systems, DIC concentration is governed by chemical, physical, and biological processes. Spatial and temporal variation of DIC could be related to the catchment area characteristics and variability in hydrology. The spatial and temporal variability of DIC is mostly due to the weathering of the bed rock in the drainage basin; however, biological activity also has a large influence in controlling the DIC values in Chilika lagoon (Gupta et al. 2008). Exchange of CO2 with atmosphere, pH, salinity, carbonates, respiration, and photosynthesis are some of the factors that govern DIC concentration in a water body. Photosynthetic uptake of CO2 and precipitation of calcareous material lowers DIC (Muduli et al. 2013).
The spatial distribution values of DIC for both the seasons have been presented in Fig. 4. DIC shows a higher average value in pre-monsoon season when compared to the post-monsoon season. The higher DIC during the summer may be due to its lesser assimilation by the biological systems. Moderate to high values of DIC has been observed in most parts of southern sector and some parts of central sector during the pre-monsoon season as these regions do not have higher plants which make use of dissolved carbon for photosynthesis. Lower values of DIC observed in the post-monsoon season could be a result of primary production and bacterial respiration of carbon which causes variations in DIC concentration (Muduli et al. 2012; Kanuri et al. 2013).
Dissolved organic carbon
The mean dissolved organic carbon (DOC) value in the pre-monsoon period was 5.65 mg/l (3.87–7.89 mg/l). The mean DOC value for post-monsoon period was 5.85 mg/l (3.97–9.75 mg/l). The chief sources of organic carbon to these environments are allochthonous materials exported from the land through rivers and autochthonous production of organic matter by algae through photosynthesis and intertidal vegetation (Kanuri et al. 2013). The combination of primary production of plant matter and decomposition rates controls the amount of DOC in water. Spatial distribution of DOC has been presented in (Fig. 5). The lower DOC values observed in pre-monsoon season when compared to the post-monsoon season may be a result of DOC being converted to CO2 (Kanuri et al. 2013). Slightly higher DOC during the post-monsoon period can be attributed to heavy downpour and freshwater influence on the entire lagoon which has contributed to the carbon from terrestrial sources. DOC fluxes from sediments are also an important carbon source in many estuaries and benthic remobilization is expected to be an internal source of DOC in shallow systems (Clark et al. 2006). Rivers draining into Chilika carry higher DOC concentration, thus Chilika has appreciable DOC load (Gupta et al. 2008).
Dissolved oxygen
The mean dissolved oxygen (DO) value for pre-monsoon season was 7.34 mg/l (2.34–13.43 mg/l) and 7.94 mg/l for post-monsoon (2.30–17.39 mg/l). The spatial distribution of DO shows higher values in the northern sector during pre-monsoon when compared to the other sectors except near the Balugaon town in the central sector (Fig. 6). Northern sector is dominated by a large number of freshwater macrophytes which engage in active photosynthesis. Oxygen is released as a by-product of photosynthesis. Therefore, northern sector remains well oxygenated. Higher DO concentration near the Balugaon region could be a result of high concentrations of nutrients like NO3+ and PO43+ entering the lagoon from Balugaon township which gives rise thick algal mats. Presence of plants leads to photosynthetic activity and release of oxygen (Bose et al. 2012).
In the post-monsoon season also a similar pattern is observed in DO values in the entire lagoon (Fig. 6). Slightly higher DO values in the post-monsoon season could be a result of the photosynthetic activity of micro and macrophytes along with DO-rich freshwater reaching the system due to the large amount of precipitation which occurred due to Phailin (Srichandan et al. 2015a, b). Greater values of DO in the post-monsoon season could also be a result of wind-induced enhanced vertical mixing due to Phailin and lower water temperature which holds more DO (Fawzy et al. 2012; Barik et al. 2017).
Nitrite
Mean value for nitrite (NO2−) was 0.43 mg/l (0–1.67 mg/l) and 0.72 mg/l (0–2.34 mg/l) for pre and post-monsoon seasons, respectively. NO2− is the intermediate oxidation state between ammonia and nitrate; therefore, it can appear as an intermediate transient species by the oxidation of ammonia or by the reduction of nitrate. NO2− being an unstable species does not remain as such and it gets readily reduced to NO3−. The higher average values of NO2− were observed during post-monsoon season. Higher values ranging from 0.3 to 1.32 mg/l are observed in the southern sector during the pre-monsoon season, whereas values ranging from 0.7 to 2.2 mg/l of NO2− are observed in few patches in a post-monsoon season. NO2− is added into the system due to the phytoplankton excretion, oxidation of ammonia, and reduction of nitrate and by recycling of nitrogen and bacterial decomposition of planktonic debris (Prabu et al. 2008; Satpathy et al. 2010a, b).
The spatial distribution of NO2− has been shown in (Fig. 7). A gradual increase in NO2− concentration is observed moving toward south from the northern part of the lagoon. This may be due to higher NO3− concentration in a southern sector which gets converted to NO2−. We observe very low NO2− in northern sector in spite of higher NO3−. This may be due to rapid assimilation of NO3− by a large number of submerged and floating macrophytes. In post-monsoon season, a very variable trend is observed in the spatial distribution of NO2−. Low concentration is observed in both northern and southern sectors. This could be due to rapid conversion of NO2− to NO3− and its assimilation by the intrinsic vegetation.
Nitrate
Mean value for nitrate (NO3−) was 2.2 mg/l (0.2–7.5 mg/l) for pre-monsoon, whereas average value for NO3− in the post-monsoon season was 5.3 mg/l (0.8–9.6 mg/l). Nitrate in aquatic environments is mainly derived from terrestrial runoff. Concentration and rate of supply of nitrate are related to land use practices in drainage basin, since NO3− moves easily through soils and is rapidly lost from land through natural drainage systems. NO3− is the most stable form of inorganic nitrogen in well-oxygenated waters. Variations in nitrate are predominantly due to biologically activated reactions (Satpathy et al. 2010a, b). It is added through nitrogenous fertilizers, industrial effluents, human and animal wastes through the biochemical activity of nitrifying bacteria, such as nitrosomonas and nitrobacter (Raju et al. 2009).
The spatial distribution of NO3− has been presented in Fig. 8. In the pre-monsoon season, highest NO3− values are observed in the southern sector. Small patches having higher NO3− concentration are also observed in northern and central sectors (Fig. 8). These patches may be witnessing NO3− inputs due to agricultural runoff, untreated sewage discharge, aquaculture ponds, and other household wastes being directly dumped into the lagoon water. Organic nitrogen present in domestic sewage is bound to carbon-containing compounds as proteins (R-NH2). Decomposition of organic nitrogen in the sewage soil by a variety of microorganisms slowly transforms the organic nitrogen to ammonia (NH3) by a process called “mineralization” (Raju et al. 2009). Further during nitrification process, ammonia gets converted to NO2− first and then NO3−. NO2− is unstable therefore its gets oxidized to NO3−. Nitrate is quite soluble in water and being stable does not get adsorbed to soil particles as well. The majority of agricultural fertilizers contain nitrogen in the forms of ammonium and nitrate, often as ammonium nitrate (NH4NO3), which could be the prime source of nitrogen species in the coastal water (Newton and Mudge 2005). High NO3− concentration in the post-monsoon season may be due to large amount of nitrate brought about from the agricultural fields by the large freshwater flow into the lagoon. One of major reasons for high nitrates in certain pockets during post-monsoon may be due to aquaculture ponds and domestic wastes directly released into the water by the fishing villages around the lagoon. Moreover, in winter, the rates of nitrification and denitrification become slow which may have led to NO3− accumulation at some places in the lagoon (Zhang et al. 2014). Higher nitrate concentration in post-monsoon season may also be due to release of NO3− by the death and decay of macroflora. A patch of high NO3− is observed near the Kalijai temple during post-monsoon (Fig. 8). Low water levels and large amount of waste dumped by the visitors to the temple could be the possible reason for high NO3− values in this region. Moderately higher values of NO3− observed in the post-monsoon is a consequence of heavy freshwater discharge during the cyclone Phailin carrying agricultural runoff into the lagoon.
Phosphate
The mean value of phosphate (PO43−) was 0.03 mg/l (0.0–0.09 mg/l) in the pre-monsoon season and 0.04 mg/l (0.01–0.09 mg/l) in the post-monsoon. Phosphate is one of the major nutrients responsible for biological production in the aquatic ecosystems. PO43− is also the limiting nutrient in most of the aquatic ecosystems. Phosphate is mainly derived from agricultural runoff and domestic sewage, part of it is also derived from weathering of P bearing minerals as apatite and fluroapatite (Nazneen and Raju 2017). Domestic sewage is a major source of ammonium and phosphate. Phosphate is also added through fertilizers which contain di-ammonium phosphate (Chauhan and Ramanathan 2008). Low phosphate concentration may be due to its rapid assimilation by the micro and macrophytic vegetation in the lagoon or due to its adsorption on the surface of sediments. Other reasons for PO43− removal from the water column is its microbial incorporation. The spatial distribution of PO43− shows a higher value in some parts of the northern sector and in small patches in the central sector during pre-monsoon (Fig. 9). This is also the place where a large number of birds assemble. The release of phosphate from guano of birds is also responsible for the higher concentration of PO43− near Nalabana island in the central sector of the lagoon. The congregation of birds adds 33.8 t of nitrogen and 10.5 t of phosphorous (in the form of guano) to the lagoon which helps in high biomass production of macrophytes and lucrative fisheries in Chilika (Wetlands International Report 2011). Highest PO43− concentrations ranging from 0.07 to 0.09 mg/l observed in the inner portion of the lagoon during pre-monsoon season might have been primarily influenced by external sources as well as contributions from sediment fluxes (Silva et al. 2013; Barik et al. 2017). There could be a result of PO43− addition from the river runoff in the northern and western boundaries of the lagoon. During post-monsoon, moderately higher values ranging from 0.04 to 0.06 mg/l prevails in most parts of the lagoon. The values observed in both the seasons are comparable to the values observed in previous studies (Panigrahi et al. 2007, 2009).
Dissolved silica
The mean concentration of dissolved silica (DSi) was 3.33 mg/l (0.3–10.3 mg/l) in pre-monsoon and 6.53 mg/l (0.99–9.46 mg/l) in post-monsoon seasons respectively. The spatiotemporal variations of DSi in coastal water is influenced by several factors, including the proportional physical mixing of seawater with fresh water (Purushothaman and Venugopalan 1972), adsorption of reactive silicate into sedimentary particles, chemical interaction with clay minerals (Gouda and Panigrahy 1992), co-precipitation with humic compounds, and iron and biological removal by phytoplankton, especially by diatoms and silicoflagellates (Beucher et al. 2004). The DSi values are lower in the pre-monsoon or summer season; this could be a result of silicate assimilation by the diatoms (Silva et al. 2013). Increased concentration of DSi has been observed in the post-monsoon period over the entire lagoon as compared to pre-monsoon period (Fig. 10). This could be due to increased river discharge after heavy rainfall during tropical cyclone Phailin (Sahoo et al. 2014; Barik et al. 2017). The elevated value of DSi in post-monsoon season is because of freshwater intrusion in the entire lagoon which carries higher DSi load. Another reason for the higher concentration of DSi during the post-monsoon season could be a result of death and decay of diatoms due to low salinity values prevailing in the lagoon. The death of diatoms causes subsequent release of biogenic silica locked up into their frustule into the water column.
Chloride
In the pre-monsoon season, mean value of cchloride (Cl−) was 14,160 mg/l (1200–22,200 mg/l), whereas it was 3467 mg/l (500–9000 mg/l) in the post-monsoon season. Chloride is the most dominant anion in the seawater. NaCl is the major salt present in the seawater. Therefore, Cl− exhibits a similar spatial variation like salinity in both the seasons. In the pre-monsoon season, highest concentration of Cl− is observed in the outer channel region (Table 1), followed by central and southern sectors. The presence of Cl− is largely due to saltwater intrusion; however, evaporate dissolution could also be one of the prominent sources (Raju et al. 2016). The Cl− values are lowest in the northern sector which has major river discharge, and least salinity values. The Cl− values remain quite low in the post-monsoon season when compared to the pre-monsoon season. Chilika receives a large amount of precipitation due to the southwest and northeast monsoons which brings down the salinity to lower values in the subsequent months. The salinity remains very low during the December and January months. In October 2013, cyclone Phailin hit the east coast of India which brought down heavy discharge which further lowered the salinity values in Chilika. Therefore in the post-monsoon season, the highest Cl− of 9000 mg/l value is observed only in the outer channel region near the inlet to Bay of Bengal (Table 1). Southern sector has slightly higher Cl− values due to lesser discharge of freshwater in this sector when compared to others. Southern sector is also connected to the sea by Palur canal and has a constant supply of saline water. Another pathway of saltwater intrusion is due to its connection to Rambha Bay through Rushikulya estuary (Ray 2011).
Sodium and potassium
A similar trend like salinity is observed for sodium and potassium (Na+ and K+) in both the seasons as sea water is the major contributor of these ions. Na+ had a mean value of 7396 mg/l (590–11,900 mg/l) in the pre-monsoon. The post-monsoon mean value for Na+ was 1611 mg/l (140–4850 mg/l). Mean value of K+ in the pre-monsoon season was 271 mg/l (10–520 mg/l) and 50.6mg/l (2.7–136.3 mg/l). Both the cations have a similar distribution like salinity as these ions are the major constituents of seawater. The mean value of Na+ was higher in pre-monsoon season owing to higher salinity values. The other sources of Na+ and K+ could also be due to weathering, erosion, and agricultural drainage in the catchment area which adds these ions into the lagoon (Raju et al. 2012). Clay minerals like illite are also a source of K+ in aquatic bodies. Khandelwal et al. (2008) have mentioned that the southern sector remains relatively undisturbed because of much slower water renewal, and hence brackish water conditions prevail in this zone even during monsoons. This region also has greatest depth, least freshwater discharge, and constant supply of saline water through Palur canal (Muduli et al. 2012). The more stable ion chemistry in the southern sector is reminiscence of geological past of the Chilika basin and can be attributed to several palaeochannels in this region (Misra and Jena 2015).
Calcium and magnesium
Mean calcium (Ca2+) was 630.8 mg/l (22.5–1000 mg/l) in pre-monsoon and 159 mg/l (44–528 mg/l) in the post-monsoon season respectively. Average value of magnesium (Mg2+) was 3449.2 mg/l (83.5–5437.5 mg/l) in pre-monsoon and 688 mg/l (92–2064 mg/l) in post monsoon season respectively. Seawater is the major contributor of these cations in Chilika lagoon. Though freshwater has a considerable amount of Ca2+ and Mg2+ ions, but in Chilika the dominant source of these ions is sea water.
Conservative and non-conservative behavior of nutrients along the salinity gradient
In the estuarine system, the nutrients behave either conservatively or non-conservatively depending on their chemistry, seasonal and biological influence, and several other factors (Liu et al. 2011). An element is conservative if its ratio to salinity is constant. If a material moves through the estuarine system in direct proportion to its relative abundance in relation to some non-reactive material like salinity, then this material is said to be “conservative” with respect to salinity. This implies that the system exerts no control on the fate of the material throughout its passage through the system. If the material moves disproportionately relative to a conservative tracer, it is said to be “non-conservative” with respect to the tracer. Materials, which are non-conservative, are of biogeochemical and ecological interest. Conservative elements have very low chemical reactivity in the ocean and marine systems and their distributions in the ocean interior are determined only by currents and mixing. Nutrients like carbon, phosphorus, and nitrogen are non-conservative in the coastal environment and are essential for the biological productivity of the system. All the nutrients (NO3−, PO43−, DSi) showed a nonlinear behavior with salinity in both the seasons (Fig. 11a) which marks mixing in the lagoon. On the other hand, all major cations like Na+, K+, Ca2+, and Mg2+ show a strong positive correlation with salinity. The anions like Cl−, SO42− is used by sulphate reducing bacteria to form HS- or H2S yet we do not observe a nonlinear behavior for these anions, which is due to their high abundance in comparison to their utilization.
The input of terrigenous materials from agricultural runoff is responsible for the higher concentration of nutrients like NO3− and PO43−. The nonlinear behavior of nutrients with salinity indicates their non-conservative behavior. This kind of non-linear behavior points toward a good amount of mixing in the lagoon beside biological uptake (Wang et al. 2014). The other parameters like Na+, K+, Ca2+, Mg2+, and Cl− do not show much scatter (Fig. 11b). We observe a strong linearity in these parameters with salinity. These ions are governed by the seawater intrusion into the lake. Freshwater is not a major source of these ions; therefore, they behave in a conservative manner. In the present study, NO3− shows a non-conservative behavior but it shows a conservative behavior in Delaware estuary, whereas a non-conservative behavior for NO3− has been observed in Godavari estuary due to removal at lower salinity zone by the uptake from phytoplanktons and addition in high salinity zone due to remineralization (Padmavathi and Satyanarayana 1999).
Correlation analysis
A bivariate correlation analysis is applied to describe the relationship between the two geochemical parameters. A correlation value above 0.5 between two parameters is considered good, whereas a correlation value around 1 (either positive or negative) between two parameters renders them very high relative relationship. The correlation between various parameters has been presented in during the pre and post-monsoon seasons have been presented in Tables 2 and 3.
In pre-monsoon season, a strong positive correlation between salinity (Sal), EC, Cl−, Na+, K+, Ca2+, and Mg2+ indicates that seawater is the major source of these ions. The correlation value of salinity denotes a moderate negative correlation with DO and DSi. Dissolved oxygen decreases with salinity because high salinity waters are low in DO. Negative relation indicates that DSi is mainly brought by the freshwater since tropical rivers have high DSi concentration (Li et al. 2014). A strong positive correlation between NO2− and NO3− is because NO2− being unstable is converted to NO3− in aquatic systems. A positive correlation between PO43− and DSi indicates their terrigenous source. The probable sources of PO43− could be fertilizer residue from the agricultural field. Studies have indicated SiO2 reaching water bodies from the agricultural field along with other nutrients. DSi has a negative correlation with Cl−, Na+, K+, Ca2+, and Mg2+ which shows its freshwater source, as the other ions are predominantly from the seawater. Different sources of PO43− (freshwater) and Mg2+ (seawater) could be a reason for the negative correlation between PO43− and Mg. Salinity and EC are positively related to Cl−, Na+, K+, Ca2+, and Mg2+. All these ions are present in major quantity in seawater. This relation is also an indication of seawater dominance in the lagoon. The geochemical studies on Chilika reveal that Montmorillonite, illite, Kaolinite with Chlorite are present in the sediment sample as major soil is alluvium in Chilika basin. These minerals are also the sources of major ions in Chilika water (Rai 1985).
In post-monsoon season, a good correlation of EC and salinity with SO42−, Cl−, Na+, K+, Ca2+, and Mg2+ indicates that these anions and cations are mainly governed by the seawater since these ions are in major quantity in seawater. NO2− shows a strong positive correlation with NO3− because NO2− is an intermediate state of NO3− in aquatic systems. SO42− is strongly correlated with DSi which could be due to SO42− and DSi release from the death and decay of the diatoms. DSi also shows a positive correlation with K+ which could be due to the simultaneous release of silica and K+ from clay minerals.
Principal component analysis
Principal component analysis (PCA) is a technique for taking high dimensional data and reducing it to more manageable, lower dimensional form without loosing significant information. The PCA enables a reduction in data and description of a given multidimensional system by means of a small number of new variables (Loska and Wiechuła 2003; Garali et al. 2011). Relevant components are those whose eigenvalue is higher than 1 (Kaiser 1960). The application of varimax rotation of standardized component loadings enables us to obtain a clear system as a result of the maximization of component loadings variance and elimination of less valid components (Chakrapani and Subramanian 1993; Mustapha et al. 2013). In the pre-monsoon season, three factors are formed which show 74% of the total variance. PCA 1 is explained by 47.7% of the variance. PC2 and PC3 show 13% of variance each (Table 4).
Factor 1—this factor accounts for 47.7% of the total variability. Factor 1 shows positive loadings of salinity, EC, Cl−, Na+, K+, Ca2+, and Mg2+. This factor can be explained by the influence of seawater. EC is governed by the concentration of ions in a solution. Na+, Cl−, Ca2+, and Mg2+ are the dominant ions in seawater; hence, they show a positive loading with salinity and EC.
Factor 2—current factor shows a positive loading of NO2−, NO3−, and SO42−. A high positive loading between NO2− and NO3− is an indication of the oxidizing environment which oxidizes unstable nitrite to nitrate. Slightly positive loading of NO2−, NO3−, and SO42− could be an indication of their anthropogenic source. Like NO3−, SO42− can also be added to the system from agricultural fields (Singh et al. 2015).
Factor 3—this factor depicts high positive loadings of DOC, PO43−, and DSi. A positive relation between these components suggests their allochthonous source. DOC is mainly transported into estuarine systems by rivers which carry carbon in the dissolved form to the coastal waters (Bouillon and Dehairs 2007., Gupta et al. 2008). Similarly, PO43− and DSi are usually brought by the land and river runoff into the lagoon.
In the post-monsoon season, PCA is explained by 90% of the variance. PC 1 shows 42% variance. PC2 is explained by 17% variance. PC3 by 13%, PC4 by 9%, and PC5 by 8% (Table 5).
Factor 1: factor 1 shows strong positive loadings of salinity, EC, SO42−, Cl −, K+, Ca2+, and Mg2+. This is due to influence of marine water which contains very high concentration of these ions.
Factor 2: factor 2 shows strong positive loadings of DSi and SO42−. This could be the result of terrigenous input of these anions into the system besides release from the death and decay of diatoms and algae.
Factor 3: this factor has positive loadings on NO2− and NO3−. Since NO2− is an intermediate state of NO3−, we observe a positive correlation between these two nutrients.
Factor 4: factor 4 shows a positive relationship between DO and PO43−. This relation can be explained by the deep intrusion of freshwater into the lagoon which brings DO and PO43− load to the lagoon. A positive loading of DO and PO43− suggests PO43− assimilation by the flora and in turn release of DO.
Factor 5: strong positive loadings of DIC and DOC are observed in this factor. The concentrations of both these components are governed by catchment characteristics and aquatic vegetation. There increase or decrease is governed by plant uptake and release.
Heavy metal distribution in water
Heavy metal concentration in Chilika water was analyzed for pre and post-monsoon seasons and compared with standard values of metals for surface water determined by EPA 2009 (the United States Environmental Protection Agency). The range and average values of dissolved metals have been presented in Table 6. Some of the heavy metals like Zn, Cd, and Cr were non-detectable in the water in both the seasons, whereas the water samples at many locations exceeded the EPA standard values for other metals like Pb, Cu, Ni, Co, Mn, and Fe (Table 7).
In general, the heavy metal values were higher in the pre-monsoon season. Cu has a value higher than the standard in all the samples during pre-monsoon, whereas 50% and 85% of Ni and Mn respectively have higher values compared to standards (Table 7). There are several sources of Cu in coastal waters including fertilizers from agricultural fields, municipal and industrial wastewater, and antifouling paints used in the boats (Gardham et al. 2015). Copper is acutely toxic causing deleterious affect on primary producers and decomposers, which are crucial players in essential processes such as the nutrient cycling in aquatic ecosystems (Gardham et al. 2015). In the post-monsoon season due to dilution, heavy metals values were lower. However, Pb was detected in more number of samples in the post-monsoon season (Table 7). This could be due to precipitation during monsoon which washes the Pb present in the vehicular dust. The dissolved metal concentration in Chilika waters is higher and can impose a risk to the fishes and other organisms. The presence of Cu, Co, Ni, Fe, and Mn could be an indicator of corrosion-resistant paints used in fishing boats, fertilizers, and pesticides used in the agricultural fields (Singh et al. 2015; Satapathy and Panda 2015; Nazneen et al. 2018). Elevated Fe levels could be due to dissolution of ferruginous minerals and domestic sewage discharges (Raju et al. 2016).
The wetlands are mostly polluted through agricultural runoff and discharge of untreated sewage and other wastes from urban areas (Bassi et al. 2014; Sarkar et al. 2017). Chilika lagoon receives significant anthropogenic input from Daya and Bhargavi rivers which join the lagoon in its northern periphery. Untreated domestic waste from the capital city of Bhubaneshwar reaches the lagoon through these rivers. Ghosh et al. 2006 have reported that 550MLD of sewage water reaches Chilika lagoon along with domestic wastes from nearly 150 villages in the catchment basin of Chilika lagoon. There are also fish processing units and other small scale industries in the Balguon township which add significant waste at the western periphery. Besides this, a large number of fishing boats ply in Chilika which often use low-grade paints and antifouling agents which contain metals like Pb, Cu, and Zn (Barik et al. 2018; Nazneen et al. 2018). The overall elevated concentrations of the metals in the water samples may be attributed to mobilizing factors like oversiltation, intensive use of mechanized boat for transport using low-grade fuels, tourism and fishing, untreated discharges from the fish processing units, and aquaculture which has been highlighted in the recent studies on sediments of Chilika lagoon (Nazneen and Patel 2016; Barik et al. 2018; Nazneen et al. 2018).
Impact of Phailin on water quality of Chilika lagoon
The water quality of Chilika lagoon was significantly affected by the advent of very severe cyclone Phailin. The study was undertaken 3 months before and 3 months after the cyclone hit the Odisha Coast. There was drastic change observed in salinity post Phailin as the salinity values remained very low. There was a remarkable impact on nutrient biogeochemistry. The NO3− and PO43− values were higher than the pre-Phailin period. DSi values also increased significantly. An increase in DO and pH values was also observed. The pH became more alkaline as a result of enhanced photosynthesis which withdrew CO2 from the system, thus shifting the pH toward alkaline. The NS showed larger changes in water quality due to maximum addition of freshwater from river discharge; eutrophication was evident with thick mats of macrophytic growth. The advent of Phailin lowered down salinity in the entire lagoon which gave rise to changes in other parameters.
Conclusions
A marked spatial and temporal variability is observed in the Chilika lagoon water. The water quality of Chilika lagoon in this study shows the influence of very severe cyclone Phailin in particular and lagoon vegetation, increased agriculture, domestic sewage discharge, and fishing in general. The biological activity plays a major role in governing the water quality parameters. Nutrients like NO3−, PO43−, and DSi show higher values in the post-monsoon season due to heavy freshwater discharge in particular due to very severe cyclone Phailin bringing these from terrestrial sources. Higher nutrient concentration observed in the northern sector of the lagoon is leading to heavy macrophytic infestation. PCA revealed a major influence of seawater besides geological factors in both the seasons as can been seen by the values of Na+, K+, Ca2+, and Mg2+. The other parameters like DSi show that freshwater entering the lagoon also has significant influence on the water quality. The non-conservative behavior of nutrients indicates a good amount of mixing besides biological uptake. Heavy metals like Cu, Pb, Ni, Co, Mn, and Fe exceeded EPA standards at many locations in both the seasons. To control the influx of excess nutrients and heavy metals, the soil from agricultural runoff needs to be reduced. The dumping of untreated domestic waste from the peripheral villages’ lagoon has to be checked. Another important aspect is management and treatment of the urban waste from the capital city of Bhubaneshwar entering the lagoon from Daya and Bhargavi rivers. The number of boats plying in the lagoon need to be restricted and the fishermen community should be made aware of the perils of heavy metal pollution. Further to reduce the heavy metal concentration, the approach needed to be followed should be holistic catchment area treatment in terms of agricultural management and reduction of silt reaching the lagoon.
References
Balasubramanian CP, Pillai SM, Ravichandran P (2004) Zero-water exchange shrimp farming systems (extensive) in the periphery of Chilka lagoon, Orissa, India. Aquac Int 12:555–572
Barik SK, Muduli PR, Mohanty B, Behera AT, Mallick S, Das A, Samal RN, Rastogi G, Pattnaik AK (2018) Spatio-temporal variability and the impact of Phailin on water quality of Chilika lagoon. Cont Shelf Res 136:39–56
Barik SK, Muduli PR, Mohanty B, Rath P, Samanta S (2018) Spatial distribution and potential biological risk of some metals in relation to granulometric content in core sediments from Chilika Lake, India. Environ Sci Pollut Res 25:572–587
Bassi N, Kumar MD, Sharma A, Pardha-Saradhia P (2014) Status of wetlands in India: a review of extent, ecosystem benefits, threats and management strategies. J Hydrol Reg Stud 2:1–19
Beltrame MO, De Marco SG, Marcovecchio JE (2009) Dissolved and particulate heavy metals distribution in coastal lagoons. A case study from mar Chiquita lagoon, Argentina. Estuar Coast Shelf Sci 85:45–56
Beucher C, Tr ´e P, Corvaisier R, Hapette AM, Elskens M (2004) Production and dissolution of biosilica, and changing microphytoplankton dominance in the bay of Brest (France). Mar Ecol Progr Ser 267:57–69
Bose R, De A, Sen G, Mukherjee AD (2012) Comparative study of the Physico chemical parameters of the coastal waters in Rivers Matla and Saptamukhi: impacts of coastal water coastal pollution. Journal of Water Chemistry and Technology 34(5):246–251
Bouillon S, Dehairs F (2007) Biogeochemistry of the Tana estuary and delta (Northern Kenya). Limnol Oceanogr 52(1):46–59
Burford MA, Alongi DM, McKinnon AD, Trott LA (2008) Primary production and nutrients in a tropical macrotidal estuary, Darwin Harbour, Australia. Estuar Coast Shelf Sci 79:440–448
Chakrapani GJ, Subramanian V (1993) Heavy metal distribution and fractionation in sediments of the Mahanadi River basin, India. Environ Geol 22:80–87
Chauhan R, Ramanathan AL (2008) Evaluation of water quality of Bhitarkanika mangrove ecosystem, Orissa, east coast of India. Indian Journal of Marine Sciences 37(2):153–158
Clark LB, Gobler CJ, Sanudo-Wilhelmy SA (2006) Spatial and temporal dynamics of dissolved trace metals, organic carbon, mineral nutrients, and phytoplankton in a coastal lagoon: great South Bay, New York. Estuar Coasts 29(5):841–854
Das SK, Routh J, Alakendra N, Roy Choudhury J, Klump V (2008) Major and trace element geochemistry in Zeekoevlei, South Africa: a lacustrine record of present and past processes. Appl Geochem 23:2496–2511
Fawzy MA, El-sayed BN, El-Khatib A, Abo-El-Kassem A (2012) Heavy metal biomonitoring and phytoremediation potentialities of aquatic macrophytes in River Nile. Environ Monit Assess 184:1753–1771. https://doi.org/10.1007/s10661-011-2076-9
Garali AB, Ouakad M, Gueddari M (2011) Geochemistry of Bizerte lagoon affluent water Northern Tunisia: principal component analysis. Arab J Geosci 4:475–481. https://doi.org/10.1007/s12517-010-0128-z
Gardham S, Chariton AA, Hose GC (2015) Direct and indirect effects of copper-contaminated sediments on the functions of model freshwater ecosystems. Ecotoxicology 24:61–70. https://doi.org/10.1007/s10646-014-1355-y
Ghosh AK, Pattnaik AK, Balatore TJ (2006) Chilika Lagoon: Restoring ecological balance and livelihoods through re-salinization. Lakes & Reservoirs: Research and Management. 11: 239–255
Gouda R, Panigrahy RC (1992) Seasonal distribution and behavior of silicate in the Rushikulya estuary, east coast of India. Indian J Mar Sci 24:111–115
Gupta GVM, Sarma VVSS, Robin RS, Raman AV, Kumar MJ, Rakesh M, Subramanian BR (2008) Influence of net ecosystem metabolism in transferring riverine organic carbon to atmospheric CO in a tropical coastal lagoon (Chilka Lake, India). Biogeochemistry 87:265–285
Henneman MC, Petrucio MM (2011) Spatial and temporal dynamic of trophic relevant parameters in a subtropical coastal lagoon in Brazil. Environ Monit Assess 181:347–361. https://doi.org/10.1007/s10661-010-1833-5
Jayakumar R, Steger K, Chandra TS, Seshadri S (2013) An assessment of temporal variations in physicochemical and microbiological properties of barmouths and lagoons in Chennai (southeast coast of India). Mar Pollut Bull 70(1–2):44–53. https://doi.org/10.1016/j.marpolbul.2013.02.005
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Measure 20:141–151
Kanuri VV, Muduli PR, Robin RS, Kumar BC, Lovaraju A, Ganguly D, Patra S, Rao GN, Raman AV, Subramanian BR (2013) Plankton metabolic processes and its significance on dissolved organic carbon pool in a tropical brackish water lagoon. Cont Shelf Res 61-62:52–61
Khandelwal A, Mohanti M, Garcı’a-Rodrı’guez F, Scharf BW (2008) Vegetation history and sea level variations during the last 13,500 years inferred from a pollen record at Chilika Lake, Orissa, India. Veget Hist Archaeobot 17:335–344. https://doi.org/10.1007/s00334-007-0127-5
Lara RJ, Dittmar T (1999) Nutrient dynamics in a mangrove creek (North Brazil) during the dry season. Mangrove Salt Marshes 3:185–195
Li RH, Liu SM, Li YW, Zhang GL, Ren JL, Zhang J (2014) Nutrient dynamics in tropical rivers, lagoons, and coastal ecosystems of eastern Hainan Island, South China Sea. Biogeoscience 11:481–506. https://doi.org/10.5194/bg-11-481-2014
Liu SM, Li RH, Zhang GL, Wang DR, Du JZ, Herbeck LS, Zhang J, Ren JL (2011) The impact of anthropogenic activities on nutrient dynamics in the tropical Wenchanghe and Wenjiaohe estuary and lagoon system in East Hainan, China. Mar Chem 125:49–68
Loska K, Wiechuła D (2003) Application of principal component analysis for the estimation of source of heavy metal contamination in surface sediments from the Rybnik reservoir. Chemosphere 51:723–733
Misra SP, Jena J (2015) Morphological reconstruction of southern Mahanadi Delta and Chilika lagoon, India – a critical study. Int J Adv Res 3(5):691–702
Mohanty PK, Panda US (2009) Circulation and mixing processes in Chilika lagoon. Indian J Mar Sci 38(2):205–214
Muduli PR, Kanuri VV, Robin RS, Kumar BC, Patra S, Raman AV, Rao GN, Subramanian BR (2012) Spatio-temporal variation of CO2 emission from Chilika Lake, a tropical coastal lagoon, on the east coast of India. Estuar Coast Shelf Sci 113:305–313
Muduli PR, Kanuri VV, Robin RS, CharanKumar B, Patra S, Raman AV, Rao GN, Subramanian BR (2013) Distribution of dissolved inorganic carbon and net ecosystem production in a tropical brackish water lagoon, India. Cont Shelf Res 64:75–87
Mustapha A, Aris AZ, Juahir H, Ramli MF (2013) Surface water quality contamination source apportionment and physicochemical characterization at the upper section of the Jakara Basin, Nigeria. Arab J Geosci 6:4903–4915. https://doi.org/10.1007/s12517-012-0731-2
Nayak L, Behera DP (2004) Seasonal variation of some physicochemical parameters of the Chilika lagoon (east coast of India) after opening the new mouth, near Sipakuda. Indian Journal of marine Sciences 33(2):206–208
Nazneen S, Patel P (2016) Distribution and fractionation of heavy metals in surface sediments of Chilika lagoon, east coast of India. IOSR J Environ Sci Toxicol Food Technol 10(6):63–71
Nazneen S, Raju NJ (2017) Distribution and sources of carbon, nitrogen, phosphorus and biogenic silica in the sediments of Chilika lagoon. J Earth Syst Sci:126–213. https://doi.org/10.1007/s12040-016-0785-8
Nazneen S, Singh S, Raju NJ (2018) Heavy metal fractionation in core sediments and potential biological risk assessment from Chilika lagoon, Odisha state. India. Quaternary International. https://doi.org/10.1016/j.quaint.2018.05.011
Newton A, Mudge SM (2005) Lagoon-sea exchanges, nutrient dynamics and water quality management of the Ria Formosa (Portugal). Estuar Coast Shelf Sci 62:405–414
Padmavathi D, Satyanarayana D (1999) Distribution of nutrients and major elements in riverine, estuarine and adjoining coastal waters of Godavri, Bay of Bengal. Indian J Mar Sci 28:345–354
Panigrahi S, Chandra AB, Panigrahy RC, Nayak BK, Banarjee K, Sarkar SK (2007) Anthropogenic impact on water quality of Chilika lagoon RAMSAR site: a statistical approach. Wetlands Ecol Manage 15:113–126. https://doi.org/10.1007/s11273-006-9017-3.
Panigrahi S, Wikner J, Panigrahy RC, Satapathy KK, Acharya BC (2009) Variability of nutrients and phytoplankton biomass in a shallow brackish water ecosystem (Chilika Lagoon, India). Limnology 10:73–85. https://doi.org/10.1007/s10201-009-0262-z.
Prabu VA, Rajkumar M, Perumal P (2008) Seasonal variations in physico-chemical parameters in Uppanar estuary, Cuddalore (southeast coast of India). J Mar Biol Ass India 50(2):161–165
Purushothaman A, Venugopalan VK (1972) Distribution of dissolved Silicon in the Vellar Estuary. Indian J Mar Sci 1:103–105
Rai LB (1985) Sedimentology of Lake Chilka. M. Phil Dissertation, Jawaharlal Nehru University
Rajawat AS, Gupta M, Acharya BC, Nayak S (2007) Impact of new mouth opening on morphology and water quality of the Chilika Lagoon—a study based on Resourcesat-1 LISS-III and AWiFS and IRS-1D LISS-III data. Int J Remote Sens 28(5):905–923
Raju NJ, Ram P, Dey S (2009) Groundwater quality in the Lower Varuna River Basin, Varanasi District, Uttar Pradesh. J Geol Soc India 73:178–192
Raju NJ, Dey S, Gossel W, Wycisk P (2012) Fluoride hazard and assessment of groundwater quality in the semi-arid Upper Panda River basin, Sonbhadra district, Uttar Pradesh, India. Hydrological Sciences Journal. 57:7, 1433–1452. https://doi.org/10.1080/02626667.2012.715748
Raju NJ, Patel P, Reddy BCSR, Suresh U, Reddy TVK (2016) Identifying source and evaluation of hydrogeochemical processes in the hard rock aquifer system: geostatistical analysis and geochemical modeling techniques. Environ Earth Sci 75:1157
Ray AK (2011) Biogeochemical fluxes of carbon and nitrogen from Chilika Lake, East Coast of India. PhD Thesis, Anna University.
Sahoo S, Baliarsingh SK, Lotliker AA, Sahu KC (2014) Imprint of cyclone Phailin on water quality of Chilika lagoon. Curr Sci 107(9)
Sahu BK, Pati P, Panigrahy RC (2014) Environmental conditions of Chilika Lake during pre and post hydrological intervention: an overview. J Coast Conserv. 18:285–297. https://doi.org/10.1007/s11852-014-0318-z
Sarkar SK, Mondal P, Biswas JK, Kwon EE, Ok YS, Rinklebe J (2017) Trace elements in surface sediments of the Hooghly (Ganges) estuary: distribution and contamination risk assessment. Env Geochem and Health. https://doi.org/10.1007/s10653-017-9952-3
Satapathy DR, Panda CR (2015) Spatio-temporal distribution of major and trace metals in estuarine sediments of Dhamra, Bay of Bengal, India—its environmental significance. EnvironMonit Assess. 187:4133. https://doi.org/10.1007/s10661-014-4133-7
Satpathy KK, Mohanty AK, Natesan U, Prasad MVR, Sarkar SK (2010a) Seasonal variation in physicochemical properties of coastal waters of Kalpakkam, east coast of India with special emphasis on nutrients. Environ Monit Assess 164:153–171. https://doi.org/10.1007/s10661-009-0882-0
Satpathy KK, Mohanty AK, Sahu G, Sarkar SK, Natesan U, Venkatesan R, Prasad MVR (2010b) Variations of physicochemical properties in Kalpakkam coastal waters, east coast of India, during southwest to northeastmonsoon transition period. EnvironMonit Assess 171:411–424. https://doi.org/10.1007/s10661-009-1287-9
Silva MAM, Eça GF, Santos DF, Guimarães AG, Lima MC (2013) Dissolved inorganic nutrients and chlorophyll a in an estuary receiving sewage treatment plant effluents: Cachoeira River estuary (NE Brazil). Environ Monit Assess:5387–5399. https://doi.org/10.1007/s10661-012-2953-x-
Singh S, Raju NJ, Nazneen S (2015) Environmental risk of heavy metal pollution and contamination sources using multivariate analysis in the soils of Varanasi environs, India. Environ Monit Assess 187:345. https://doi.org/10.1007/s10661-015-4577-4.
Srichandan S, Kim JY, Bhadury P, Barik SK, Muduli PR, Samal RN, Pattnaik AK, Rastogi G (2015a) Spatio temporal distribution and composition of phytoplankton assemblages in a coastal tropical lagoon: Chilika, India. Environ Monitoring Assess 187(47):1–17
Srichandan S, Kim JY, Kumar A, Mishra DR, Bhadury P, Muduli PR, Pattnaik AK, Rastogi G (2015b) Interannual and cyclone-driven variability in phytoplankton communities of a tropical coastal lagoon. Sci Total Environment 101(1):39–52
Sylaios G, Theocharis V (2002) Hydrology and nutrient enrichment at two coastal lagoon Systems in Northern Greece. Water Resour Manag 16:171–196
United States Environmental Protection Agency (EPA) (2009). http://www.epa.gov/scitech/swguidance/standards/current
Wang K, Chen J, Jin H, Li H, Gao S, Jie X, Huang YLD, Hao Q, Weng H (2014) Summer nutrient dynamics and biological carbon uptake rate in the Changjiang River plume inferred using a three end-member mixing model. Cont Shelf Res 91:192–200
Wetlands International Report (2011) Chilika An Integrated Management Planning Framework for Conservation and Wise Use.
Zachmann DW, Mohanti M, Treutler HC, Scharf B (2009) Assessment of element distribution and heavy metal contamination in Chilika Lake sediments (India). Lakes Reservoirs Res Manag 14:105–125
Zhang L, Du Y, Wu S, Yu C, Feng Q, Ban X, Ren X, Xue H (2014) Characteristics of nutrients in natural wetland in winter: a case study. Environ Monit Assess 184:5487–5495. https://doi.org/10.1007/s10661-011-2355-5.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial handling: T. Iwalewa
Rights and permissions
About this article
Cite this article
Nazneen, S., Raju, N.J., Madhav, S. et al. Spatial and temporal dynamics of dissolved nutrients and factors affecting water quality of Chilika lagoon. Arab J Geosci 12, 243 (2019). https://doi.org/10.1007/s12517-019-4417-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-019-4417-x