Abstract
The variability in surface water chemistry within and between aquatic ecosystems is regulated by many factors operating at several spatial and temporal scales. The present study was carried out on one of the famous shallow wetlands of Kashmir known as Hokersar wetland. As the wetland is under tremendous pressure of anthropogenic activities besides suffering from internal wetland processes, it becomes imperative to study the seasonal dynamics of various physico-chemical characteristics of water. For this purpose, six study sites were chosen for the collection of water samples from September 2013 to August 2014. Among the parameters recorded, lower values of depth (0.9 m), dissolved oxygen (2.4 mg/L), higher values of chloride (44 mg/L), ammonical nitrogen (251 µg/L), nitrate nitrogen (688 µg/L) and both the forms of phosphorus reflect that the wetland is under the heavy influence of sewage, silt and unmanaged agricultural activities in the surrounding catchment which seems to be the foremost cause of deterioration of the wetland waters. When the present study is compared with the previous studies in terms of physico-chemical characteristics of water, it clearly reveals that some aspects depict distinct variations while some showed close proximity. Further, some of the recorded parameters of water were in range with national standards while some portray wide variations. Principal component analysis revealed that component 1 explains 82.157% of the variation, component 2 with 8.345% variation and component 3 having 3.26% variation. Among various parameters, nitrate nitrogen, total alkalinity and total hardness contribute strongly towards the seasonality of the remaining parameters in the wetland. From the present study, it seems that the wetland has still the capacity to absorb major nutrients especially the nitrogen and phosphorus that drain from the immediate catchment into the Queen wetland of Kashmir.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A wetland is defined as land that is saturated with water long enough to promote wetland or aquatic processes as indicated by poorly drained soils, hydrophytic vegetation and various kinds of biological activity which are adapted to a wet environment (National Wetlands Working Group 1988). These are waterlogged landscapes that cover approximately 8.6 × 106 km2, which amounts to 6.4 percent for the world’s land surface (Mitsch and Gosselink 1993). They cover, therefore, a much greater area than the 1.2 × 106 km2 of freshwater lakes and the 0.8 × 106 km2 of saline lakes (Shiklomanov 1993). Wetlands are among the most important yet vulnerable ecosystems on the planet and the alterations in land use within their catchment have strongly contributed to the degradation and destruction of their buffering capacity, with profound ecological, social and economic repercussions (Rebelo et al. 2010). Wetlands function chemically to improve water quality as filters, transformers, and sinks for materials delivered to them by human activities. For instance, they can filter 60–90 percent of suspended solids from added wastewater and as much as 80 percent of sediment in runoff from agricultural fields (Richardson 1989). Wetlands are sometimes described as “the kidneys of the landscape” because they function as the downstream receivers of water and waste from both natural and human sources (Williams et al. 2000). As they act as sinks for carbon, sulphur and nitrogen, they retain a good deal of these materials when added from human by-products (Bayley et al. 1987), agricultural runoff, and sewage wastewater (Richardson 1989).
Surface water chemistry is regulated by a complex suite of processes and mechanisms operating at varying spatial and temporal scales. Early work by lake ecologists focused on the importance of geographic position as a strong predictor of lake water chemistry. For instance, in the early 1900s, Thienemann (1925) and Naumann (1932) developed lake trophic classification schemes that basically recognized differences between lowland, nutrient-rich (eutrophic) and alpine, nutrient-poor (oligotrophic) ecosystems.
The values of water quality parameters change daily, monthly, seasonally or annually because of difference in agricultural activities, land use types, degradation of streamside vegetation, seasons and rain events in the agricultural watershed (Poudel 2006), and the water quality parameters exhibit a high level of multicollinearity (Poudel and Simon 2008). The parametric values also change due to various biogeochemical processes, atmospheric deposition and hydrological changes. Due to high level of multicollinearity among the water quality parameters, it is possible to explain the variability in surface water quality just by studying few important water parameters (Poudel et al. 2013). Therefore, the specific objectives of this study were to (1) assess seasonal variability of surface water quality, (2) understand the relationships between surface water quality parameters and (3) to identify factors associated with surface water quality variability so as to emphasize the importance of sustainable water resources management and protection of wetlands.
Materials and methods
The physico-chemical parameters of water were collected from the six study sites differing in catchment characteristics, nearness of inlet and outlet, depth ranges and accessibility (Table 1). The parameters were analysed on monthly basis for a period of one year from September 2013 to August 2014. The parameters such as pH (pH meter; Model 335 systronics), temperature, depth and transparency (Golterman et al. 1978) were monitored on the spot while the remaining parameters like dissolved oxygen (Winkler’s Iodometric method), free carbon dioxide, total alkalinity, total hardness (1998), chloride (Argentometric method), calcium and magnesium contents (EDTA Titrimetric method), ammonical nitrogen (Spectrophotometric method; APHA1998), nitrate nitrogen (Spectrophotometric method CSIR 1974) orthophosphate phosphorus and total phosphorus (Stannous chloride method; American Public Health Association APHA 1998) were determined by different standard globally accepted procedures. All Statistical analyses were performed by SPSS (Version 22).
Study area
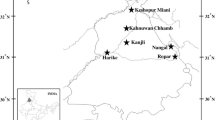
Hokersar, the Queen of wetlands in Kashmir Himalaya, is a natural perennial wetland (34º05′N–34º06′N latitude and 74°8–74°12′E longitude) positioned 12 km to the west of Srinagar on Srinagar–Baramullah Highway in the northern most part of Doodhganga catchment (Fig. 1). The wetland, situated at an altitude of 1,584 m (amsl), was once spread over an area of 19.5 km2, and has presently got reduced to about 13.26 km2at present (Ahmad et al. 2014). The famous wetland, designated as Ramsar Site on 08 November, 2005, is under the control of Government since 1945 (Wildlife Protection Department of Jammu and Kashmir Government). It harbours 0.4–0.5 million overwintering migratory waterfowl (ducks, geese and rails) which migrate from Palaearctic region, extending from northern Europe to central Asian, to Kashmir wetland flying over great Himalayan massif (Pandit 1999; Ahmad et al. 2014). Hokersar alone harbours about 80–90% of the total population of the migratory birds that visit Kashmir valley during winter. Sixty-eight avian species have been inventoried from the area, including IUCN Red-listed endangered white-eyed pochard (Aythya nyroca). The wetland is fed by two inlet streams, Doodhganga (from east) and Sukhnag Nalla (from west). Hokersar wetland is colonized by rooted floating-leaf types like Nymphoides peltata and Trapa natas, besides thick strands of Sparganium ramosum and Phragmites australis. The excessive siltation and addition of wastes aggravate the problem of eutrophication of the waterbody. Such disturbances have not only led to the shrinkage in the area but also reduced its average depth, consequently leading to the colonization of some stress- and pollution-tolerant species like Sparganium ramosum,Myrophyllum verticillatum, Hydrochoris dubia and Lemna–Salvinia complexes.
Results
Temperature
Water temperature depicted a distinct seasonal trend during the entire study period in the wetland. It ranges from a minimum of 6 °C in winter to a maximum of 30 °C in summer with an average of 18.6 ± 8.06 °C. On temporal scale, intermediate temperatures were noticed in spring (18.8 °C) and autumn (19.3 °C).
Depth
During the period of study, low depth ranges were registered in autumn and high in spring at all the study site. Evident Spatial and temporal variations were noticed in the entire study and fluctuates from 0.9 m at site V to 3.2 m at site VI being the outlet of the wetland. The average depth reported in the wetland was 1.8 ± 0.68 m during the entire study period.
Transparency
Water was less turbid due to shallow nature of the wetland and ranged from 25 to 100% throughout the study. Among the study sites, the greater water transparency was recorded in summer (88.8%), followed by autumn (85.5%), winter (61%) and decreasing to the lowest during spring (41.6%). During the study, the sites I and VI were more turbid, being located near the inlet and outlet of the wetland, respectively.
Hydrogen ion concentration, pH
pH concentration showed narrow differences spatially as well as temporally. A gradual increase in pH was noticed from winter to summer and fluctuates from 7.5 to 8.3 with the annual mean concentration of 7.9 ± 0.68 during the entire study (Table 2).
Dissolved oxygen
During the study period, there was significant temporal and spatial variations in the dissolved oxygen (DO) concentrations in the lake with the mean value of 7.2 ± 3.03 mg/L ranging from a minimum of 2.4 mg/L in summer to a maximum of 11.5 mg/L. Previous studies on DO values showed comparably higher concentrations than the present study (Table 3).
Dissolved free carbon dioxide
Higher values of free carbon dioxide were noticed in winter (29.7 mg/L) at site IV; however, the corresponding lower values were observed in summer (3.7 mg/L) at sites II and III. However, annual mean concentration was reported to be 16.2 mg/L.
Total alkalinity
Total alkalinity values fluctuated between a minimum of 697 mg/L at site II in summer and maximum of 229.7 mg/L at site I in winter being located near the inlet of the wetland. In general, the sites with luxuriant growth of macrophytes showed lower values of total alkalinity as compared to the sites with lesser number of macrophytes.
Total hardness, calcium and magnesium contents
During the entire study period of investigation, an evident spatial and temporal variations were recorded among the said parameters. Higher values were observed in winter and lower in summer at all study sites. Among the sites studies, site V witnessed minimum value registering 75 mg/L, while site VI registering maximum value 206.3 mg/L. Temporarily, a similar trend as that of total hardness were observed for calcium and magnesium during the entire study period.
Chloride
The chloride concentration depicted little variations in the wetland. However, the sites which are in close vicinity to the human settlement recorded higher values of the ion in the study. Higher values of chloride were observed during spring (44 mg/L site VI) and corresponding lower values in winter (13 mg/L; site III).
Nutrient salts: nitrogenous compounds
Ammonical nitrogen (NH4-N) and nitrate nitrogen (NO3-N)
A perusal of data revealed distinct spatial and temporal variations in ammonical nitrogen (NH4-N) concentration, registering lower values in summer and higher in winter throughout the study period. Higher concentrations (251.0 µg/L) of ammonical nitrogen were noticed in winter at site VI. However, lower concentration (113 µg/L) was observed in summer at site II. Similar trend was observed in the nitrate nitrogen registering lower values in summer (235 µg/L at site III) and higher values in winter (688 µg/L at site IV).
Phosphorus compounds
The concentration of both the forms of phosphorus revealed clear spatial and temporal variations during the entire period of study. Lower concentrations of orthophosphate phosphorus were observed in winter and higher in summer. Among the sites, site VI depicted lower concentration (45.7 µg/L) and site IV registered higher concentration (167.7 µg/L). Likewise, total phosphorus concentration fluctuates between a low of 103.3 µg/L in winter at site I to a high of 271.3 µg/L in summer at the same site.
Statistical analysis
Principal component analysis is primarily used for data reduction and reduces the number of variables (here 15) to various components or factors that accounts for most of the variance in the number of variables. Here component 1 explains 82.157% of the variation, component 2 explains 8.345% variation and component 3 explains 3.26% variation (Table 4; Fig. 2). Among the various components, component 1 has significant correlation with nitrate nitrogen (0.989), total alkalinity (0.901) and total hardness (0.909) while in component 2 showed significant correlations with total phosphorus (0.827) and orthophosphate phosphorus (0.523), and nitrate nitrogen (0.117).
ANOVA was also performed between various study sites and showed significant correlation with each other (Table 5). Pearson correlation matrix depicted that water temperature showed significant negative correlations with dissolved oxygen (r = 0.950; P < 0.01), free carbon dioxide (r = 0.690; P < 0.01), total alkalinity (r = 0.891; P < 0.01), total hardness (r = 0.786; P < 0.01), calcium content (r = 0.796; P < 0.01), magnesium content (r = 0.796; P < 0.01), ammonical nitrogen (r = 659; P < 0.01) and nitrate nitrogen (r = 0. 831; P < 0.01). At the same time, water temperature showed significant positive correlations with orthophosphate phosphorus (r = 0. 873; P < 0.01) and total phosphorus (r = 0.611; P < 0.01). Among the other recorded parameters, water depth showed negative correlation with transparency (r = 0.705; P < 0.01) and positive correlation with chloride (r = 0.508; P < 0.05). Total alkalinity showed positive correlation with total hardness (r = 0.796; P < 0.01), calcium content (r = 0.880; P < 0.01), magnesium content (r = 0.880; P < 0.01), ammonical nitrogen (r = 0.730; P < 0.01), nitrate nitrogen (r = 0.837; 0. P < 0.01) and negative correlation with both the forms of phosphorus (Table 6).
Discussion
Globally, shallow water ecosystems are much more abundant than deep lakes and play pivotal role for drinking purposes and nutritional requirements (Dai et al. 2012). These provide appropriate habitat for fishes and migratory birds by providing them food, fodder, shelter and breeding ground for the same. Kashmir Himalaya is rich in such habitats which are under the direct influence of anthropogenic activities as well as natural pressures. As a result, these aquatic ecosystems are more vulnerable towards cultural eutrophication (Pandit 1999). For lakes in general and wetlands in particular, the magnitude of eutrophication varies greatly, ranging from oligotrophic to mesotrophic and finally to eutrophic stages. The high-altitude lakes operating more or less solely under natural forces are oligotrophic, while the low-altitude valley lakes under a multitude of cultural eutrophication are evolving at a much faster pace with most of them being already categorized as eutrophic ones (Zutshi et al. 1980; Pandit 1999).
The present study was carried out on Hokersar, a typical wetland of Kashmir Himalaya being recognized as Ramsar Site (Wetland of International Importance) which is located in the floodplains of River Jhelum and as such is situated at the end of the drainage system. Water is a requisite medium between varied factors that plays a decisive role in wetland ecology and the quality and management of water is a fundamental part for wetland conservation. Further, the importance of water for aquatic life as the fulcrum of biochemical metabolism rests on its physical and chemical properties. The present study when compared with the previous reports in terms of physico-chemical characteristics of water, clearly reveals that some aspects depict distinct deviations reflecting that the wetland is degrading at an alarming speed.
Water temperature
Temperature (in general) must be viewed as the main factor affecting almost all physico-chemical equilibriums and biological reactions. It is well known that all physico-chemical constants vary with temperature, and frequently increasing endothermic reactions. Consequently, several transformations or effects related to water will be favoured by water temperature increase such as dissolution, solubilization, complexation, degradation, evaporation, etc. Furthermore, majority of the biochemical processes, being temperature dependent, have very frequently been used as important parameters. As is usual for temperate climates, the atmospheric temperature during the period of present investigation depicted a definite seasonal trend, with maximum values being recorded in summer and minimum in winter. However, water temperature did not reveal much deviation from that of previous reports. The water temperature corresponds to the air temperature, as is common for the shallow water bodies (Wetzel 1975).
Depth
Depth of water body plays a substantial role and sometimes acts as a controlling factor influencing the quality of water as well as the biotic setup in these systems. There were evident spatial and temporal variations regarding the depth in the wetland. Higher depth values were registered in spring that can be attributed directly to higher precipitation rate resulting in greater water discharge from the inlet in the wetland (Kumar and Pandit 2007; Shah and Pandit 2012). However, lower depth values were recorded in autumn during the entire study period. Water level is maintained by weir and lock system located on the outlet of the wetland especially during winters as the wetland receives millions of migratory birds during this season (Kumar and Pandit 2007; Ahmad et al. 2014). The process of continuous siltation brought through the feeding channel not only leads to the shrinkage of the wetland but also damages the littoral zones there in (Pandit 1999, 2002; Shah et al. 2013). Further, on the basis of the lowest mean depth ranges, a warning sign of an evolutionary progression corresponding with higher trophic status of the wetlands is also opined by Rawson (1955) and Hayes (1957) and Pandit (2002). Furthermore, Bayley et al. (2013a) are of the opinion that wetlands with more agricultural area in the surrounding catchment become shallower with time and the same holds true for the wetland under study.
Transparency
Water transparency is believed to be one of the most essential physical factors operating in lake waters (Chowdhury and Mazumder 1981). Water in Hokersar was least turbid for most of the time period. In general, the greater water transparency was recorded in summer, followed by autumn, winter and decreasing to the lowest during spring. The higher values of transparency in autumn as compared to winter could be due to lesser depth ranges. Moreover, lesser winter transparency values in the wetland may be attributed to the fact that the wetland is hosted by millions of migratory birds which by their activities like wading, diving, feeding, etc. make water less transparent.
Hydrogen ion concentration, pH
The water in the wetland was generally on alkaline side (pH > 7) at all the study sites during the entire investigation period. There were narrow differences regarding spatial and temporal variations as far as pH concentration is considered. A gradual increase in pH was noticed from winter to summer which can be attributed to the removal of carbon dioxide from the water column by phytoplankton and macrophytes via photosynthesis, thereby shifting the equilibrium between the carbonic acid and less soluble carbonates and monocarbonates with consumption of protons thereby increasing pH in summer (Wetzel 1973). This fact gains further support from the studies of Verspagen et al. (2014) opining that increase in summer pH is related to dense algal growth that often depletes the dissolved CO2 and raises pH in summer over a wide range of atmospheric carbon dioxide conditions (Channar et al. 2014). The recorded observations were much closer to those of Pandit and Kumar (2006). The pH of wetland water samples during the study period (pH range: 7.5–8.3) were within the limits defined by BIS guidelines (2012).
Dissolved oxygen
Dissolved oxygen is of great limnological significance in aquatic ecosystems as it acts as a regulator of metabolic processes of the community and organisms, thereby determining their composition and as an indicator of lake conditions (Wetzel 2001). Besides, the role of oxygen as a critical factor in aquatic ecosystem is due to its slow rate of diffusion in water than in air. The amount of oxygen dissolved in unit volume of water is usually considerably less than present in an equivalent volume of atmosphere. The rate at which the atmospheric oxygen passes across the air–water interface is dependent upon the physical factors of the partial pressure of the gas in contact with water. During the entire study, dissolved oxygen concentration recorded higher values in winter and lower in summer, thereby reflecting an inverse relation with temperature (Naz and Turkmen 2005; Idowu et al. 2013; Shah and Pandit 2013). Temperature plays a significant role by affecting the solubility of the gases in water thus period of high temperature coincides with the low oxygen content and vice versa (Kaul and Handoo 1987). Channar et al. (2014) attributed low levels of dissolved oxygen in summer to the respiration of microorganisms and other animals. Further, lower levels of oxygen, falling below 2.5 mg/L in summer, can be attributed to its small water volume in relation to the areas of contact with sediment resulting in fast utilization of oxygen which in turn is dependent upon the rate at which the oxygen is transported down to the sediment and the reverse transport of reduced substances (Edberg and Hofsten 1973; Kaul and Trisal 1984). On comparing the previous results, Rather et al. (2001) reported much higher concentration of dissolved oxygen (15.20 mg/L) indicating deterioration of water quality owing to anthropogenic activities in the immediate catchment.
Dissolved free carbon dioxide
Free carbon dioxide (CO2) is an important parameter in the aquatic ecosystems as majority of the autotrophs depend on it via photosynthesis. The levels of the gas, varying from place to place, depend upon several factors like temperature, rainfall, photosynthesis, respiration of biota, etc. Temperature, being a significant factor, determines the concentration and also the solubility of gases in the aquatic ecosystems. During the entire study, free CO2 recorded higher values in winter and lower values in summer reflecting inverse relation with temperature (Wetzel 2001). Low free CO2 in summer may be due to high primary productivity in eutrophic systems as compared to oligotrophic ones (Lazzarino et al. 2009; Balmer and Downing 2011). Further, the increase in photosynthetic activity by aquatic plants leads to decrease in free CO2 in summer season (Wetzel 2001; Shah and Pandit 2012; Sharma et al. 2013). Negative correlation was found between pH and free CO2 in the present study which confirms the view point of Jindal and Rumana (2000) and Shah and Pandit (2012).
Total alkalinity
Alkalinity plays an important role in determining the ability of water to support algal growth and aquatic life. Thomas (1953) used the difference between summer and winter in the epilimnetic waters as a lake eutrophication index. The greater the difference, the more eutrophic system is. In general, total alkalinity depicted two- to three-fold increase in average seasonal concentration between the higher and lower values, reflecting higher trophic index in the wetland under study. Total alkalinity also depicted clear spatial and temporal variations during the entire study period. In general, lower values were recorded in summer to early autumn and higher values were maintained in winter months (Kumar and Pandit 2007; Shah and Pandit 2012; Mushatq et al. 2013; Bhat and Pandit 2014). On the basis of total alkalinity values, the wetland can be categorized as hard water type (>90 mg/L) as per the classification of Moyle (1945). Total alkalinity values registering summer low due to removal of CO2, HCO3 during photosynthesis by primary producers in the wetland, witnessed a trend opposite to that of pH (Wetzel 1960, 1973; Cole 1975). Further, bicarbonates serve as the essential carbon source for macrophytes especially for submerged angiosperms which according to Raven (1970) and Wetzel and Rich (1973) have developed a close affinity for bicarbonates and has become adaptive feature of the submerged vegetation. The total alkalinity was found to be slightly higher in the wetland waters exceeding the BIS-proposed drinking water quality criteria (Bureau of Indian Standards BIS 2012).
Total hardness, calcium and magnesium contents
In aquatic ecosystems, hardness of freshwater is mainly due to the presence of carbonates and bicarbonates of calcium and magnesium ions (Cole 1983). The factors which directly influence hardness of water are soil type, watershed bedrock in the catchment and the water coming in contact with these minerals (Tepe et al. 2005). Total hardness revealed a general trend registering higher values in winter and lower in the wetland. The plausible reasons may be the luxuriant growth of plants like phytoplankton and macrophyte infestation (Fig. 3, 4, 5) which use the salts for their development and various metabolic processes (Wetzel, 2001). Calcium is generally the dominant cation in Kashmir lakes on account of predominance of lime-rich bed rocks in the catchment area (Zutshi et al. 1980). A number of calcium-rich lakes have already been reported from Kashmir (Zutshi 1968; Kaul et al. 1980; Zutshi et al. 1980). On the basis of calcium content remaining generally high throughout the study, the wetland could be placed in Ca-rich category of Ohle (1934).
Magnesium content recorded lower values than calcium and depicted lower values in summer and higher values in winter. Decalcification of water column, being pH dependent, is directly influenced by the photosynthesis of aquatic plants leading to decrease in summer concentration of the ion (Kumar and Pandit 2007). These facts are in consonance with the findings of Zutshi (1968), Kaul et al. (1980), Zutshi et al. (1980), Pandit (1999), Kumar and Pandit (2007), Mushatq et al. (2013) and Bhat and Pandit (2014). Calcium, magnesium and total hardness have slightly higher values as compared to their permissible level of drinking water (BIS 2012). However, previous studies depict lesser values than the present study reflecting that the wetland is under tremendous pressure from the catchment.
Chloride
The chloride content depicted higher values in spring and lower in winter during the entire period of study. The sites which are in close environs of human tenancy in the wetland receive domestic sewage registering maximum values of the ion. Thresh et al. (1976) opined that organic pollution is the primary cause of elevated chloride concentration in aquatic ecosystems (Fig. 5). According to Unni (1985), the vital factors which tend to increase chloride concentration in water are human faeces and sewage of domestic origin. However, Pandit (1999) credited higher chloride content in warmer periods to both allochthonous and autochthonous substances organic in nature (Shah and Pandit 2012). The range of chloride in the present study is in close agreement with the previous reports of Rather and Pandit (2002).
Nutrient salts: nitrogenous compounds
Ammonical nitrogen (NH4-N) and Nitrate nitrogen (NO3-N)
Unplanned agricultural activities are responsible for consistent increase in nitrogen and phosphorus export to the aquatic ecosystems causing cultural eutrophication (Pandit, 1999; Schlesinger, 2009). The mobilization of nitrogen through agricultural activities and wastewater discharges produces detrimental impacts on the aquatic environment as well as on the human health (Lavelle et al. 2005; Billen et al. 2011; Durand et al. 2011; Shah et al. 2014). Besides, Khan et al. (2004) and Khan and Shah (2004) attributed this situation to leaching from agricultural fields in the catchment (2430 ha), in which large quantities (403.1 tonnes) of fertilizers are used annually in this particular wetland (Khan 2015).
Diffuse source (agricultural inputs) of nitrogen is the major cause of nutrient enrichment in Kashmir lakes and wetlands. In addition to these, increase in population also has a tremendous effect in enhancement of nitrogen inputs in these waterbodies (Erisman et al. 2011). The chemical characteristics of an aquatic ecosystem play a major role in defining its trophic status.
The winter high of ammonical nitrogen at all the study sites may be explained on the basis of relatively low nitrification of ammonical nitrogen due to low temperatures, which retards the nitrification process (Kayranli et al. 2010). Ammonical nitrogen depicted higher variations as compared to previous records of Rather et al. (2001), Rather and Pandit (2002) and Pandit and Kumar (2006). NO3-N showed seasonal higher values in winter which is the reflection of low denitrification rate and also non-utilization by plants in this season.
Phosphorus compounds
Phosphorous is an important element which controls the reproduction and growth of aquatic organisms. Many organisms utilize both organic and inorganic forms of phosphorous, however, inorganic phosphorous seems to be more appreciated by plants than organic phosphorous is (Riley and Chester, 1971). Seasonal variations in concentrations of both the forms of phosphorus in the present study reflect a significant influence by internal lake processes registering higher concentrations in summer and lower in winter (Jeppesen et al. 2000; Shah et al. 2015). In summer, the rise in temperature enhances the microbial activity which consequently results in the increase of diffusion process of P from the sediments to the overlying water column (Maassen et al. 2005). Still further, seasonal variations in phytoplankton productivity are the other factors ascribed for higher peaks of P (Søndergaard 1989). Likewise, TPP concentrations, showing more pronounced summer peaks, are attributable to increase in trophic level (Xie 2006).These results are in conformity with the recent findings of Shah et al. (2014, 2015). However, comparable results of orthophosphate phosphorus concentration were recorded with the previous works of Rather and Pandit (2002).
Conclusions
It can be concluded from the study that the rigorous biotic intrusion coupled with anthropogenic activities along the drainage basin are the contributory factors for deteriorating conditions of the wetland that leads to cultural eutrophication. Wetland restoration evidently has potential for reducing nutrient discharges coming from the immediate catchment as the higher values of both the forms of nitrogen and phosphorus were very high at sites which are nearer to the inlet of the wetland. However, it is difficult to predict the quantitative effect of wetland restoration because nutrient removal differs greatly among wetlands and varies greatly with brief occurrences of high flow, inter-annual differences in rainfall and long-term changes in wetland development. Therefore, it becomes imperative to restore the health of the deteriorating ecosystems based on sound management tools. For this purpose, some of the conservation measures are suggested as (1) it is required to construct siltation beds (settling basins) near the inlet which will help in settling the major portion of silt and sediment which remains the main problem for the nutrient enrichment in the wetland; (2) constructions of sewage treatment plants (STPs) especially near the entry site of Doodgangha stream into Hokersar wetland will help to a great extent to curtail the maximum nutrient load in the wetland; (3) areas encroached upon should be reclaimed back by the concerned authorities at an earliest to prevent them from further shrinkage of the wetland; and (4) major policies should be implemented at an earliest for the protection of wetlands for the sustainable water resources management and fortification of surfaces waters.
References
Ahmad SS, Reshi ZA, Shah MA, Rashid I, Ara R, Andrabi SMA (2014) Phytoremediation potential of Phragmites australis in Hokersar Wetland—a Ramsar Site of Kashmir Himalaya. Int J Phytorem 16(12):1183–1191. doi:10.1080/15226514.2013.821449
American Public Health Association APHA (1998) Standard Methods for the Examination of Water and Waste Waters. American Public Health Association ed., Washington DC, USA
Balmer MB, Downing JA (2011) Carbon dioxide concentrations in eutrophic lakes: under- saturation implies atmospheric uptake. Inland Waters 1:125–132
Bayley SE, Vitt DH, Newbury RW, Beaty KG, Behr R, Miller C (1987) Experimental acidification of a Sphagnum-dominated peatland: first year results. Can J Fish Aquat Sci 44(Supplement 1):194–205
Bayley SE, Wong AS, Thompson JE (2013a) Effects of agricultural encroachment and drought on wetlands and shallow lakes in the boreal transition zone of Canada. Wetlands 33:17–28
Bayley SE, Wong AS, Thompson JE (2013b) Effects of agricultural encroachment and drought on wetlands and shallow lakes in the boreal transition zone of Canada. Wetlands 33:17–28. doi:10.1007/s13157-012-0349-x
Bhat SA, Pandit AK (2014) Surface water quality assessment of Wular lake, A Ramsar Site in Kashmir Himalaya, using discriminant analysis and WQI. Journal of Ecosystems 2014:1–18. doi:10.1155/2014/724728
Billen G, Silvestre M, Grizzetti B. et al (2011) Nitrogen flows from European watersheds to coastal marine waters. In: Sutton MA, Howard CM, Erisman JW et al (eds) The European Nitrogen Assessment Cambridge University Press
Bureau of Indian Standards BIS (2012) Drinking water specification (Indian standards) Second Revision (IS 10500) Manak Bhavan, 9 Bahadur Shah Zafar Marg New Delhi 110002
Channar AG, Rind AM, Mastoi GM, Almani KF, Lashari KH, Qurishi MA, Mahar N (2014) Comparative study of water quality of Manchar lake with drinking water quality standard of world health organization. Am J Environ Prot 3(2):68–72
Chowdhury SH, Mazumder A (1981) Limnology of Lake Kaptai: physico-chemical features. Bangladesh J. Zool. 9(1):59–72
Cole GA (1975) Textbook of Limnology. The C. V Moslbey Company, Saint Louise
Cole GA (1983) Textbook of Limnology. Waveland Press, Prospect Heights
CSIR (1974) Analytical Guide (Laboratory Techniques). CSIR, Pretoria, South Africa
Dai GZ, Shang JL, Qiu BS (2012) Ammonia may play an important role in the succession of cyanobacterial blooms and the distribution of common algal species in shallow freshwater lakes. Glob Change Biol 18:1571–1581. doi:10.1111/j.1365-2486.2012.02638.x
Durand P, Breuer L, Johnes PJ, Billen G, Butturini A, Pinay G, Grinsven HV, Garnier J, Rivett M, Reay DS, Curtis C, Siemens J, Maberly S, Kaste O, Humborg C, Loeb R, Klein JD, Hejzlar J, Skoulikidis N, Kortelainen P, Lepisto A, Wright R (2011) Nitrogen processes in aquatic ecosystems. In: Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P, Grinsven HV, Grizzetti B (eds) European Nitrogen Assessment. Cambridge University Press, Cambridge, Cambridge, p 126–146
Edberg N, Hofsten BV (1973) Oxygen uptake of bottom sediments studied in situ and in the laboratory. Water Res 7:1285–1294
Erisman JW, Grinsven HV, Grizzetti B, Bouraoui F, Powlson D (2011) The European nitrogen problem in a global perspective. In: Sutton MA, Howard CM, Erisman JW, Billen G, Bleeker A, Grennfelt P, Hansen J eds.The European Nitrogen Assessment. Cambridge, UK: Cambridge University Press
Golterman HL, Clymo RS, Ohnstand MAM (1978) Methods for physical and chemical analysis. IBP Handbook No 8, 2nd edn. Blackwell Scientific Publication, Oxford, p 172
Hayes FR (1957) On the variation in bottom fauna and fish yield in relation to trophic level and lake dimensions. J Fish Res Bd Canada 14(l):l–32
Idowu EO, Ugwumba AAA, Edward JB, Oso JA (2013) Study of the seasonal variation in the physico-chemical parameters of a tropical reservoir. Greener Journal of Physical Sciences 3(4):142–148
Jeppesen E, Jensen JP, Sødergaard M, Lauridsen T, Landkildehus F (2000) Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient. Freshw Biol 45:201–218
Jindal R, Rumana HS (2000) Biomonitoring of water pollution in western Yamuna Canal at Yamunanagar, Haryana. J Punjab Acad Sci 2(1):177–182
Kaul V, Handoo JK (1987) Chemical parameters useful in the evaluation of eutrophication and ecological state of lakes of Jammu and Kashmir. pp. 363–397. In: Agarwal SK, Garg RK (eds). Environmental Issues and Researches in India. Udaipur, India. Himanshu Publications
Kaul V, Trisal CL (1984) Chemical and physical characteristics of some wetland waters Kashmir. Acta Hydrochem et Hydrobiol 12(2):137–144
Kaul V, Trisal CL, Kaul S (1980) Mineral removal potential of some macrophytes in two lakes of Kashmir. J Indian Bot Soci 55:113–123
Kayranli B, Scholz M, Mustafa A, Hedmark A (2010) Carbon storage and fluxes within freshwater Wetlands: a critical review. Wetlands 30:111–124
Khan MA (2015) Environmental characteristics and phytoplankton productivity of a shallow Ramsar-site floodplain in the western Himalaya. Lakes Reserv 20:69–76
Khan MA, Shah MA (2004) Land-use pattern in the catchment and its impact on the ecology of a sub-urban wetland, Kashmir Himalaya. In: Khan MA, Zargar MY (eds) Agriculture and Environment pp. 281–8. APH Publishing Corporation, New Delhi
Khan MA, Shah MA, Bashir S, Mir SS (2004) The environmental status of a Kashmir Himalayan wetland game reserve: aquatic plant communities and ecorestoration measures. Lakes Reserv Res Manage 9:125–132
Kumar R, Pandit AK (2007) Physico-chemical characteristics of water in Hokersar wetland in Kashmir Himalaya. Pollution Research 26(4):73–79
Lavelle P, Dugdale R, Scholes R, Berhe AA, Carpenter E, Codispoti L, Izac AM, Lemoalle J, Luizao F, Scholes M, Tréguer P, Ward B (2005) p. 154–187. In: Hassan R, Scholes R, Ash N (eds) Nutrient Cycling: Ecosystems and Human Well-being: Current State and Trends Findings of the condition and trends working group of the millennium ecosystem assessment. Island Press
Lazzarino JK, Bachmann RW, Hoyer MV, Canfield DE (2009) Carbon dioxide super saturation in Florida lakes. Hydrobiologia 627(1):169–180
Maassen S, Uhlmann D, Roske I (2005) Sediment and pore water composition as a basis for the trophic evaluation of standing waters. Hydrobiologia 543:55–70
Mitsch WJ, Gosselink JG (1993) Wetlands, 2nd edn. Wiley, New York
Moyle JB (1945) Some chemical factors influencing the distribution of aquatic plants in Minnesota. Amer Midl Natl 34:402–426
Mushatq B, Raina R, Yaseen T, Wanganeo A, Yousuf AR (2013) Variations in the physico-chemical properties of Dal Lake, Srinagar, Kashmir. African J Environ Sci Technol 7(7):624–633
National Wetlands Working Group (1988) Wetlands of Canada. In Canada’s wetlands. Map folio Energy Mines Resour. Can. Environ. Can, Ottawa
Naumann E (1932) Grundzuge der regionalen Limnologie. Die. Binnengewässer 11:1–176
Naz M, Turkmen M (2005) Phytoplankton biomass and species composition of Lake Golbasi (Hatay-Turkey). Turkish J Biol 29:49–56
Ohle W (1934) Chemische und Physikalische Untersuchungen Norddeutscher. Arch Hydrobiol 26:386–464
Pandit AK (1999) Freshwater ecosystems of the Himalaya. Parthenon Publishing, New York
Pandit AK. (2002) Trophic evolution of lakes in Kashmir Himalaya In: Pandit AK(ed) p 175–222. Natural Resources of Western Himalaya. Valley Book House, Srinagar-190006, J&K
Pandit AK, Kumar R (2006) Comparative studies on ecology of Hokersar Wetland, Kashmir: present and Past. J Himal Ecol Sustain Dev 1:73–81
Poudel DD (2006) Challenges for improving surface water quality in an agricultural watershed in Louisiana. In :Coastal Environment and Water Quality, Proceedings of the AIH 25th Anniversary Meeting and International Conference, Challenges in Coastal Hydrology and Water Quality, Water Resources Publications,Highlands Ranch, CO, eds. Xu Y.J., Singh V.P., 251–261
Poudel DD, Simon MJ (2008) Pasture-based dairy and water hyacinth (Eichornia crassipes) for reduced biological oxygen demand in dairy discharge water. Outlook Agric 37(2):135–142
Poudel DD, Thakur RP, Duex T, Blakewood G, Singh A, DeRamus A, Chapagain B, Acharya K, Adhikari S, Gramling RB, Sharma N (2013) Adapting livestock production systems to climate change in Nepal: Challenges and opportunities. In: Michalk DL, Miller GD, Badgery WB, Broadfoot KM (eds) Proceedings of the 22nd International Grassland Congress, Sydney, Australia, New South Wales Department of Primary Industry, Kite St. Orange New South Wales, Australia, pp 1362–1367
Rather SA, Pandit AK (2002) Phytoplankton dynamics in Hokarsar wetland. Kashmir J Res Dev 2:25–46
Rather SA, Bhat SA, Pandit AK (2001) Water quality of Hokarsar, a typical wetland of Kashmir. J Res Dev l:39–44
Raven JA (1970) Exogenous inorganic carbon sources in plant photosynthesis. Biol Rev 45:167–221
Rawson DS (1955) Morphometry as a dominant factor in the productivity of large lakes. Verh Internat Verein Limnol 12:164–175
Rebelo LM, McCartney MP, Finlayson CM (2010) Wetlands of sub-Saharan Africa: distribution and contribution of agriculture to livelihoods. Wetlands Ecol Manage 18:557–572
Richardson CJ (1989) Freshwater wetlands: transformers, filters, or sinks? In: Sharitz RR, Gibbons JW (eds) Freshwater wetlands and wildlife. US Dep. of Energy Office of Sci. and Tech. Info, Oak Ridge, pp 25–46
Riley JP, Chester R (1971) Introduction to marine chemistry. Acad. Press, London, p 465
Schlesinger WH (2009) On the fate of anthropogenic nitrogen. Proc Natl Acad Sci 106:203–208
Shah JA, Pandit AK (2012) Physico-chemical characteristics of water in Wular lake-A Ramsar Site in Kashmir Himalaya. Int J Geol, Earth Environ Sci 2(2):257–265
Shah JA, Pandit AK (2013) Relation between physico-chemical limnology and crustacean community in Wular lake of Kashmir Himalaya. Pak J Biol Sci 16(19):976–983
Shah JA, Pandit AK, Shah GM (2013) Distribution, diversity and abundance of copepod zooplankton of Wular Lake, Kashmir Himalaya. J Ecol Nat Environ 5(2):24–29
Shah JA, Pandit AK, Shah GM (2014) Spatial and temporal variations of nitrogen and phosphorus in Wular lake leading to eutrophication. Ecologia 4(2):44–55. doi:10.3923/ecologia
Shah JA, Pandit AK, Shah GM (2015) A research on rotifers of aquatic ecosystems of Kashmir Himalaya for documentation and authentication. Proc Natl Acad Sci India Sect B Biol Sci 85(1):13–19. doi 10.1007/s40011-014-0334-7
Sharma S, Solanki CM, Sharma D, Tali I (2013) Population dynamics of Planktons in river Narmada at Omkareshwar. Int J Adv Res 1(1):11–15
Shiklomanov IA (1993) World water resources. In: Gleick PH (ed) Water in Crisis, Oxford University Press, New York and Oxford
Søndergaard M (1989) Phosphorus release from a hypertrophic lake sediment: experiments with intact sediment cores in a continuous flow system. Arch Hydrobiol 116:45–59
Tepe Y, Turkmen A, Mutlu E, Ates A (2005) Some physico-chemical characteristics of Yarselli Lake, Turkey. Turkish J Fish Aquat Sci 5:35–42
Thienemann A (1925) Die binnengewasser Nittelewopas Eine limnologische Einfuhrung. Binnengegewasses 1:1–225
Thomas EA (1953). Empirische and experimentalla inter Suchungen Zur Kinntnis der minimum stoffe in 46 seender Schweiz
Thresh JC, Suckling EV, Baele JF (1976) In: Taylor EW (ed) The Examination of Water Supplies 6th edn
Unni KS (1985) Comparative limnology of several reservoirs in central India. Int Revue ges Hydrobiol 70(6):845–856
Verspagen JMH, Van de Waal DB, Finke JF, Visser PM, Huisman J (2014) Contrasting effects of rising CO2 on primary production and ecological stoichiometry at different nutrient levels. Ecol Lett 17:951–960
Wetzel RG (1960) Marl encrustation on hydrophytes in several Michigan lakes. Oikos 11:223–236
Wetzel RG (1973) Productivity investigations of interconnected lakes.I. The eight lakes of the Oliver and walters chains, north-eastern Indiana. Hydrobiol. Stud. 3:91–143
Wetzel RG (1975) Limnology. Saunders Company, Philadelphia-London-Toronto, p 743
Wetzel RG. (2001) Limnology: Lakes and Rivers. Academic Press; A Harcourt Science and Technology Company, 525B Street, Suite 1900, San Diego, California, 1000 p (Third edn)
Wetzel RG, Rich PH (1973) Carbon in freshwater systems. pp. 241-263. In: Woodwell GM, Pecan EV (eds) Carbon and the Biosphere Brookhaven Symposium in Biology no. 24. Technical Information Center, U.S. Atomic Energy Commission, CONF-720510, Brookhaven, N.Y
Williams M, Eugster W, Rastetter EB, McFadden JP, Chapin FSIII (2000) The controls on net ecosystem productivity along an arctic transect: a model comparison with flux measurements. Global Change Biol. 6((Suppl)):116–126
Xie P (2006) Biological mechanisms driving the seasonal changes in the internal loading of phosphorus in shallow lakes. Sci China Ser D Earth Sci 49:14–27
Zutshi DP (1968) Ecology of Some Kashmir lakes. Ph.D. thesis University of Kashmir Srinagar-190006 J and K India
Zutshi DP, Wanganeo A, Raina R (1980) Limnology of a man-made lake. Geobios 7:320–324
Acknowledgements
Thanks are due to the Director, Centre of Research for Development and Head, Environmental Science, University of Kashmir for providing necessary laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests
Rights and permissions
About this article
Cite this article
Shah, J.A., Pandit, A.K. & Shah, G.M. Dynamics of physico-chemical limnology of a shallow wetland in Kashmir Himalaya (India). Sustain. Water Resour. Manag. 3, 465–477 (2017). https://doi.org/10.1007/s40899-017-0115-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-017-0115-6