Abstract
To date, few studies have examined the circadian pattern of motor activity in children and adolescents newly diagnosed with attention-deficit/hyperactivity disorder (ADHD). The objective was to study the circadian pattern of motor activity in subjects with ADHD (medication naïve) and to investigate the relationships between alterations in circadian patterns, the ADHD subtype (combined or inattentive), sleep disturbances and body mass index (BMI). One-hundred twenty children and adolescents (60 medication naïve ADHD and 60 controls) were included in a gender- and age-matched case–control study. ADHD was diagnosed according to the DSM-IV-TR, the Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version, and the Conner’s Parents Rating Scale-Revised. Circadian rhythms of motor activity and sleep parameters were measured using actigraphy and the Sleep Disturbance Scale for Children. BMI and dietary intake were also evaluated. ADHD patients showed a trend towards eveningness and greater sleep disturbances than controls. Additionally, patients with ADHD-combined had significantly higher mean values of motor activity and showed a significant delay in bedtime. Furthermore, among ADHD-C patients hyperactivity symptoms were significantly associated with the least 5 h of activity. Regarding patients with ADHD-inattentive, increased fragmentation of the circadian pattern was associated with inattention symptoms, and they also showed a significant increase in BMI of 2.52 kg/m2 [95% CI 0.31, 4.73] in comparison with controls. Our findings highlight the potential use of actigraphy as a clinical tool to aid in the diagnosis of ADHD. It should be noted that evaluating motor activity variables could also allow the differentiation between ADHD subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Attention Deficit Hyperactivity Disorder (ADHD) is a childhood neurodevelopmental disorder and is one of the most common disorders in this stage in life [1], which can often persist into adulthood [2]. Systematic reviews indicate that the worldwide prevalence of ADHD is between 2% and 7%, with an average of around 5% [3]. ADHD is characterized by three nuclear symptoms: inattention, impulsivity, and hyperactivity [4, 5]. Furthermore, ADHD also exhibits high comorbidity with sleep disorders that are generally associated with circadian rhythm abnormalities, in both children and adults [6,7,8,9,10,11].
The circadian clock is responsible for the generation of 24-h rhythms of behavior and physiology (also known as circadian rhythms) and has a key role in determining the rhythm of the sleep/wake cycle [12]. Several studies have reported that ADHD is strongly associated with disturbances of circadian rhythms [4, 10, 13]. This includes alterations of circadian rhythms at endocrine and behavioral levels [8, 12, 14], desynchronization between melatonin secretion and sleep timing [15], increased nocturnal motor activity [9], altered body temperature [16] and disrupted expression of circadian clock genes [12, 16, 17]. Furthermore, ADHD is associated with a delay in the chronotype and, therefore, patients tend to be more evening-oriented [18, 19]. All of these factors have an impact on the health status of ADHD patients, which have already been shown to influence comorbidities of this disease, such as defiant opposition disorder [14] and higher levels of aggression [17]. In addition, alterations in circadian rhythms in ADHD patients are related to poor sleep quality, delayed sleep, shorter sleep, more nocturnal wake time, and insomnia, as well as greater sleep latency and deficiency [6,7,8, 10].
On the other hand, several studies have reported a significant association between obesity and ADHD, which seems paradoxical since the motor activity is greater among these patients [20,21,22,23]. Biological rhythms are known to be driven by a self-sustained internal network of molecular clocks, with the master pacemaker located in the suprachiasmatic nucleus [24]. Molecular clocks and the master pacemaker must be synchronized with each other and with external environmental agents, for example, exposure to light, resting patterns, or scheduled food intake [25]. However, when the internal order is disturbed, circadian misalignment (also known as chronodisruption) may occur, which is an etiological factor of several pathologies, among them metabolic syndrome and obesity [26].
However, despite all this knowledge, few studies have explored the 24-h rhythm of activity in children and adolescents with ADHD without prior pharmacological treatment or medication naïve [6, 7]. Therefore, our objective was to study possible alterations in circadian rhythms in subjects with ADHD and to establish possible relationships between these circadian alterations, sleep disorders, obesity, and ADHD symptoms. In addition, we have independently studied two subtypes of ADHD: Combined (ADHD-C) and Inattentive (ADHD-I). We hypothesize that if circadian rhythm disruption is associated with ADHD symptoms, this may provide clinical opportunities for the development of chronobiological treatment strategies in ADHD patients, improving long-term health.
Methods
Participants
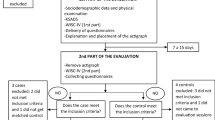
The group of ADHD patients consisted of sixty children and adolescents (ages 6–16 years) newly diagnosed with ADHD (medication naïve, no pharmacological treatment) and sixty gender- and age-matched controls. The cases were recruited in the ADHD Unit of the Department of Child and Adolescent Psychiatry and Psychology of the Hospital of Sant Joan de Déu (Barcelona, Spain) (described in detail in Ríos-Hernández et al. [27]). The cases were diagnosed for the first time and had never been evaluated for psychiatric disorders or treated with psychopharmacological medicine. The diagnosis of ADHD was made by expert child psychiatrist according to the Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV-TR) [28]. The ADHD Rating-Scale-IV (ADHD RS-IV) for parents was used as screening for the diagnosis of ADHD [29]. The Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime version (K-SADS-PL) was used to confirm the ADHD diagnosis and other comorbidities [30]. The Conners Parents Rating Scale-Revised (Short Version) (CPRS-R:S) [31] was used to evaluate the behavior of children assessed by their parents. This scale includes inattentiveness, hyperactivity domains, oppositional behavior, and ADHD index.
Exclusion criteria were IQ < 70, autism spectrum disorder, psychosis, developmental disorders, taking any medication treatment for ADHD or nutrient complement (mineral/vitamin) before or during the study. We also excluded subjects in whom the severity of symptoms was significant and in whom a symptomatic treatment was needed urgently (e.g. anxiolytic, antipsychotic) before completing the entire study.
Controls were recruited from the ADHD patients’ classmates (40%) and from patients attending in other hospital services (60%) (i.e. minor surgery ambulatory, or similar ones). Controls were screened for the absence of ADHD symptoms and the same exclusion criteria as patients applied to controls.
Ethical standards
The study was performed in accordance with the ethical guidelines of the Declaration of Human Studies of Helsinki and approved by the Ethical Committee of the Hospital of Sant Joan de Deu. Written informed consent was obtained from the parents of the participants, and verbal assent was obtained from the participants.
Anthropometric measures
Participants underwent a physical examination, which included height and weight. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared, and BMI was standardized to BMI z score using age and gender, and compared with the parameters of child growth from the World Health Organization [32].
Actigraphy
The 24-h rhythm of motor activity was measured with actigraphy. Therefore, we used the actigraph ActiSleep (ActiGraph, Pensacola, FL, USA) programmed to collect motor activity data each minute for seven consecutive days and nights [33]. Children and adolescents were required to wear the actigraph on the non-dominant wrist and only took it off while bathing. A validated algorithm was used to calculate: time in bed (hh:mm), time out bed (hh:mm), total sleep time (min), total time in bed (min), latency (min), efficiency (%), wake after sleep onset (min), awakenings and average of awakenings (min). The energy expenditure for the physical activity of participants was obtained also throughout the actigraph (kcal/day). Moreover, activity data were grouped into 1 h bouts, to study the hourly differences between the groups.
Assessment of sleep disturbances
Sleep disturbances were measured with the Sleep Disturbance Scale for Children (SDSC) [34], a well-validated parental report instrument. Parents were asked to answer 26 questions which are grouped into six sleep factors: Behavioral Sleep problems of Initiating and Maintaining Sleep (BSP); Sleep Breathing Disorder (SBD); Parasomnias-Arousal Disorders (AD) such as nightmares and night terrors, and Sleep–Wake Transition Disorders (SWTD), such as sleepwalking, sleep talking and bruxism; Excessive Daytime Somnolence (EDS); Sleep Hyperhidrosis—night sweating (HYH); and Total Sleep Problems (TSP) which results of the sum of all individual questions scores.
Chronotype
As chronotype measure, we used the mid-sleep on free days corrected for accumulated sleep debt during the week (Monday to Friday) (MSFsc) according to the instructions accompanying the Munich Chronotype Questionnaire [35].
Analyses of motor activity daily rhythm
First, motor activity data were filtered to eliminate erroneous measurements, such as those produced by temporarily removing the actigraph. Subsequently, data were analyzed using “el Temps” (v293) (www.el-temps.com), an integrated package for chronobiological analysis (Diez-Noguera, University of Barcelona, Barcelona, Spain). The rhythmic variables mesor, amplitude, and acrophase (defined as the time of the maximum value of motor activity) were determined by fitting the data to a 24-h cosinusoidal curve. In addition, non-parametric circadian analyses were performed. In this case, the intradaily variability, the stability of the rhythm (Rayleigh test and interdaily stability), the 10 h of maximum motor activity (M10) and the least 5 h of motor activity (L5) were calculated [36]. The definitions of all the rhythmic variables are provided in Table 1.
Statistical analyses
Continuous variables were expressed as mean (standard deviation; SD) whereas categorical variables were expressed as a percentage. Normality was confirmed in all variables by histograms and Q–Q plots. Partial correlations controlled for age and gender were conducted to study the associations between ADHD symptoms and rhythmic variables of motor activity among cases. In addition, differences between cases and controls were analyzed with paired t tests or McNemar–Bowker’s test. In the case of multiple comparisons, p values were corrected using the Benjamini–Hochberg method, assuming a false discovery rate (FDR) of 5%. Analyses were performed using the SPSS 25.0 statistical software package (SPSS Inc., Chicago, Ill., USA) and a p < 0.05 was considered statistically significant.
Results
Table 2 compares the general characteristics between cases and controls. There were statistically significant differences associated with ADHD for BMI (and its z score) and physical activity. As expected, cases showed higher ADHD symptom values (inattention, hyperactivity, and oppositional behavior) than the controls. The K-SADS-PL confirmed the diagnosis of all cases. Of the 60 participants with ADHD, 38 were diagnosed as ADHD-combined (ADHD-C), while 22 were diagnosed as ADHD-inattentive (ADHD-I). Regarding comorbid diagnoses, 33.3% of patients with ADHD met cutoff criteria for oppositional defiant disorder, 23.3% for anxiety, 3.3% for conduct disorder, and 1.7% for depression. Regarding rhythmic variables of motor activity, patients with ADHD showed a significant delay in the acrophase.
Variables of the activity rhythm are associated with ADHD symptomatology in children and adolescents
First, we examined partial correlations between circadian variables of the motor activity rhythm and ADHD symptoms of inattention, hyperactivity, and oppositional behavior (ADHD RS-IV and CPRS-R:S). Analyses were conducted with all 60 subjects with ADHD and adjusted for age and gender (see Online Resource 1 for all coefficients). We found significant correlations between the Rayleigh test and ADHD hyperactivity symptoms according to the CPRS-R:S scale (r = − 0.282, p = 0.032). Additionally, we noted that the L5 was significantly correlated with ADHD hyperactivity symptoms according to both the ADHD RS-IV and CPRS-R:S scales (r = 0.314; p = 0.004 and r = 0.467; p < 0.001, respectively). Finally, we found a positive correlation between the L5 and the symptoms of oppositional disorder (r = 0.263; p = 0.046).
Second, partial correlation analyses (adjusted for age and gender) between ADHD symptoms and rhythmic variables of motor activity based on ADHD subtype (inattentive [ADHD-I] or combined [ADHD-C], Online Resource 1) showed that among ADHD-C patients, L5 was significantly associated with symptoms of hyperactivity according to both scales (r = 0.386, p = 0.020 and r = 0.409, p = 0.013, respectively). While among ADHD-I patients, the intradaily variability was associated with inattentive symptoms according to both scales (r = 0.449, p = 0.047 and r = 0.625; p = 0.003).
Differences in rhythmic variables of motor activity are associated with ADHD subtypes
Table 3 summarizes the differences in the circadian variables of motor activity between the ADHD subtypes (ADHD-C and ADHD-I) and their respective healthy controls. First, regarding differences between ADHD-C patients and healthy controls, we observed that mesor was significantly higher (p = 0.039) in patients compared to controls. In addition, we noted that ADHD-C patients presented a delay of ~ 45 min (p = 0.002) in acrophase. Second, concerning the comparison between ADHD-I patients and their respective healthy controls, no differences were found for the values of mesor. However, we found that ADHD-I patients also showed a delay of ~ 29 min in acrophase (p = 0.020).
The daily profile of motor activity shows a shift to eveningness in ADHD-C patients
Differences in the daily profile of motor activity were also found (Fig. 1). Interestingly, we observed that ADHD-C patients had greater motor activity levels between 19:00 and 23:00 h compared with controls and also, and to a lesser extent, between 04:00 and 05:00 h (Fig. 1a). In contrast, ADHD-C patients showed lower motor activity at 07:00 h than healthy controls. On the other hand, no significant differences were found in the daily profile of motor activity between ADHD-I patients and healthy controls (Fig. 1b).
Mean daily profile of motor activity in a patients with ADHD-Combined (ADHD-C) versus healthy controls and b patients with ADHD-Inattentive (ADHD-I) versus healthy controls. Data are presented as mean (SEM). General linear model were used to compare differences in the 24-h activity rhythm (i.e., mean number of movements in 1-min epoch in one recording hour) between ADHD patients (combined or inattentive) with healthy controls. Significant p values ** < 0.01; *** < 0.001. P values were corrected using the Benjamini–Hochberg method, assuming a false discovery rate of 5%
Late sleep timing and more sleep disturbances are associated with ADHD subtypes
Regarding sleep variables, we observed that both time in bed and time out of bed were significantly delayed among ADHD-C patients when compared to healthy controls (p = 0.006 and p = 0.005, respectively) (Table 4). Additionally, we observed that ADHD-C patients showed a trend towards a later chronotype when compared with healthy controls (p = 0.073). Furthermore, we observed that ADHD-C patients presented greater sleep disturbances than healthy controls. Specifically, individuals with ADHD-C showed greater: behavioral sleep problems of initiating and maintaining sleep, sleep breathing problems, sleep–wake transition disorders, excessive daytime somnolence, and sleep hyperhidrosis.
Concerning the comparison between sleep variables and sleep disturbances among ADHD-I patients and their respective healthy controls, we observed that sleep timing, duration, latency, and efficiency were similar between the groups. However, we did find that cases showed greater sleep disturbances than the controls. In this case, we noted that ADHD-I patients had increased: behavioral sleep problems of initiating and maintaining sleep, sleep–wake transition disorders and excessive daytime somnolence.
In addition, we compared sleep variables between ADHD patients with low and high scores on the Children’s Sleep Disorders scale (Online Resource 2). Interestingly, we observed that patients with high scores in the sleep breathing disorder subscale had lower sleep efficiency (− 3.56% [95% CI − 6.03, − 1.1]) and a higher duration average of awakenings (0.51 min [95% CI 0.07, 0.96]). We also noted that ADHD patients with high scores on the sleep hyperhidrosis subscale had a shorter duration of sleep (− 0.59 h [95% CI − 1.17, − 0.02]). Besides, these patients woke less frequently at night (− 4.57 [95% CI − 8.91, − 0.226]), but when they did, the awakenings lasted longer [0.77 min (95% CI 0.15, 1.39)].
Higher values of body mass index are related to ADHD subtype
As shown in Table 5, BMI (kg/m2 and z score) and dietary intake were similar between ADHD-C patients and healthy controls. However, the energy expenditure of physical activity was higher among ADHD-C patients. In contrast, BMI (kg/m2 and z score) was significantly higher among ADHD-I patients compared to healthy controls (p = 0.027 and p = 0.038, respectively). Note that although dietary intake and energy expenditure from physical activity was similar between groups, being an ADHD-I patient was associated with a significant increment in the BMI of 2.52 kg/m2 [95% CI 0.31, 4.73].
Discussion
To the authors’ knowledge, this is one of the few studies that has described the daily profile of motor activity in children and adolescents with ADHD (medication naïve) and has compared it to healthy controls, as well as with ADHD symptomatology. According to our results, the acrophase was delayed in both groups of patients (ADHD-C and ADHD-I) compared to their healthy controls, indicating a tendency to an evening chronotype among patients. On the other hand, only ADHD-C patients showed significantly higher mean values of motor activity (expressed as mesor) compared to healthy controls. A difference not seen in ADHD-I patients. We also observed that ADHD-C and ADHD-I patients had greater sleep disturbances than controls, while only ADHD-C patients showed a significant delay in sleep time. All this validates the usefulness of actigraphic methods in the evaluation of ADHD patients. Interestingly, ADHD-I patients showed a significant positive association between inattention and fragmentation of the daily profile motor activity (expressed as intradaily variability). Such association was not found in the ADHD-C.
To our knowledge, few studies have been conducted in the field of circadian patterns and ADHD [6,7,8,9, 37]. Interestingly, Dane et al. [37] reported that children with ADHD showed greater activity in the afternoon, but not in the morning, which is consistent with our findings. In Dane’s study, children only wore the actigraph for 24 h (one day), while our study included seven consecutive days of actigraphy and the results were analyzed according to the ADHD subtype. In this regard, we show that motor activity increased in the evening in ADHD-C patients (activity higher from 19:00 to 23:00 h) compared to healthy controls. Curiously, in ADHD-I patients, a certain increase in motor activity was also observed compared with controls; however, this difference was not statistically significant. This could be related with a real delay in the circadian pacemaker itself or to the fact that children after school carried out more physical exercise. In any case, this could interfere with sleep onset and proper sleep habits [6, 7].
Along the same lines, we observed a slight increase in motor activity from 04:00 to 06:00 h at night in ADHD-C patients, although these differences were not as significant as those that occurred in the evening. Tonetti et al. [9] also found that motor activity was significantly higher between 4:00 and 6:00 h in a sample of adult ADHD patients (some of them under medication). As a first approach, we hypothesize that increased motor activity during this night segment could be related to sleep disturbances, which are common in ADHD patients [38, 39]. Which is also in agreement with our results. Interestingly, we showed that ADHD patients had increased behavioral sleep problems of initiating and maintaining sleep, sleep–wake transition disorders, and excessive daytime somnolence. Furthermore, Gruber et al. [40] demonstrated that compared to controls, children with ADHD show a shorter duration of REM sleep and a smaller percentage of total sleep time spent in REM sleep. It seems plausible that the increased activity at the end of the night could be caused by the decrease in REM sleep, which is characterized by muscular atony. This suggests a relationship between the mechanisms underlying the pathophysiology of ADHD and REM sleep regulation [40].
Furthermore, delayed sleep patterns have also been associated with increased symptoms of hyperactivity and impulsivity in adults with ADHD [15]. This is also consistent with the fact that ADHD-C patients presented a significant delay in their sleep schedules (shown by the time in bed and out of bed) and a trend toward a later chronotype (given by MSFsc: midpoint of sleep in free days). In this regard, ADHD has been associated with late sleep and melatonin onset [8, 15]. Furthermore, Bijlenga et al. [15] pointed out that the circadian pattern of motor activity and body temperature were also delayed in adults with ADHD, suggesting that these patients were more evening-oriented. It is important to note that eveningness has an impact on the comorbidity of this disease, such as a higher prevalence of oppositional defiant disorder [14] or higher levels of aggression [17]. Note that in our patients, we only found a significant association between the least 5 h of motor activity (expressed as L5) and oppositional behavior.
In addition, we observed a delay in the acrophase of the daily profile of motor activity among the cases which could indicate that ADHD patients are more evening-oriented. Although Baird et al. [12] found no difference in acrophase between controls and ADHD cases, there is stronger and most consistent evidence in the literature for the association between later chronotype (evening preference) with ADHD and its symptoms [4, 10, 16, 38]. In this line, Vogel et al. [41] reported that ADHD was associated with eveningness and with a higher prevalence of delayed sleep phase disorder. Furthermore, evening preference is linked to higher rates of inattention among adults with ADHD [38, 42]. In our study, delay in the daily profile of motor activity was associated with both ADHD subtypes: inattentive and combined. Thus, it is plausible that increased activity in the afternoon and evening could exacerbate the symptoms of this disorder and increase sleep disturbances among ADHD patients. This would be in line with previous studies that showed a relationship between delayed sleep onset and circadian rhythm disturbances in ADHD patients [6,7,8].
It is noteworthy that our data showed a positive correlation between hyperactivity symptom score and the amount of activity in the least active 5 h (expressed as L5) in ADHD patients. This suggests that increased activity during the sleep period, is probably related to the higher degree of sleep disturbances and an increase in the severity of hyperactivity symptoms [39]. Among other outcomes, we showed that the lower the stability of the circadian pattern of motor activity (given by the Rayleigh test), the greater the symptoms of hyperactivity. It is important to highlight that a stable activity rhythm is characterized by a 24-h profile that remains similar day to day [8, 43]. Hence, low stability would indicate a decrease of the internal temporal organization, which could lead to suboptimal physiological functioning [43]. Interestingly, low stability of the daily motor activity profile is associated with eveningness [44], as well as sleepiness, depression, cognitive deficits, obesity, and a 20% risk of all-cause mortality among adults (< 45 years old) [43]. Furthermore, this trend to eveningness would be reflected in the lower activity in the early morning, which could cause more difficulties to wake up in the morning (perhaps interfering with school performance).
Finally, our data showed that increased fragmentation of the circadian pattern of motor activity (expressed as intradaily variability) was associated with symptoms of inattention among ADHD-I patients. Noteworthy, the fragmentation of the daily rhythm of motor activity is considered a marker of chronodisruption [45], and has been linked to cognitive impairment, symptoms of depression, and obesity among adults [46, 47]. In adolescents, the fragmentation of the daily rhythm of activity has been related to obesity and central adiposity [48]. This is consistent with our observation that ADHD-I showed higher values of body weight parameters (BMI and its z score) compared to healthy controls. It is worth mentioning that Vogel et al. [41] stated that physicians should pay special attention to physical activity in ADHD patients with symptoms of inattention in order to prevent weight gain. Note that ADHD-I patients tend to be more sleepy during the day [8, 49], implying that these patients may be less physically active.
Strengths and limitations
Some limitations of our design and methods should be acknowledged, since the design of our study limited our ability to assess cause-and-effect associations. In addition, other circadian parameters such as melatonin, cortisol, and temperature could have been evaluated. Nonetheless, this study has several strengths, including the fact that all of the included cases were medication naïve and controls were selected one by one to fit with the patient’s characteristics. It has been suggested that certain medications, especially the psychostimulants often used in the treatment of ADHD, can affect biological rhythms such as diurnal behaviors [50, 51], and may also have an important role in the neurophysiology of suprachiasmatic nucleus (NSQ) [51] and diurnal patterns of clock gene expression [52].
Conclusions
We have demonstrated that the circadian pattern of motor activity in children and adolescents with ADHD is different than those of control subjects showing a clear trend towards eveningness. Remarkably, our results showed that motor activity was higher in ADHD-C patients than controls, while such association was not found in ADHD-I patients. This observation sheds light on the potential use of actigraphy as a clinical tool to aid in the diagnosis of ADHD and subtypes. It should be noted that the circadian variables of motor activity could also allow the differentiation between ADHD subtypes: inattentive and combined. In addition, both ADHD-C and ADHD-I patients had greater sleep disturbances than controls, while only ADHD-C patients showed a significant delay in sleep timing. Finally, increased fragmentation of the circadian pattern of motor activity was associated with symptoms of inattention among ADHD-I patients, which could be related to obesity. Further research on modulating circadian rhythm alterations in ADHD may be beneficial in understanding the underlying causes of the symptomatology and may serve to aid in the appropriate and efficacious treatment of the disorder.
References
Polanczyk GV, Salum GA, Sugaya LS et al (2015) Annual research review: a meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry Allied Discip 56:345–365. https://doi.org/10.1111/jcpp.12381
Sibley MH, Swanson JM, Arnold LE et al (2017) Defining ADHD symptom persistence in adulthood: optimizing sensitivity and specificity. J Child Psychol Psychiatry Allied Discip 58:655–662. https://doi.org/10.1111/jcpp.12620
Sayal K, Prasad V, Daley D et al (2018) ADHD in children and young people: prevalence, care pathways, and service provision. Lancet Psychiatry 5:175–186. https://doi.org/10.1016/S2215-0366(17)30167-0
Coogan AN, McGowan NM (2017) A systematic review of circadian function, chronotype and chronotherapy in attention deficit hyperactivity disorder. ADHD Atten Deficit Hyperact Disord 9:129–147. https://doi.org/10.1007/s12402-016-0214-5
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association Publishing, Washington, DC
Van Der Heijden KB, Smits MG, Gunning WB (2006) Sleep hygiene and actigraphically evaluated sleep characteristics in children with ADHD and chronic sleep onset insomnia. J Sleep Res 15:55–62. https://doi.org/10.1111/j.1365-2869.2006.00491.x
Van Der Heijden KB, Smits MG, Van Someren EJW, Gunning WB (2005) Idiopathic chronic sleep onset insomnia in attention-deficit/hyperactivity disorder: a circadian rhythm sleep disorder. Chronobiol Int 22:559–570. https://doi.org/10.1081/CBI-200062410
Van Veen MM, Kooij JJS, Boonstra AM et al (2010) Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry 67:1091–1096
Tonetti L, Conca A, Giupponi G, Natale V (2017) Circadian pattern of motor activity in adults with attention-deficit/hyperactivity disorder. Chronobiol Int 34:802–807. https://doi.org/10.1080/07420528.2017.1309660
Bijlenga D, Vollebregt MA, Kooij JJS, Arns M (2019) The role of the circadian system in the etiology and pathophysiology of ADHD: time to redefine ADHD? ADHD Atten Deficit Hyperact Disord 11:5–19. https://doi.org/10.1007/s12402-018-0271-z
Yoon SYR, Jain U, Shapiro C (2012) Sleep in attention-deficit/hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev 16:371–388
Baird AL, Coogan AN, Siddiqui A et al (2012) Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioral, endocrine and molecular levels. Mol Psychiatry 17:988–995
Kooij S, Bijlenga D (2013) The Circadian Rhythm in Attention-Deficit/Hyperactivity Disorder and Health. Expert Rev Neurother 13:1107–1116
Imeraj L, Antrop I, Roeyers H et al (2012) Time-of-day effects in arousal: disrupted diurnal cortisol profiles in children with ADHD. J Child Psychol Psychiatry Allied Discip 53:782–789. https://doi.org/10.1111/j.1469-7610.2012.02526.x
Bijlenga D, Van Someren EJ, Gruber R et al (2013) Circadian rhythms and ADHD Body temperature, activity and melatonin profiles in adults with attention-deficit/hyperactivity disorder and delayed sleep: a case—control study. J Sleep Res 22:607–616
Mcgowan NM, Voinescu BI, Coogan AN (2016) Sleep quality, chronotype and social jetlag differentially associate with symptoms of attention deficit hyperactivity disorder in adults. Chronobiol Int 33:1433–1443
Mogavero F, Jager A, Glennon JC (2018) Clock genes, ADHD and aggression. Neurosci Biobehav Rev 91:51–68. https://doi.org/10.1016/j.neubiorev.2016.11.002
Rybak YE, McNeely HE, Mackenzie BE et al (2007) Seasonality and circadian preference in adult attention-deficit/hyperactivity disorder: clinical and neuropsychological correlates. Compr Psychiatry 48:562–571. https://doi.org/10.1016/j.comppsych.2007.05.008
Lunsford-Avery JR, Krystal AD, Kollins SH (2016) Sleep disturbances in adolescents with ADHD: a systematic review and framework for future research. Clin Psychol Rev 50:159–174
De Zwaan M, Gruß B, Müller A et al (2011) Association between obesity and adult attention-deficit/hyperactivity disorder in a German community-based sample. Obes Facts 4:204–211. https://doi.org/10.1159/000329565
Cortese S, Tessari L (2017) Attention-deficit/hyperactivity disorder (ADHD) and obesity: update 2016. Curr Psychiatry Rep 19:4
Fuemmeler BF, Sheng Y, Schechter JC et al (2020) Associations between attention deficit hyperactivity disorder symptoms and eating behaviors in early childhood. Pediatr Obes. https://doi.org/10.1111/ijpo.12631
Cortese S (2019) The association between ADHD and obesity:intriguing, progressively more investigated, but still puzzling. Brain Sci 9:256
Baron KG, Reid KJ (2014) Circadian misalignment and health. Int Rev Psychiatry 26:139–154. https://doi.org/10.3109/09540261.2014.911149
Johnston JD, Ordovás JM, Scheer FA, Turek FW (2016) Circadian Rhythms, metabolism, and chrononutrition in rodents and humans. Adv Nutr 7:399–406
Garaulet M, Ordovás JM, Madrid JA (2010) The chronobiology, etiology and pathophysiology of obesity. Int J Obes 34:1667–1683. https://doi.org/10.1038/ijo.2010.118
Ríos-Hernández A, Alda JA, Farran-Codina A et al (2017) The mediterranean diet and ADHD in children and adolescents. Pediatrics 139:e20162027. https://doi.org/10.1542/peds.2016-2027
American Psychiatric Association (2000) Diagnostic and Statistical Manual of Mental Disorders: DSM-IV, 4th edn. American Psychiatric Association, Washington, DC
DuPaul GJ, Power TJ, Anastopoulos AD, Reid R (1998) ADHD rating scale-IV: checklists, norms and clinical interpretation. The Guilford Press, New York
Ulloa RE, Ortiz S, Higuera F et al (2006) Interrater reliability of the Spanish version of Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL). Actas Esp Psiquiatr 34:36–40
Conners CK, Sitarenios G, Parker JD, Epstein JN (1998) The revised conners’ parent rating scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268
World Health Organization Child growth standards. https://www.who.int/childgrowth/standards/imc_para_edad/es/. Accessed 22 May 2020
Van Someren EJW (2007) Improving actigraphic sleep estimates in insomnia and dementia: how many nights? J Sleep Res 16:269–275. https://doi.org/10.1111/j.1365-2869.2007.00592.x
Bruni O, Ottaviano S, Guidetti V et al (1996) The sleep disturbance scale for children (SDSC) construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res 5:251–261. https://doi.org/10.1111/j.1365-2869.1996.00251.x
Roenneberg T, Wirz-Justice A, Merrow M (2003) Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms 18:80–90. https://doi.org/10.1007/978-1-4419-9893-4_58
Van Someren EJW, Swaab DF, Colenda CC et al (1999) Bright light therapy: improved sensitivity to its effects on rest- activity rhythms in Alzheimer patients by application of nonparametric methods. Chronobiol Int 16:505–518. https://doi.org/10.3109/07420529908998724
Dane AV, Schachar RJ, Tannock R (2000) Does actigraphy differentiate ADHD subtypes in a clinical research setting? J Am Acad Child Adolesc Psychiatry 39:752–760
Durmuş FB, Arman AR, Ayaz AB (2017) Chronotype and its relationship with sleep disorders in children with attention deficit hyperactivity disorder. Chronobiol Int 34:886–894. https://doi.org/10.1080/07420528.2017.1329207
Chamorro M, Lara JP, Insa I et al (2017) Evaluation and treatment of sleep problems in children diagnosed with attention deficit hyperactivity disorder: an update of the evidence. Rev Neurol 64:413–421
Gruber R, Xi T, Frenette S et al (2009) Sleep disturbances in prepubertal children with attention deficit hyperactivity disorder: a home polysomnography study. Sleep 32:343–350. https://doi.org/10.1093/sleep/32.3.343
Vogel SWN, Bijlenga D, Tanke M et al (2015) Circadian rhythm disruption as a link between attention-deficit/hyperactivity disorder and obesity? J Psychosom Res 79:443–450. https://doi.org/10.1016/j.jpsychores.2015.10.002
Caci H, Bouchez J, Baylé FJ (2009) Inattentive symptoms of ADHD are related to evening orientation. J Atten Disord 13:36–41
Zuurbier LA, Luik AI, Hofman A et al (2015) Fragmentation and stability of circadian activity rhythms predict mortality. Am J Epidemiol 181:54–63. https://doi.org/10.1093/aje/kwu245
Martinez-Nicolas A, Martinez-Madrid MJ, Almaida-Pagan PF et al (2019) Assessing chronotypes by ambulatory circadian monitoring. Front Physiol 10:1–13. https://doi.org/10.3389/fphys.2019.01396
Corbalán-Tutau MD, Madrid JA, Ordovás JM et al (2011) Differences in daily rhythms of wrist temperature between obese and normal-weight women: associations with metabolic syndrome features. Chronobiol Int 28:425–433. https://doi.org/10.3109/07420528.2011.574766
Luik AI, Zuurbier LA, Hofman A et al (2013) Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int 30:1223–1230
Oosterman JM, Van Someren EJW, Vogels RLC et al (2009) Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res 18:129–135. https://doi.org/10.1111/j.1365-2869.2008.00704.x
Garaulet M, Martinez-Nicolas A, Ruiz JR et al (2017) Fragmentation of daily rhythms associates with obesity and cardiorespiratory fitness in adolescents: the HELENA study. Clin Nutr 36:1558–1566
Mayes SD, Calhoun SL, Bixler EO et al (2009) ADHD subtypes and comorbid anxiety, depression, and oppositional-defiant disorder: differences in sleep problems. J Pediatr Psychol 34:328–337. https://doi.org/10.1093/jpepsy/jsn083
Algahim MF, Yang PB, Wilcox VT et al (2009) Prolonged methylphenidate treatment alters the behavioral diurnal activity pattern of adult male Sprague-Dawley rats. Pharmacol Biochem Behav 92:93–99
Antle MC, Van Diepen HC, Deboer T et al (2012) Methylphenidate modifies the motion of the circadian clock. Neuropsychopharmacology 37:2445–2446
Baird AL, Coogan AN, Kaufling J et al (2013) Daily methylphenidate and atomoxetine treatment impacts on clock gene protein expression in the mouse brain. Brain Res 1513:61–71
Acknowledgements
The study was supported by a grant from the Subdirección General de Evaluación y Fomento de la Investigación (PI11/2009) from the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, Spain; MFZR was supported by a scholarship from Consejo Nacional de Ciencia y Tecnología (CONACYT) of México and TVCA was supported by a scholarship from Secretaría de Educación Superior, Ciencia, Tecnología e Innovación (SENESCYT) of Ecuador. We are grateful to Dr. Ramírez, Dr. Hernández and Dr. Serrano for their assistance with material collection and to the patients and families for their collaboration and implication. We are also grateful to Mr. Peter Mendoza for his assistance in the English editing of the current manuscript.
Author information
Authors and Affiliations
Contributions
JA and MIP designed the study; TVCA, EFG, and JAA acquired the data; MFZR, TVCA, TC, and MIP analyzed the data; MFZR and MIP wrote the first draft; MFZR, ADN, TC, JAA, and MIP revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared they had no conflicts of interest with respect to their authorship or the publication of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zerón-Rugerio, M.F., Carpio-Arias, T.V., Ferreira-García, E. et al. ADHD subtypes are associated differently with circadian rhythms of motor activity, sleep disturbances, and body mass index in children and adolescents: a case–control study. Eur Child Adolesc Psychiatry 30, 1917–1927 (2021). https://doi.org/10.1007/s00787-020-01659-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-020-01659-5