Abstract
Although an extensive number of studies support the efficacy and tolerability of stimulants in the treatment of attention deficit/hyperactivity disorder (ADHD), in recent years, increasing concerns have been raised about their cardiovascular safety. We investigated whether a time domain analysis of heart rate variability (HRV) recordings in 24-h ECG under medication with stimulants yielded new information about therapy control in ADHD. We analysed the HRV parameter standard deviation of all normal sinus RR intervals over 24 h (SDNN), percentage of successive normal sinus RR intervals > 50 ms (pNN50) and root-mean-square of the successive normal sinus RR interval difference (rMSSD) from 23 children diagnosed by ADHD (19 boys and 4 girls), aged 10.5 ± 2.2 years, who were consecutively referred to our outpatient clinic for paediatric cardiology. Eleven children received medication with methylphenidate (MPH), while twelve children were initially examined without medication. Of these, eight probands were re-examined after therapy with MPH was established. Controls comprised 19 children (10 boys, 9 girls) from our Holter ECG data base without any cardiac or circulatory disease. Compared to healthy controls, the ADHD children with and without MPH treatment showed significantly higher mean heart rates (ADHD without MPH: 94.3 ± 2.2; ADHD with MPH: 90.5 ± 1.8, controls: 84.7 ± 1.8). pNN50 (ADHD without MPH: 6.5 ± 2.7; ADHD with MPH: 14.2 ± 6.9, controls: 21.5 ± 9.0) and rMSSD (ADHD without MPH: 26.1 ± 4.1; ADHD with MPH: 36.7 ± 8.3, controls: 44.5 ± 10.1) were lowest in ADHD children without MPH, middle in ADHD children with MPH and highest in controls. SDNN values were not significantly different. The hourly analysis shows highly significant reduced pNN50 and rMSSD values in untreated ADHD children between 5:00 pm and 6:00 am while the pattern approaches to levels of controls during MPH treatment. Data of this pilot study indicate a decreased vagal tone with significantly diminished HRV and higher heart rates in unmedicated ADHD children. These parameters of autonomic activation are ameliorated by MPH treatment. No evidence for negative impact of MPH on HRV was detected. Further studies will clarify a potential cardio-protective effect of MPH in ADHD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention deficit/hyperactivity disorder (ADHD) is a common psychiatric disorder, affecting about 5% of children and adolescents and 3% of adults (Polanczyk et al. 2007; Schlander et al. 2010). Frequently, ADHD implicates a challenge for social and academic development of affected children and leads to increased rates of life events in adulthood and developmental comorbidities (Polderman et al. 2010; Polanczyk and Rohde 2007; Jacob et al. 2007; Taurines et al. 2010). Effective treatment often includes pharmacotherapy with agents influencing neurotransmission (Renner et al. 2008). The most prominently and successfully used substances in the pharmacotherapy of ADHD are the psychostimulants methylphendidate (MPH) and amphetamine, with increasing numbers of prescriptions (Faraone 2009; Schubert et al. 2010).
Potential side effects of stimulants are in the focus of scientific discussions, intensified by the higher number of prescriptions. An extensive number of studies support efficacy and tolerability of stimulants. However, in 2006, the U.S. Food and Drug Administration added a black box warning that stimulants may cause serious cardiovascular effects like high blood pressure up to sudden death. A general contraindication for children with known serious heart rhythm abnormalities or other severe cardiac problems that may increase their vulnerability to the sympathomimetic effects of stimulants was stated (http://www.fda.gov/ohrms/dockets/ac/oc06.htm). Since then, a controversial scientific discussion about potential cardiovascular risks of stimulant medication has been ongoing (Schelleman et al. 2011).
To minimize eventual risks, most clinical guidelines currently recommend pretreatment screening including thorough history and physical examination. Cardiologic referral and ECG is advised only in cases of abnormal findings in cardiac examination, personal history of cardiac symptoms or family history of sudden cardiac death (Leslie et al. 2008). Recently, a borderline cost-effectiveness in adding ECG pretreatment screening compared to history and physical examination in the prevention of sudden cardiac death was reported (Denchev et al. 2010).
Basal regulation of heart activity by the autonomous nervous system is one potential factor modulating cardiovascular side effects of stimulants, and altered vagal and sympathetic activation levels were reported for children affected by ADHD. However, little data on the interplay between autonomic nervous system and stimulant medication are available. In this context, we were aimed to retrieve data on basal cardiac activity in children with ADHD with and without medication in a pilot study.
As an established informative and non-invasive marker, the heart rate variability (HRV) reflects the autonomic activity of the heart (Buchhorn et al. 2002). Time domain and power spectral analyses of beat-to-beat HRV have been used to quantitatively assess the parasympathetic and sympathetic modulation of the heart in clinical as well as in basic research studies (Task Force of the European Society of Cardiology and the North American society of Pacing 1996). Therefore, in the present study, 24-h HRV was analysed, subsumed by three standard time domain parameters: Standard deviation of all normal sinus RR intervals over 24 h (SDNN), root-mean-square of the successive normal sinus RR interval difference (rMSSD) and the percentage of successive normal sinus RR intervals > 50 ms (pNN50). These measures reflect different autonomic modulation: rMSSD and pNN50 are markers for parasympathetic modulation, while SDNN mostly reflects sympathetic modulation.
In a previous study, it has been reported that HRV analysis in 24-h Holter ECG recordings might be a valuable tool in improving the risk stratification for heart rhythm disturbance and sudden cardiac death (Fei et al. 1994).
In the present, study we used HRV analysis in children diagnosed with ADHD who were referred to an outpatient clinic for paediatric cardiology in 24-h Holter ECG examinations. ADHD patients prior to medication, medicated by methylphenidate (MPH) and healthy controls were included.
Methods
Subjects
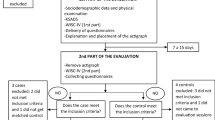
All children referred between 8/2005 and 3/2010 to the outpatient clinic for paediatric cardiology of Caritas hospital Bad Mergentheim by paediatricians or child and adolescent psychiatrists were consecutively included in the study. ADHD diagnoses were confirmed prior to referral by child and adolescent psychiatrists. The motivation for cardiac examination was anamnestic signs for potential cardiac disease, conspicuous standard ECG’s or concerns of the parents about cardiac safety of medical treatment. Twenty-four-hour ECG recordings were obtained for data analysis from eleven ADHD children medicated by methylphenidate (MPH) and twelve children without medication. Eight probands were re-examined after therapy with MPH was established. Six further admitted children with ADHD had to be excluded from analysis: Five children had more than 20 premature ventricular contractions (PVC) per hour, causing methodical reasons since only normal RR Intervals represent autonomic regulation; another child was excluded due to hyperthyroidism suffering from Grave′s disease. In addition, 19 healthy controls took part.
All children received a cardiac ultrasound from a paediatric cardiologist, and sinus rhythm was confirmed by standard twelve channel ECG. Controls comprised 19 age-matched children from our Holter ECG data base without any cardiovascular disease.
To assess potential co-factors, height and weight were measured, and the body mass index (BMI) and BMI percentiles were calculated.
Processing and analysis of 24-h Holter recordings
Autonomic control of cardiovagal function was tested by time domain analysis of 24-h ambulatory digital recordings of the electrocardiogram. A two channel Holter monitor (Pathfinder™, Spacelabs, Germany) was used for recording while the children followed their normal daily routines. All Holter recordings were reviewed by an experienced cardiologist (R.B.) and were edited to validate the system’s QRS labelling in order to exclude artefacts. Measures of HRV were calculated employing only normal-to-normal intervals. The Holter ECG’s were analysed as average values from the entire 24 h of analysable data and additionally for circadian rhythm analysis as mean hourly values with regard to the time of day. Numbers of pairs of adjacent NN intervals differing by more than 50 ms were given as absolute value per hour (sNN50) or as the percentage of the total number of all NN intervals during 24 h (pNN50).
Measurement and physiological interpretation of HRV parameters were performed according to the standards of the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (Table 1) (Task Force of the European Society of Cardiology and the North American society of Pacing 1996). rMSSD, pNN50 and heart rate reflect predominantly a response to changes in vagal tone. SDNN is dually influenced by cholinergic and adrenergic activity, as well as other physiological inputs.
Statistical analysis
All results are reported as mean ± standard deviation. Because most clinical variables were normally distributed, parametric techniques were used. Differences between the patients groups and controls were evaluated with ANOVAs with the between-subject factor group. Hourly analyses of the HRV parameters were analysed with repeated-measures ANOVAs with time as within-subject factor and group as between-subject factor. Greenhouse–Geisser corrections were applied when required. Follow-up t-tests were used to specify the significant effects. Due to the preliminary nature of the data and the impossibility to refer only on those eight ADHD children who were assessed two times with and without MPH because of the statistical power, analyses were calculated with group as between-subject factor including in the ADHD groups children that were partially assessed two times. Presumably, the found significant group differences between the ADHD groups, therefore, should be rather strong. To verify the results reported, we conducted an additional analysis in advance. We established a variable describing whether subjects were tested one or two times as covariate within the original analyses. However, all effects remained and there was never a significant effect of the covariate. Therefore, analyses were conducted as described above. For all parameters, a value of p < 0.05 was considered statistically significant. The data analyses were performed using SPSS version 18.
Results
Clinical and demographic data
Clinical covariates including age, sex, bodyweight, body length and body mass index (BMI) were analysed. As shown in Table 2, no significant statistical difference between the three groups was found.
Average HVR parameters in 24-h Holter ECG
Average HRV parameters from 24-h ECG showed significant differences between groups (mean heart rates: F(2, 47) = 6.1, p = .004; pNN50: F(2, 47) = 16.4, p < .001; rMSSD: F(2, 47) = 18.0, p < .001). Heart rates were higher in both ADHD children groups as compared to controls (ADHD without MPH: 94.3 ± 2.2; ADHD with MPH: 90.5 ± 1.8, controls: 84.7 ± 1.8, both ts > 2.2, ps < .033), while the ADHD groups did not differ (t (29) = 1.7, p = .107) (see Fig. 1). The pNN50 and rMSSD, both predominantly reflecting changes in vagal tone, were lower in ADHD patients as compared to controls (pNN50: ADHD without MPH: 6.5 ± 2.7; ADHD with MPH: 14.2 ± 6.9, controls: 21.5 ± 9.0, both ts > 2.8, ps < .008; rMSSD: ADHD without MPH: 26.1 ± 4.1; ADHD with MPH: 36.7 ± 8.3, controls: 44.5 ± 10.1, both ts > 2.6, ps < .013) and lower in ADHD patients without MPH as compared to ADHD patients with MPH (pNN50: t(29) = 4.4, p < .001; rMSSD: t(29) = 4.1, p < .001). rMSSD values are given in Fig. 2. Groups did not differ in average SDNN (F(2, 47) = 0.9, p = .402).
Circadian analysis of heart rate
Heart rate analysis (Fig. 3a) showed significant main effects of time (F(23, 1,081) = 64.7, p < .001) and group (F(2, 47) = 3.4, p = .043), while the interaction time x group just missed significance (F(46, 1,081) = 1.7, p = .064). Follow-up t-tests could indicate that heart rates were lowest from 2 to 5 am (all ts > 2.1, ps < .037), increasing from 5 to 9 am (all ts > 4.1, ps < .001) with a maximum and plateau phase from 9 am to 7 pm (all ts < 1.8, ps > .074), and then decreasing from 8 pm until 2 am (all ts > 2.1, ps < .042). The group effect was due to higher heart rates in both ADHD groups as compared to controls as mentioned above. Descriptively, the marginal significant interaction group x time was due to an extraordinary increase in heart rate from 2 to 7 pm in ADHD children without MPH, while the pattern of ADHD children with MPH more reassembled that of healthy controls.
Circadian analysis of heart rate variability parameters
For sNN50 (Fig. 3b) and rMSSD (Fig. 3c), ANOVA revealed a significant main effect of time (sNN50: F(23, 1,081) = 16.7, p < .001; rMSSD: F(23, 1,081) = 23.3, p < .001) due to an increase in sNN50 and rMSSD during evening and night-time beginning in the early afternoon. In addition, there was a main effect of group (sNN50: F(2, 47) = 18.0, p < .001; rMSSD: F(2, 47) = 17.6, p < .001; controls > ADHD with MPH > ADHD without MPH, as reported above) as well as a significant interaction time x group (sNN50: F(46, 1,081) = 2.5, p = .003; rMSSD: F(46, 1,081) = 2.1, p = .022).
This interaction was statistically tested by repeated-measures ANOVAs of the time effect for each group separately as well as with separate ANOVAs for each time of measurement and group as between-subject factors. While the time effect was significant for all groups (all Fs > 2.9, ps < .025), the interaction was due to a stronger raise in sNN50 and rMSSD in controls followed by ADHD children with MPH as compared to ADHD children without MPH. Comparisons between groups could indicate that group differences were less in the morning hours from 8 to 10 am (Fs < 2.9, ps > .065), increasing from 11 am to 4 pm with a differentiation between controls and both ADHD groups (Fs > 3.6, ps < .035, the same for 7 am for sNN50 and 6 and 7 am for rMSSD) followed by maximal group differences (maximum at 2 am) and an approximation between ADHD children with MPH and controls and stronger differentiation from ADHD children without MPH beginning at 5 pm up to 5 am (ADHD without MPH < ADHD with MPH < or = controls, Fs > 7.7, ps < .018, for SNN50 also for 6 am).
Discussion
In the present pilot study, we aimed to contribute data to the ongoing discussion about potential cardiovascular risks in children with ADHD especially under medication with stimulants. Parameters of the heart rate variability reflecting autonomic heart regulation were analysed in 24-h Holter ECG recordings in children diagnosed with ADHD with and without medication, as well as in healthy controls. We found significant differences in circadian heart rates, as well as in the markers reflecting vagal tone, sNN50 and rMSSD, in between the groups. ADHD children showed a diminished vagal tone during night-time with lower levels of pNN50 and rMSSD and elevated heart rates, especially during sleep. Medication with MPH shifted the markers towards levels of normal controls. This implicates that instead of destabilizing the autonomic heart regulation, stimulants might lead to a normalization of the tested heart rate variability parameters. A difference in sympathetic activation represented by the parameter SDNN was not detected.
Our findings on baseline heart activity in ADHD are in line with results of recent studies (Huang and Tsai 2011). Imeraj et al. also report higher heart rates of children with ADHD during the night-time (Imeraj et al. 2011) in a circadian study. An independent group found, besides of increased heart rates, lower rMSSD in short-term HRV analyses during resting states and orthostatic provocation (Tonhajzerova et al. 2009). These results further endorse the assumption of a basically altered autonomic nervous system in children with ADHD, pointing to a decreased parasympathetic activity at least during the night-time. However, sample sizes in these studies are small, and the studies were designed to answer different aspects, and there are controversial reports stating a hypo-sympathetic activation in children with ADHD (Negrao et al. 2011).
Cardiovascular effects of sympathomimetic amines like MPH have been thoroughly described in the medical literature, and these agents are reported to substantially increase the heart rate and blood pressure in healthy probands. Most of all measurements were undertaken during daytime and not during sleep (Hammerness et al. 2009).
However, in ADHD children medicated by MPH, we found no increase in heart rate during school time after MPH was given but lower heart rates during sleep when MPH should be degraded. A potential explanation for this phenomenon after medication with psychostimulants could be indicated by the known increase in vagal tone in children during the night after maximal exercise training. This effect correlates with degradation of exercise-induced catecholamines (Al Haddad et al. 2009), and an increase in the catecholaminergic tone is also considered the correlate in neurotransmission under medication with psychostimulants.
In respect to the discussion about higher cardiovascular risk and risk of sudden death in patients with ADHD, as reported by Gould et al. (2009), our data indicate that higher heart rates and reduced vagal tone might occur in the pathophysiology of ADHD. This implicates that ADHD patients might have an enhanced baseline risk of cardiovascular complications, independent from MPH treatment (Singh et al. 2002; Vaseghi and Shivkumar 2008). In line, McCarthy and colleagues also could not confirm stimulant medication as a risk factor in a recent cohort study (McCarthy et al. 2009).
Additional data are urgently needed for further clarification of the risk of cardiovascular mortality among children with ADHD with and without stimulant medication. Especially in respect with the FDA ‘black box’ warning expecting adverse effects of psychostimulants, an automatic transferability of cardiac effects by sympathomimetic agents in healthy humans to ADHD patients is not underpinned by our data. In the contrary, we found that MPH seems to ameliorate the abnormal HRV parameters in ADHD children, which indicates a potential cardio-protective role of stimulants in these patients.
Interestingly, other interventions ameliorating ADHD symptoms ranging from pharmacological central sympathicolysis with clonidine to enhanced physical activity or discussed alternative approaches by nutrial supplementation also have influence on the HRV (Arnsten 2009; Singh et al. 2001). Whether a change in the HRV might be a correlate to a direct pathophysiologically relevant alteration of the autonomic system or rather reflect an epiphenomenon will be an interesting topic in further research.
In the evaluation of the results of the present pilot study, several limitations have to be considered. First, our study is a retrospective study from Holter ECG data obtained during clinical routine in children diagnosed with ADHD, who were referred to our outpatient clinic for paediatric cardiology for cardiac evaluation. Thus, the preselection could imply that our sample represents a risk subsample of children with ADHD. Additionally, ADHD in all children was diagnosed by child and adolescent psychiatrists in private practice; a uniform diagnostic study manual was not applied. If the night-time changes of HRV were not expected, we have no data about sleep quality of the study patients. Also, the group sizes are rather small and replication in an independent sample with standardized study manuals is needed.
In conclusion, the results of the present pilot study using 24-h Holter ECG indicate alterations in basal autonomic activity in children with ADHD, which are shifted towards normal levels by medication with MPH. Regarding this normalization, our data rather point to cardio-protective effects of psychostimulants than to an increase in a sudden heart death risk. This influence on basal autonomic activation could be a potential explanation of the very recently published population-based cohort studies, reporting association of psychostimulant medication with reduced incidence of serious cardiovascular events (Cooper et al. 2011; Habel et al. 2011). Due to the limitations of the study design, the reported results have to be considered as preliminary data, which contribute to the actual data on autonomic effects of psychostimulants and channel continuative studies.
References
Al Haddad H, Laursen PB, Ahmaidi S, Buchheit M (2009) Nocturnal heart rate variability following supramaximal intermittent exercise. Int J Sports Physiol Perform 4:435–447
Arnsten AF (2009) Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs 23(Suppl 1):33–41
Buchhorn R, Hulpke-Wette M, Nothroff J, Paul T (2002) Heart rate variability in infants with heart failure due to congenital heart disease: reversal of depressed heart rate variability by propranolol. Med Sci Monit 8:CR661-6
Cooper WO, Habel LA, Sox CM, Chan KA, Arbogast PG, Cheetham TC, Murray KT, Quinn VP, Stein CM, Callahan ST, Fireman BH, Fish FA, Kirshner HS, O’Duffy A, Connell FA, Ray WA (2011) ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med 365:1896–1904
Denchev P, Kaltman JR, Schoenbaum M, Vitiello B (2010) Modeled economic evaluation of alternative strategies to reduce sudden cardiac death among children treated for attention deficit/hyperactivity disorder. Circulation 121:1329–1337
Faraone SV (2009) Using Meta-analysis to Compare the Efficacy of Medications for Attention-Deficit/Hyperactivity Disorder in Youths. P T 34:678–694
Fei L, Anderson MH, Katritsis D, Sneddon J, Statters DJ, Malik M, Camm AJ (1994) Decreased heart rate variability in survivors of sudden cardiac death not associated with coronary artery disease. Br Heart J 71:16–21
Gould MS, Walsh BT, Munfakh JL, Kleinman M, Duan N, Olfson M, Greenhill L, Cooper T (2009) Sudden death and use of stimulant medications in youths. Am J Psychiatry 166:992–1001
Habel LA, Cooper WO, Sox CM, Chan KA, Fireman BH, Arbogast PG, Cheetham TC, Quinn VP, Dublin S, Boudreau DM, Andrade SE, Pawloski PA, Raebel MA, Smith DH, Achacoso N, Uratsu C, Go AS, Sidney S, Nguyen-Huynh MN, Ray WA, Selby JV (2011) ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 306:2673–2683
Hammerness P, Wilens T, Mick E, Spencer T, Doyle R, McCreary M, Becker J, Biederman J (2009) Cardiovascular effects of longer-term, high-dose OROS methylphenidate in adolescents with attention deficit hyperactivity disorder. J Pediatr 155:84–89
Huang YS, Tsai MH (2011) Long-Term Outcomes with Medications for Attention-Deficit Hyperactivity Disorder Current Status of Knowledge. Cns Drugs 25:539–554
Imeraj L, Antrop I, Roeyers H, Deschepper E, Bal S, Deboutte D (2011) Diurnal variations in arousal: a naturalistic heart rate study in children with ADHD. Eur Child Adolesc Psychiatry 20:381–392
Jacob CP, Romanos J, Dempfle A, Heine M, Windemuth-Kieselbach C, Kruse A, Reif A, Walitza S, Romanos M, Strobel A, Brocke B, Schäfer H, Schmidtke A, Böning J, Lesch K (2007) Co-morbidity of adult attention-deficit/hyperactivity disorder with focus on personality traits and related disorders in a tertiary referral center. Eur Arch Psychiatry Clin Neurosci 257:309–317
Leslie LK, Alexander ME, Trikalinos TA, Cohen JT, Parsons SK, Newburger JW (2008) Reexamining the emperor’s new clothes: ambiguities in current cardiac screening recommendations for youth with attention deficit hyperactivity disorder. Circ Cardiovasc Qual Outcomes 1:134–137
McCarthy S, Cranswick N, Potts L, Taylor E, Wong ICK (2009) Mortality associated with attention-deficit hyperactivity disorder (ADHD) drug treatment: a retrospective cohort study of children, adolescents and young adults using the general practice research database. Drug Saf 32:1089–1096
Negrao BL, Bipath P, van der Westhuizen D, Viljoen M (2011) Autonomic correlates at rest and during evoked attention in children with attention-deficit/hyperactivity disorder and effects of methylphenidate. Neuropsychobiology 63:82–91
Polanczyk G, Rohde LA (2007) Epidemiology of attention-deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry 20:386–392
Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007) The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry 164:942–948
Polderman TJ, Boomsma DI, Bartels M, Verhulst FC, Huizink AC (2010) A systematic review of prospective studies on attention problems and academic achievement. Acta Psychiatr Scand 122:271–284
Renner T, Gerlach M, Romanos M, Herrmann M, Reif A, Fallgatter A, Lesch K (2008) Neurobiologie des Aufmerksamkeitsdefizit-/Hyperaktivitätssyndroms. Nervenarzt 79:771–781
Schelleman H, Bilker WB, Strom BL, Kimmel SE, Newcomb C, Guevara JP, Daniel GW, Cziraky MJ, Hennessy S (2011) Cardiovascular Events and Death in Children Exposed and Unexposed to ADHD Agents. Pediatrics 127:1102–1110
Schlander M, Trott GE, Schwarz O (2010) The health economics of attention deficit hyperactivity disorder in Germany. Part 1: Health care utilization and cost of illness. Nervenarzt 81:289–300
Schubert I, Köster I, Lehmkuhl G (2010) The changing prevalence of attention-deficit/hyperactivity disorder and methylphenidate prescriptions: a study of data from a random sample of insurees of the AOK Health Insurance Company in the German State of Hesse, 2000–2007. Dtsch Arztebl Int 107:615–621
Singh RB, Weydahl A, Otsuka K, Watanabe Y, Yano S, Mori H, Ichimaru Y, Mitsutake G, Sato Y, Fanghong L, Zhao ZY, Kartik C, Gvozdjakova A (2001) Can nutrition influence circadian rhythm and heart rate variability? Biomed Pharmacother 55(Suppl 1):115s–124s
Singh RB, Kartik C, Otsuka K, Pella D, Pella J (2002) Brain-heart connection and the risk of heart attack. Biomed Pharmacother 56(Suppl 2):257s–265s
Task Force of the European Society of Cardiology and the North American society of Pacing (1996) Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 17:354–381
Taurines R, Schmitt J, Renner T, Conner AC, Warnke A, Romanos M (2010) Developmental comorbidity in attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord 2:267–289
Tonhajzerova I, Ondrejka I, Adamik P, Hruby R, Javorka M, Trunkvalterova Z, Mokra D, Javorka K (2009) Changes in the cardiac autonomic regulation in children with attention deficit hyperactivity disorder (ADHD). Indian J Med Res 130:44–50
Vaseghi M, Shivkumar K (2008) The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis 50:404–419
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Buchhorn, R., Conzelmann, A., Willaschek, C. et al. Heart rate variability and methylphenidate in children with ADHD. ADHD Atten Def Hyp Disord 4, 85–91 (2012). https://doi.org/10.1007/s12402-012-0072-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12402-012-0072-8