Abstract
This paper is a review of the recent studies on Maillard reaction products, the formation mechanism for these compounds and melanoidin structure, the undesirable consequences in food especially in fruit juice processing, the desirable effects and the biological properties related to the beneficial health. Melanoidins are compounds generated in the late stages of the Maillard reaction from reducing sugars and proteins or amino acids during food processing and preservation. Recently, the effects of melanoidins on human health and the chemical characterization of the beneficial components have gained a lot of attention, and their implications on several levels, sensory, nutritional, toxicological and technological were investigated. Food melanoidins have been reported to be anionic, coloured compounds, and some of their key chromophores have been elucidated. The antioxidant activity and other biological effects of melanoidins from real foods and model systems have been widely studied. Despite this, very few different melanoidin structures have actually been described, and specific health effects have yet to be linked with chemically distinct melanoidins. The variety of Maillard reaction products formed during the reaction, in conjunction with the difficulty in purifying and identifying them, makes a thorough analysis of melanoidins challenging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Maillard reaction has been named after the French chemist Louis Maillard who first described such conversion reactions in 1912 [112], but it was only in 1953 that the first coherent scheme was put forward by Hodge [73]. The Maillard reaction, which is a non-enzymatic browning reaction, is a complex network of reactions involving carbonyl and amino compounds, such as reducing sugars and amino acids [36, 172]. It is the main reaction responsible for the transformation of precursors into colorants and flavour compounds during food processing [169].
Melanoidins are polymeric and coloured macromolecules originated by the Maillard reaction and formed primarily by interactions between carbohydrates (typically reducing sugars) and compound characterized by a free amino group, such as amino acids [123]. Studies on melanoidins are mainly focused on their chemical characterization chemistry of reaction and identification of various pathways [124, 134] evaluation of the impact of different reaction parameters (pH, temperature, time, sugar reactivity, reagent concentration and water activity). Many research efforts have been done to determine the structure and chemical properties of melanoidins but since none has been isolated and characterized yet, this information is still lacking [151]. The most important roles of melanoidins, as for most biopolymers, related to flavour and texture, are strongly affected by physical changes and structure transitions [148, 172]. However, few references are found in the literature with regard to the physical structure of melanoidins, possibly due to their complexity and heterogeneity [127, 147, 163].
The importance of melanoidins is due to their wide presence in foods and to the effects that these compounds could have on food quality [116]. Depending on the extent of heat induced reaction, melanoidins could impair or improve the overall quality of food products [9, 186]. Melanoidins are responsible for the colour in cooked and processed foods, and they are present in widely consumed dietary components (e.g. coffee, bread, and roasted malt) [1, 17, 125]. Artificial maple syrup is often made through a careful combination of corn syrup and amino acids under heat, gets its distinctive brown colour from the Maillard reaction [37, 53]. The Maillard reaction is also used to create artificial flavourings, based on the hundreds of complex amino/acid sugar combinations formed after the process [143]. A typical sample of roasted meat alone is said to have over 600 different flavours, for example. Food scientists use the Maillard reaction to duplicate these flavours in laboratories. [128].
In melanoidins characterization there is three ways for isolation based on their molecular weight; (a) bath dialysis in a cellulose dialysis tubing [1] (i.e. 33 mm of flat width, 12.4 kDa of molecular weigh cut-off (MWCO). (b) gel-filtration chromatography (HPGPC) [124] and ultrafiltration (from 1 to 300 kDa molecular cut-off) [80].The main advantages of ultrafiltration process, are the high yield/rate of sample processed and low contamination [44].
In the past, many scientific works focused on the negative biological effects of the Maillard reaction. The formation of antinutritional and toxic Maillard reaction products (MRPs) has been reported frequently. In vitro studies revealed some harmful effects including mutagenic, carcinogenic [40] and cytotoxic effects [184]. Excessive glycation has also been stated to cause the destruction of essential amino acids, decreased digestibility, inactivation of enzymes, inhibition of regulatory molecule binding, cross-linking of the glycated extra-cellular matrix, decreased susceptibility to proteolysis, abnormalities of nucleic acid function, altered macromolecular recognition and endocytosis inhibitory [181] activity and increased immunogenicity [160].
During recent years, interest in these compounds has increased because of their nutritional antiradical, antimutagenic, chelating properties [120], antimicrobial, antihypertensive and anti-browning activities [76]. MRPs containing antioxidant, antiallergenic and cytotoxic properties are amongst others mostly detected [65]. Many studies focused on the high antioxidant capacity of MRPs in model systems and foods such as beer [155], coffee [9] and bakery products [24]. In those studies it was shown that MRPs can contribute greatly to the shelf-life of heat-treated foods [149] In vitro studies demonstrated that MRPs may offer substantial health-promoting activity as they can act as reducing agents [30] metal chelators and radical scavengers [36]. It appears that especially low molecular weight MRPs exhibit antioxidant effects in the organism after they get absorbed by the small intestine [163, 182], decrease protein of digestibility and possible formation of toxic and mutagenic compounds, but can also be improved by the formation of antioxidative products [116].
Due to this growing interest in the effect of melanoidins of the human diet and their feasible nutritional, biological and health implications, the European Union has launched a specific COST (Co-operation in Science and Technology) action entitled Melanoidins in Food and Health (COST, 1998) [39, 163].
This review discusses a research approach designed to increase the understanding of (a) the chemistry of the reaction and its influence on food properties like colour, flavour and nutritional value, and (b) the desirable and the desirable properties in food especially in fruit juice processing and (c) the desirable effects and the biological properties related to the beneficial health.
Non-Enzymatic Browning Reactions (NEB) Pathways
Browning reactions, which are some of the most important phenomena occurring in food during processing and storage, represent an interesting research area for the implications in food stability and technology, as well as in nutrition and health. They can involve different compounds and proceed through different chemical pathways. The major groups of reactions leading to browning are enzymatic phenol oxidation and non-enzymatic browning [114]. The non-enzymatic browning reactions which occur in food may be caused by (a) the degradation of ascorbic acid, (b) lipid peroxidation, (c) sugar–sugar caramelization and (d) Maillard reaction [144]. Ascorbic acid undergoes a reaction chemically similar to that of sugars except that amino acids are not necessary for browning. Since ascorbic acid is very reactive, it degrades by two pathways, both of which lead to the formation of dicarbonyl intermediates and subsequently to form browning compounds [163].
Lipid peroxidation occurs by the action of oxygen and reactive oxygen species on the fatty acids, especially unsaturated fatty acids. These are oxidized to form aldehydes and ketones which then react with amino acids to form brown pigments, as in the Maillard reaction. It is possible that peroxidation products induce the browning reaction of the Amadori products [12, 185]. In foods, this reaction takes place essentially between the monosaccharides, glucose and fructose or the disaccharides, maltose and lactose as well as in some cases reducing pentoses and amino acids and/or proteins [70].
Mechanism of the Maillard Reaction
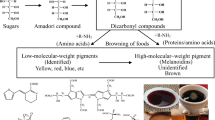
John Hodge published his consolidated scheme which summarized the chemical reactions which were understood to comprise the Maillard reaction at that time [73]. The Hodge scheme remains widely used today (Fig. 1). In essence, it states that a reducing sugar, such as glucose, condenses with a compound possessing a free amino group, such as an amino acid, to give a condensation product [4, 178]. Subsequently, a range of reactions takes place, including cyclizations, dehydrations, retroaldolizations, rearrangements, isomerizations and further condensations, which ultimately lead to the formation of brown nitrogenous polymers and co-polymers, known as melanoidins [33].
Formation of advanced MRPs from Maillard reaction (adapted from Martins [116])
The Maillard reaction is actually a complex series of reactions; the first stage involves the initial condensation of the sugar with protein and the isomerization of the resulting products to form Amadori or Heyns products.
The Maillard reaction is initiated by a condensation reaction between the carbonyl group of the aldose and the free amino group of an amino acid to give an N-substituted aldosylamine. This is the result of a nucleophilic attack group by the NH2 group of the amino acid on the electrophilic carbonyl groups of sugar [18]. It is basically an amine assisted dehydration reaction of sugar [13]. The condensation product rapidly loses water as a product and is converted into a Schiff base, this reaction is reversible and acid base catalysed. [35, 73]. The resulting Schiff base cyclizes in the case of pentoses and hexoses to the corresponding glycosylamine, which then undergoes an Amadori rearrangement. If the aldose and amino acid glycine, then the Amadori product is 1-amino-1-deoxy-2-fructose (monofructoseglycine) [68, 184], with fructose the reaction is quite similar but the rearrangement is termed the Heyns rearrangement and is generally shown as giving substituted 2-amino-2-deoxyldoses. The Amadori rearrangement is considered to be the key step in the formation of major intermediates for the browning reaction [176]. Aminoaldoses are not very stable and readily react forming the Amadori compound (no browning occurs at that stage). [105].
The stage two in the Maillard reaction, particularly in foods, is usually depicted as starting with the decomposition of Amadori and Heyns adducts to form deoxydicarbonyl sugars [69].
The Amadori product degrades by one of three main pathways depending on the conditions. (a) The free hydrogen of the amino group of the ketosamine may react with a second molecule of aldose to form a diketosamine. This compound is less stable than the monoketosamine and decomposes to give a monofructoseamine and nitrogen-free carbonyl compounds, for example, 3-deoxyosuloses and the cis-and the trans-forms of 3,4-dideoxyosulos-3-ene [180], which are probably the most important intermediates in the Maillard reaction. The decomposition of the diketosamine has a maximum rate at pH 5.5 [105]. (b) At pH 7 or below, an enolization of the Amadori product results in the formation of furfural (when pentosans are involved) or hydroxymethylfurfural (HMF) (when hexoses are involved). At pH above 7, the degradation of Amadori compound N-(1-Deoxy-d-fructos-l-yl)-glycine (DFG) by 1, 2-enolization and 2,3 enolization takes place, which involves formation of 3-deoxy-2- hexosulose and 3-deoxy-2, 3-hexodiulose, respectively. [116]. (c) Along with enolization reactions, the Amadori product and its dicarbonyl derivates can undergo concurrently retro-aldol reactions producing more reactive C2, C3, C4 and C5 sugar fragments, such as hydroxyacetone derivatives, glyceraldehydes and diketones. This reaction is called as Strecker degradation and it is characterized by the production of CO2 [33]. The aldehydes formed may be important as auxiliary flavour compounds and they also contribute to melanoidin formation. Strecker degradation products especially during cooking, and many of the heterocyclic compounds that cause flavour and aroma are formed at this stage [56].
Finally, the condensation of some of the products formed in this step is produced either among them or with amino compounds to form brown pigments and polymers [34, 135, 185]. The amino acids could react with the unsaturated carbonyl structures to form melanoidins branched with compounds. The formation of melanoidins is the result of the polymerization of the highly reactive intermediates that are formed during the advanced Maillard reaction [146]. Although the Hodge scheme is very useful, it has some drawbacks. First, the scheme is simply a summary of the reactions that take place. Secondly, despite that in recent years the Maillard reaction has been investigated, the chemistry of these compounds is not well-known and their formation mechanism also remains undefined [3, 51]. The molecular weight of these compounds increases as browning proceeds, until eventually they become insoluble high molecular weight species [147]. The chemical reactivity of MRPs and the reactants play an essential role in the type of melanoidin polymer formed. In model systems consisting of limited numbers of substrates, three main proposals for the structure of melanoidin have been put forward until now: (a) melanoidins were reported to be low molecular weight coloured substances which crosslink with free amino groups in proteins (Arg, Lys) leading to high molecular weight coloured structures [8]. (b) Second, it was proposed that melanoidins are macromolecular structures of repeating units that are built up from polycondensated furan-like and pyrrole-like structures [9, 127]. (c) Third, it was reported that the melanoidin skeleton is mainly built up from sugar degradation products which are formed in the early stages of the Maillard reaction, polymerized and linked by amino compounds [23, 57]. It has been demonstrated in aqueous systems that melanoidins are formed by aldol condensations of highly reactive a-dicarbonyl compounds, which are the main intermediates during the early stages of the MRPs, and partially branched by amino compounds [22, 135] in addition to the transglycosylation reactions of saccharides in water-free conditions produced the postulated carbohydrate-based skeleton shown in Fig. 2a. Amino acids could react with the unsaturated carbonyl structures of this structure to form melanoidins branched with amino compounds (Fig. 2b) [22, 174].
Proposal of a carbohydrate-based melanoidin structure (adapted from Wang et al. [174])
Although melanoidins are chemically diverse, many studies report that melanoidins are negatively charged in both real foods and in model systems. Melanoidins found in coffee were determined to be negatively charged but heterogeneous with respect to their polyanionic behaviour [10]: high molecular weight (HMW) melanoidins in coffee were found to be more negatively charged than low molecular weight (LMW) melanoidins [9]. Chlorogenic acids have been hypothesized to be the source of the negative charges in coffee melanoidins [174], though melanoidins obtained from sugar-amino acid model systems also showed anionic characteristics in the absence of chlorogenic acids [134]. For example, Kwak et al. [103] showed that melanoidins prepared by refluxing glucose and lysine could be separated into 14 bands over a pH range of 3.5–4.85, indicating that melanoidins were negatively charged at neutral pH. Under these conditions, the type of amino acid present during the reaction determined the anionic properties of the melanoidins.
Melanoidins in Model Systems and Food Products
The aqueous model browning reaction between glucose or fructose and asparagine was extensively investigated [2, 26, 27, 99]. It can be observed that in most model systems under consideration, the development of Maillard reaction was followed while changing one single variable, such as heating time [27, 183] initial pH [103], molecular weight of MRPs fractions [80] and reactants ratio. Wines and/or liquors have been widely used to prepare meals, but there is limited information about whether the Maillard reaction in the ethanolic solution occurs to the same extent as in an aqueous solution. Shen and Wu [158] studied the Maillard reaction in ethanol model solution and found that ethanol accelerated the rate of browning reaction. Shen et al. [157] further conducted purification analysis and confirmed that MRPs produced in an ethanolic system were different from those produced in an aqueous system.
On the other hand the juice is treated at high temperatures during a long time period while it is flowing through the evaporation stage. That is why, in the determination of the non-enzymatic browning kinetic models, it is convenient to perform studies with sufficient long treatment times, assuring the possibility to interpolate the residence time of the juice while it flows through the multiple-effect evaporator; they are useful for getting a complete kinetic description of the browning reaction [81]. In food systems, like coffee beans, the composition of melanoidins is likely to be far more complex due to the presence of many more possible reactants [8, 9, 127]. Therefore, it is likely that all the proposed structures for melanoidins can be found in coffee melanoidins, and they may even occur within the same melanoidin complex [163]. A good way to investigate the non-enzymatic browning reaction in heated foods is the use of model systems in which sugars and amino acids react under simplified conditions. Each system has to be studied taking into account its own physical and chemical characteristics and their evolution during the reaction itself. These proposals provide valuable information on what melanoidins might look like. However, these suggested melanoidin structures are mostly based on model studies [8, 73].
Model reactions using other amino acids [26], other monomeric sugars, or polymeric carbohydrates instead of glucose [129] were also conducted. However, there are 2 important reasons why research should not be restricted to model systems (a) the reaction products of these model systems are still very complex (b) model systems do not resemble the Maillard reaction in foodstuffs because the compositional complexity of foodstuffs is not taken into account in model reactions, while it is assumed that other food compounds (e.g. chlorogenic acid, polysaccharides, different types of amino acid and monosaccharide) also play a role in the Maillard reaction [10, 117]. The composition of foodstuffs is far more complicated than any simple model systems. In coffee, for example, lipids, chlorogenic acids, sugars, free amino acids, and proteins are also present [1, 10, 112]. As described above, it is likely that at least some of these components will coincide with Maillard reaction processes. It must be stated though that model systems can provide valuable information, but they should preferably be used in addition to research on the Maillard reaction within complex foodstuffs.
Methods for Isolate Melanoidins
Different methods have been employed to isolate and purify melanoidins from food products, for example, from coffee [9, 10, 16, 127], vinegar [173] bread [56], meat [136, 178] and dark beer [102] the chromatographic and electrophoretic methods have been used In coffee brew, a common method for melanoidins isolation is their dialysis Bekedam et al. [9] isolated the high and low molecular weight of coffee brew by membrane dialysis (MW cut-off 12–14 kDa), the ultrafiltration by the use of membranes with a molecular weight cut-off limit ranging from 1 to over 300 kDa in a model system [80]. Different types of column chromatography were employed for further melanoidin purification, such as gel filtration, anion-exchange chromatography, copper-chelating chromatography, Sephadex LH-20, or hydrophobic-type chromatography on octyl Sepharose [134]. Coffee melanoidins of different degrees of polysaccharide conjugation were successfully separated by their solubility in ethanol [20]. These intense studies have provided a set of facts concerning the structure and composition of coffee melanoidins; Bekedam et al. [10] and Nunes et al. [134] have established the presence of a polysaccharide backbone (galactomannans and arabinogalactans) to which phenolic compounds (hydroxycinnamtes) are covalently bound, together with proteins and amino acid-derived compounds. The origin of volatile compounds responsible for flavour is still relatively difficult to determine, due to their multiple origin. The interest shown by food industry stems from a desire to produce and control the characteristic aromas and colours obtained on cooking, baking and roasting. Once the analytical technique of combined gas-chromatography-mass spectrometry was developed for the separation and identification of relatively volatile substances. Most of the publications on foodstuff melanoidins dealt with specific food products. The fact that most attention was given to melanoidins in coffee brew can be attributed that coffee is a food product with high levels of water-soluble melanoidins which makes research less complicated [9].
Morales [124] isolated different water-soluble melanoidins from both heated (100 °C/24 h) carbohydrate/amino acid model systems and medium-roasted coffee. Capillary zone electrophoresis (50 mM sodium tetraborate, pH 9.3) He used to determine the saturated or aromatic character of the isolated melanoidins. Melanoidins obtained from different solutions after an equivalent heating treatment possess similar apparent molecular weight but different charge/mass ratio, suggesting differences in their degree of saturation. Melanoidins isolated from model systems containing lysine showed a lower saturation than those isolated from either coffee or other model systems containing glycine, alanine or tryptophan. He founded that depending on the type of amino acid as reactant, melanoidins could be mainly constituted by a stable structure or common core which is changing according to the thermal conditions applied through a more saturated structure.
Factors Affecting the Maillard Reaction
The non-enzymatic browning is influenced by many factors, including reactant concentration, time, initial pH, and water activity as well as the nature and the ratio of the reactants [118], chemical composition of the food system, but the most important factor on the velocity of the reaction is temperature [25]. Thermal process optimization during the heat processing of foods requires kinetic data on several quality related factors such as nutrients, colour, flavour, texture, etc. It may be desirable to prevent degradation of natural colours such as chlorophylls, carotenoids, etc., or the formation of colours during browning reactions [66].
Temperature and Heating Time
Temperature and duration of heating were studied by Maillard (1912) himself, who reported that the rate of the reaction increases with temperature [168]. An increase in temperature leads to an increase of the reactivity between the sugar and the amino group. The Arrhenius model has often been used to evaluate the dependence of the Maillard reaction rate on temperature: the activation energy of the reaction has been found within a range of 3–50 kcal/mol depending on reactants nature, moisture, pH [115] and on the specific indicator chosen to follow the reaction. The temperature dependence of a reaction rate constant k is often described by the well-known Arrhenius equation [84]:
where k is the rate constant; A the so-called frequency factor; E a the activation energy; R the gas constant (8.314 Jmol−1 K−1) and T is the absolute temperature (K).
Type of Sugar and Amino Acid
Browning rate is significantly influence by the type of reducing sugar involved in the reaction. The order of reactivity is follows: aldopentoses > aldohexoses > ketohexoses > disaccharides [137]. A terminal pyranose group at the C-4 position of the reducing end of disaccharides retarded further reactions of protein-disaccharide adducts. Generally, aldoses are intrinsically more reactive than ketoses [147]. There have been conflicting reports on relative reactivity of glucose compared with fructose. Jakas and Horvat [92] reported that glucose is more reactive than fructose but Knol et al. [95] reported that at high temperature and pH 5.5 fructose is more reactive than glucose.
Concentration of the Sugar and Amino Acid
The ratio of reducing sugar to amino acid has been suggested as being an important factor in determining the rate of Maillard browning and would have important implications from: a food formulation standpoint and in the case of in vivo glycation of proteins. The excess of reducing sugar over amino compound promotes the rate of Maillard browning [133]; there are mechanistic differences in the destruction of the sugar compared with the amino acid. Carabasa-Giribet and Ibarz-Ribas [27] reported that the increase in glucose concentration led to the decrease in induction time in the aqueous model systems containing glucose and aspartic acid or asparagine.
The Influence of pH
The non-enzymatic browning reaction of a model system is usually initiated in the neutral or weakly alkaline pH region and the pH is gradually decreased in the course of the reaction [103]. Since such a great decrease in pH is not common in food processing and preservation, it is necessary to investigate the effect of pH control on the intermediates and melanoidins in a model system in order to match the model melanoidin with food melanoidin. Just as for temperature, the reactivity of the sugar and amino group is also highly influenced by the pH [116]. The open chain form of the sugar and the unprotonated form of the amino group, considered to be the reactive forms, are favoured at higher pH dependence of the Maillard reaction can be related to the amount in protonated form of the amino group (the reactive form), which is favoured at high pH [164], as described in the following equation:
The amino group reacts with the group of the sugar through a nucleophilic attack. At basic conditions the nucleophile, that is, the amino group will be more likely deprotonated, and therefore more nucleophilic. However, under acidic conditions the amino group becomes a weaker nucleophile, which requires that the group be activated prior to the nucleophilic attack [115]. Ajandouz et al. [2] revealed that the pH in Maillard reaction, highly UV-absorbing and colourless compounds and brown polymer are formed at higher pH in the fructose-lysine aqueous model system. The lower the pH, the more protonated amino group is present in the equilibrium and therefore, less reactive with the sugar. In the case of a model system consisting of 2 mol/L glucose, 2 mol/L glycine, and 0.2 mol/L NaHCO3, the initial pH value of about 7.5 resulted in less than 4.0 after 4 h of reflux by heating [167].This decreased is considered attributable to reduction formation. Since such a great decrease in pH is not common in food processing and preservation, it is necessary to investigate the effect of pH control on the intermediates and melanoidins in a model system in order to match the model melanoidin with food melanoidin [100, 103].
Water Activity (aw)
Water activity has been used to assess microbial growth, lipid oxidation and non-enzymatic and enzymatic activity following the manufacture of food products. Water activity provides a general guideline for predicting the stability of food systems and is used extensively throughout the food industry as a quality and safety indicator. The effect of water on chemical reactions in foods, whether enzymatic or non-enzymatic, is difficult to attribute to a single mechanism because the reaction mechanisms are complex [94].
The measure of water activity (aw) is useful to describe a thermodynamic equilibrium state and for predicting reaction rate [159]. Browning rate is strongly influenced by environmental factors, such as temperature and water activity. With respect to moisture content, the accepted scenario is that the rate of browning increases from the dry state, starting at a critical aw of 0.2–0.3 for most foods, to a maximum at water activity of 0.5–0.8 and then decreases at higher water activities as a result of dilution of the reactant species [104]. Non-enzymatic browning (NEB) is a common mode of quality loss in low-moisture foods. At lower a w ’s, the reactions proceed more slowly, and this has been attributed to diffusional limitations. Changes in diffusion constants may in turn be related to glass transition [96, 97] studied the effect of glass transition on non-enzymatic browning of dehydrated vegetables and model systems (composed of amino acids and sugars reacting in matrices with different physical characteristics) Glass transition temperature (Tg) was determined by differential scanning calorimetry (DSC) technique. The rates of non-enzymatic browning for vegetables and for model systems were determined by measuring absorbance at 280 and 420 nm. Rate constants were analysed as a function of temperature (T) and of (T − Tg). Heat flow as a function of temperature is recorded when samples are heated at 5 °C/min. The results shows the DSC scans for selected systems at different aw’s. Browning below Tg was very slow. Changes in activation energy (which were affected by structural changes) were detected near the glass transition. In all of the systems Tg decreased with increasing aw and m (moisture content), reflecting the plasticizing effect of water [162]. They can also be shown at the same conditions of aw and m that crystallization temperatures decreased. Water plasticizes the systems, lowers the Tg and increases the mobility of reactants. Beyond lowering of the Tg there seem to exist additional effects of moisture on the chemistry of the browning reaction. They could also observed that browning reactions took place very slowly even below the glass transition temperature, when mobility of reactants is presumed to be very low. Many researches have been investigated the effect of aw in fruit juice. Vaikousi et al. [170]. studied the effect of aw (in the range of 0.74–0.99) and/or reactant concentration on brown pigment formation monitored under isothermal heat treatment at four temperatures (60, 70, 80 and 90 °C) in apple juice solutions having either the same or different concentrations of reactant solutes. The extent of the Maillard reaction was evaluated by spectrophotometric measurements at 420 nm (A420). The results showed that browning rates by the Arrhenius model was the most applicable for describing the temperature and water activity dependence of non-enzymatic browning reaction rate. Also Ibarz et al. [86]. studied the temperature influence on non-enzymatic browning kinetics and also the effect of temperature and water activity in the kinetic of non-enzymatic browning of peach juices, with different soluble solids contents at various temperatures the results shown that the browning developed in the juice fitted to a first-order kinetics. The obtained results shown that the values of the activation energy of the water of the juices decreases as the water activity of juices decreases, or what is the same with the soluble solids content increased. [77, 78].
Methods for Determination of Melanoidins
There are three procedures that are used to describe or measure the extent of melanoidin formation. First, the amount of melanoidins is determined by difference, which means that the amount of melanoidins is the percentage of compounds that can not be accounted in a food product (% of known compounds) [31, 32]. Second, the brownness can be quantified in order to measure the colour potency of MRPs [25, 73]. Third, to make possible to follow the Maillard reaction both in its early and final stages it is very important to find suitable indicators. Among the most commonly used indicators of the Maillard reaction are spectrophotometric measurements at 280 nm for pyrazine compounds and 420 nm for brown pigment detection, colorimetric evaluations and formation of 5-(hydroxymethyl)-2-furfural (HMF), which is an important intermediate formed in the early stage of non-enzymatic browning [26].
The main quality impact by which the consumers take the decision to acquire a product is its visual appearance. The colour of products can be specified by three coordinates in the colour space which can be obtained directly with a tristimulus colorimeter. The L*, a* and b* system is the more frequently used scale to measure the colour of food products [44]. Changes in food colour can be associated with its previous heat treatment. Various reactions such as pigment destruction (carotenoids and chlorophylls) and non-enzymatic browning (MR), can occur during heating of fruits and vegetables and therefore affect its colour [60]. The retention of total colour can be used as a quality indicator to evaluate the extent of deterioration due to thermal processing [88].
Several researchers have published work on modelling of thermal degradation kinetics of colour in the temperature range of sterilization conditions. CIELab, colour parameters have previously proved valuable in describing visual colour deterioration and providing useful information for quality control in fruits and fruit products [108, 179]. Colour deterioration during heating of pulp includes (a) non-enzymatic browning (NEB) reaction between reducing sugars and amino acids, which has usually been assumed to follow zero-order kinetics, as in the case of fruit juice and intermediate moisture fruit products and (b) destruction of natural fruit pigments, which degrade during heating and storage by following first-order reaction kinetics [85].
The most common method for characterizing browning is the measurement of colour development as a function of time and its expression in terms of kinetics of the reaction, which is described by the reaction rate constant. Many researchers have reported the appearance of brown pigments as following either zero-order or first-order kinetics [90].
These kinetic models are expressed by the equations:
where P is the value of the variable studied at time t, P 0 is the value of the variable studied at the initial time (t 0), k 0 is the zero-order kinetic constant and k 1 is the first-order kinetic constant [87]. It is not always possible to apply kinetics as simple as first-order or zero-order to describe the colour changes produced in fruit purees, since these changes can be due not only to the Maillard reaction but also to the thermal destruction of pigments present in the samples. Based on data for the deterioration produced by thermal treatments, a two stage mechanism is proposed. According to this combined kinetics, the non-enzymatic browning process can be expressed by the Eq. 5.
In the case of HMF, this can be formed in different ways: by hexose dehydration in an acid mean and by Maillard reaction from reducing sugars and free amino groups of amino acids. Acid dehydration of hexoses is catalysed by the same HMF, and because of this the HMF formation reaction can be considered second order auto-catalytic [60] which can express the global reaction by:
where C HMF is a HMF concentration for a given time, C 0 is a total initial concentration, initial hexose concentration (fructose or glucose) + initial HMF concentration and \( C_{\text{HMF}}^{ 0} \) is a initial HMF concentration. In the case where C 0 is much larger than \( C_{\text{HMF}}^{0} \), the hexose concentration could be included in the reaction rate constant and would result in an expression corresponding to first-order kinetics [76]. Determination of the reaction kinetics and mathematical modelling systems are very important for estimating the quality criteria, like HMF [113].
Shen and Wu [152] studied the Maillard reaction in ethanol model solution and found that ethanol accelerated the rate of browning reaction. They founded that the Maillard browning and HMF in ethanolic solutions of a 0.2 M glucose 0.2 M glycine (G–G) system in a heat treatment increase with an increase in ethanol concentration within (0–50 %, v/v).The results indicate that the mechanisms of the Maillard reaction in ethanolic solution and aqueous solution are not the same. A higher hydroxymethylfurfural (HMF) content is present in the ethanolic G–G system, suggesting the involvement of ethanol in the G–G reaction to form HMF. They also found that ethanol inhibits the formation of HMF in an ethanolic solution containing glucose alone. HMF is formed in the pH 4.3 buffered G–G system in an ethanolic solution at a water activity (aw) above 0.81 or at an ethanol concentration below 30 % than in a glycerolic solution at the same water activity. Shen et al. [157] further conducted purification analysis and confirmed that MRPs produced in an ethanolic system were different from those produced in an aqueous system.
Molecular Weight of Melanoidins
Melanoidins produced in the Maillard reactions between proteins and sugars in real foods are predominantly HMW compounds. Considering that the molecular weight of melanoidins produced from MRPs is highly dependent on the heating intensity, and HMW melanoidins are produced at longer reaction times (>24 h), it is possible that in the initial stages of the MR, low molecular weight chromophoric melanoidins are formed, which subsequently polymerize or cross-link with other MRPs to produce HMW melanoidins during the later stages of the MR [74].
In real foods, most of the melanoidins have been shown to be HMW compounds [3]. For example, the browning intensity of the ethanol extracts of bread crust increased with increasing molecular weight upon heating, demonstrating that the browning is due in large part to HMW melanoidins. In coffee, 59 % of the melanoidins are HMW (>12–14 kDa). It has been found that prolonged roasting leads predominantly to the formation of HMW melanoidins [8], which show a more intense brown colour than the LMW melanoidins [9]. Similarly, polymers of HMW melanoidins isolated from sweet wines (>12 kDa) [146], roasted malt (>60 kDa) [52] and roasted cocoa beans (>5 kDa) [165], have been shown to give rise to the brown colour of these foodstuffs.
Ibarz et al. [80] obtained from melanoidins fractions of glucose/asparagine model system > 300 kDa (32.04 %), 300–150 kDa (31.90 %), 150–50 kDa (14.28 %), 50–15 (6.80 %), 15–8 (11.86 %) and 8–1 kDa (3.12 %). The 60 % percentage of the obtained melanoidins fractions, are over than 150 kDa molecular weight. There is some controversy over the size of reactive compounds involved (low versus high molecular weight melanoidin pigments) [39, 149].
Martins et al. [117] obtained the molar extinction coefficient averaged for glucose/glycine HMW melanoidins of 0.64 ± 0.031 mmol−1cm−1 at 470 nm. They observed that the chromophores produced in the non-dialysable melanoidins in the early stages of the reaction are similar to those at later stages, independently of the reaction conditions.
Leong [106] showed that at 470 nm the extinction for HMW and LMW glucose/glycine melanoidins was the same. However, the extinction coefficient that they reported was considerably higher for glucose/glycine systems, namely 0.94 l mmol−1cm−1 at 470 nm and it varied according to the type of amino acid.
Non-Enzymatic Browning in Fruit Processing
During processing and preservation of food products often heat treatments (cooking, baking, roasting, frying, sterilization) at temperatures between 90 and 220 °C are applied. One of the main reactions occurring at these temperatures is the Maillard reaction, a Non-enzymatic browning (NEB), reaction with positive as well as negative side effects on the quality of the product (colour, flavour, stability, nutritional value) [43]. Fruit juices, in particular, exhibit that effect during manufacturing and storage [82, 84]. It not only gives the sensorial characteristics (taste and aroma) but also causes the loss of nutrients and the formation of intermediate undesirable compounds, like furfural and 5-hydroxymetilfurfural [21]. According to the International Federation of Fruit Juice Producers (IFFJP) [91], an HMF content of 5 mg/L for juices and 25 mg/kg for concentrates should not be exceeded. Higher values indicate that the product has been overheated [88].
During the manufacturing process and storage of fruit juices heating processes can affect the quality of fruit juice concentrates, which leads to consumer dissatisfaction, changes take place in the structure of derivative fruit products, and thermal treatments during preservation processes can affect the quality of juices and fruit purees through non-enzymatic browning reactions, and loss of volatile compounds, destruction of vitamins and amino acids, hydrolysis of carbohydrates, development of undesirable odours and tastes and browning reactions can occur [61].
Multiple-effect evaporators were designed to concentrate juice at reduced temperatures but, in practice, temperatures become very high in the first effects and hydroxymethyl-furfural (HMF) formation can reach very high values [109, 110]. HMF is an important intermediate in non-enzymatic browning (NEB) reactions and its production during processing may accelerate browning during storage. Fruit juice concentrates containing more than 65 % total solids are normally stable from the standpoint of fermentation at any temperature but, when stored at relatively high temperatures, NEB reactions occur [108]. Non-enzymatic browning via Maillard reactions between reducing sugars and amino acids is the major route of colour formation in apple juice concentrate during processing and storage. These reactions involved the formation of reactive intermediates by a variety of pathways and these can yield both volatile flavour components and brown melanoidins of HMW [6].
Ibarz et al. [85] observed that the presence of d-galacturonic acid (AGA) increases the browning of juices. They showed the effect of (AGA) on the non-enzymatic browning of three types of clarified concentrated fruit juices (apple, pear and peach). This was evaluated by the evolution of the absorbance at 420 nm (A420) with the treatment time that these juices show. The effect of the temperature treatment on the non-enzymatic browning of these juices was quantified by fitting the variation of the kinetic constants with the Arrehnius equation, thus obtaining the corresponding activation energies. In the case of apple juice, it was observed that for the increase in AGA content the activation energy increases considerably, while for the pear and peach juices, a slight increase of the activation energy was observed with the increase in AGA.
Control of Non-enzimatic Browning in Fruit Juices
Melanoidins and polyphenols have been found to be responsible for physico-chemical deterioration of some fruit juices because they are relatively small molecules that can easily pass through the membranes during ultrafiltration processing. In order to prevent fruit juices from browning and haze formation, a reduction of melanoidins and phenolics is necessary [15, 116].
Few publications concerning the application of membrane separation for remediation of browned juices have been found. Carrin et al. [28] studied the de-coloration of browned apple and grape juice by physical separation of melanoidins with combined use of ultrafiltration (UF) and nanofiltration (NF) membranes. Proposed actions were focused to reduce decoloration agents addition to juice, in accordance with current trends in food processing.
Non-enzymatic browning (NEB) in fruit products can be inhibited or reduced by refrigeration and by the control of water activity in dehydrated fruits [104]. Two basic treatments have been used for the control or reduction of non-enzymatic reactions: the reduction of amino nitrogen in juices and the use of different chemical inhibitors [108].
The search for effective and safe inhibitors of NEB is driven by the need to: (a) prevent undesirable Maillard reactions, and (b) find alternatives to the use of sulphites [44]. The use of sulphites is restricted to certain categories of food products. Because of these restrictions, food processors have turned to a number of sulphite alternatives. Many alternative chemicals have emerged to control browning as formulations of ascorbic acid, erythrobic acid, ascorbicacid-2-phosphate, cinnamic acid, benzoic acid and chelating agents [7].
Concentration of clarified fruit juice by evaporation ideally reduces costs and increases shelf-life by removing water without changing the solid composition. A number of agents including, gelatine, bentonite, activated carbon, casein, ion-exchange waxes, resins and polyvinylpolypyrrolidone (PVPP), have been studied for the removal of polyphenols from fruit juices. The use of adsorbent resins for clear juice stabilization has been introduced as a final treatment after clarification [28]. However, these are all used in batch processes, which lead to additional costs in the existing processing line.
Ibarz et al. [81] showed the effect of elimination of the coloured compounds produced in the browning reactions in a peach juice by means of the use of an adsorbent resin. It shows the adsorption isotherms at different temperatures applying the Langmuir and Freundlich models, and the adsorption efficiency was evaluated for different concentrations of the resin. Also Ibarz et al. [79] studied the elimination of colour compounds (melanoidins) when they have been undesirably produced during the browning reaction of apple juice with a new adsorbent resin Lewatit® S 4528. Results showed that the adsorption kinetic constant was always higher than the adsorption kinetic constant, which indicates that the adsorption stage predominates the adsorption stage. The evolution of the a* and b* parameters shows a colour reduction, which continued for the b* parameter but limited for the a* parameter, which is globally appreciated as a reduction of the reddish colours.
Many of the concentrated juices are clarified juices containing only water and soluble solids. In the conventional manufacturing process of this type of juice, there is a clarification stage where the insoluble solids are removed [45]. To improve the colour of a browned clarified peach juice, an adsorption treatment using different kinds of activated carbon was investigated [25].
The Photochemical destruction of colour compounds in fruit juices is the other method to removed enzymatic and non-enzymatic browning in fruit juices. Few works have been published concerning of the effect in fruit juices irradiation. The heat-treated juices tend to change colour and lose some of its aromas and vitamins during heating process, while the juices treated with Ultra Violet (UV) radiation tend to maintain its aroma and colour [55]. Ibarz et al. [89] irradiated apple, peach and lemon juices with different soluble solids content and different browning degrees were irradiated using a lamp that emits in the ranges 250–650 nm, studying the effect on CIELab colorimetric parameters. The evolution of the a* and b* CIELab parameters, which was an indication that the pigments and compounds that give brown coloration (melanoidins) were destroyed in this type of treatment. It is important to avoid the formation of melanoidins, in processing and in storage of the juice. However, once they are in the juice it would be desirable to seek methods to eliminate these compounds from the samples. An alternative may be found in the use of UV radiation. In this way, Kwak et al. [103] studied the effect of reaction pH on the photodegradation of model melanoidins. The Cu2+/O2 and ascorbic acid/Cu2+/O2 systems were used for generating active oxygen species, using a Xe lamp that emits in the range of 200–1,000 nm, and a halogen-tungsten lamp emitting in the range of 350–1,000 nm. They found that Photodegradation of NaHCO3-melanoidins in the Cu2+/O2 system by the continuous supply of oxygen resulted in an increased decolorization rate, decreased molecular mass, production of low molecular weight compounds, release of free lysine, and pH change. The buffer- and Cu2+-melanoidins did not show changes in chemical characteristics similar to those of the NaHCO3-melanoidin.
Structure of Advanced MRPs and their Pathological Role
In recent years, several studies have mainly been focused on the effect of melanoidins on the human diet, and their feasible nutritional, biological, and health implications [4, 5, 93]. But results in the literature are difficult to compare, since models do not resemble food matrices or processing conditions at industry, so the research could not show whether melanoidin or the last stage of Maillard reaction are responsible for the studied effects [124].
The deleterious effects of dietary MRPs on human health are still unclear, except that it is well-known that MRPs intake promotes oxidative stress and contributes to diabetes and the evolution of atherosclerosis and other age-related diseases [156]. For this reason studies on MRPs absorption and metabolism with realistic designs and concentrations of the compounds are a necessary step to establish the effects of their consumption, and its relation with the endogenously formed Maillard compounds, also called advanced glycation end-products (AGEs) [161]. AGEs are complex, heterogenous molecules that cause protein cross-linking, exhibit browning and generate fluorescence. Not all AGEs have been identified and the mechanisms underlying their formation remain unclear. Given their slow formation, it was believed that only long-lived extracellular proteins accumulate AGEs, although it is now known that they can form on short-lived molecules and even intracellular growth factors [29]. Some AGEs are exogenous, being derived from foods or even tobacco [63], although their significance in diabetic pathology remains unclear. Over a dozen AGEs have been detected in tissues and can be divided into three categories: (a) fluorescent cross-linking AGEs such as pentosidine and crossline. (b) Non-fluorescent cross-linking AGEs such as imidazolium dilysine cross-links, alkyl formyl glycosyl pyrrole (AFGP) cross-links and arginine- lysine imidazole (ALI) cross-links and (c) non-cross-linking AGEs such as pyrraline and N-(carboxymethyl)lysine (CML) [130].
The traditional scheme of the Maillard reaction implicates Amadori products, the major products resulting from the condensation of glucose with primary amines, as the sole major precursor of advanced glycation end-products (AGEs) [122]. This view was challenged when Hayashi and Namiki [68] discovered that the Schiff base adduct could undergo fragmentation to form glyoxal, C-3 fragmentation products and fairly stable pyrazine radicals. Structure elucidation and kinetic studies on the formation of advanced Maillard reaction products have shown that pyrraline undergoes further modifications in vivo, especially in the presence of sulphydryl agents such as cysteine. The biological significance of this modification remains to be assessed. Intracellular glycation and glycoxidation products do not follow an expected pattern as a function of the degree of hyperglycaemia, as revealed in experimental animals [121].
Pyrraline may contribute to diabetic microvascular extracellular damage caused by cross-linking of the microvascular extracellular matrix because it has recently been proved [111] to be a crosslinking agent, particularly with cysteine residues. Although glycaemic control has been shown to affect only modestly the concentration of advanced MRPs in the extracellular matrix. Portero-Otín et al. [142] showed that it clearly influences advanced Maillard reaction in vivo in a more dynamic compartment such as plasma/urine. Thus, metabolic control may exert beneficial effects on the progression of long-term diabetes complications through modification of circulating, cross-link precursors, that is, advanced MRPs such as pyrraline.
Dietary MRPs and Melanoidins in the Intestine
Significant quantities of dietary MRPs and melanoidins enter the human intestines every day, but scarce work have been published about their metabolism therein. Neither the effects of digestive enzymes in the small intestines nor those of the bacteria in the large bowel on melanoidin degradation have been described yet. Ames et al. [3] studied the effects of faecal bacteria were also determined, in batch culture, with a combination of phenotypic and genotypic (probes) criteria being used to identify the microbial diversity involved. After a maximum time of 24 h of incubation, the total number of anaerobes, bacteroides, clostridia, and bifidobacteria increased in a batch culture fermenter containing human faecal bacteria. Simulation of peptic and pancreatic digestion showed that the melanoidins did not produce detectable amounts of low molecular mass degradation products. However, melanoidins affected the growth of gut bacteria during mixed culture growth. The effect was to cause a non-specific increase in the anaerobic bacteria enumerated. This in vitro study indicates that melanoidins can affect the growth of human large bowel bacteria and serves to demonstrate possible effects that may occur in vivo. Given the large and varied number of food items that contain MRPs, this may have relevance for lower-gut health.
Microbial degradation of melanoidins isolated from a gluten-glucose mixture heated in water for 1 h was also indicated by the stimulated growth of bifidobacteria and by the production of short chain fatty acids in a fermenter containing human faecal bacteria [42]. As the stimulated growth of bifidobacteria was inhibited when gluten-glucose mixtures prepared by 2 or 3 h heating times were tested, it might be speculated that the microbial degradation is limited to less complex melanoidin structures, which are preferentially formed in the presence of water than under roasting conditions and during shorter heating times rather than during longer heat treatment at comparable temperatures [163].
Metabolic transit data on food advanced MRPs (melanoidins), has not yet completed elucidated and it is still under investigation an open question whether isolated melanoidin structures undergo metabolic biotransformation and subsequently cause physiological effects in vivo [41]. Advanced MRPs, acting as premelanoidins, and melanoidins are formed under severe heat treatment of foods and are ingested with the habitual diet at considerable amounts. Metabolic transit data are known for Amadori compounds classified as early MRPs, like, for example, fructose-lysine. For rats and humans, the percentages of ingested free versus protein-bound fructose-lysine excreted in the urine were found within ranges of 60–80 and 3–10 %, respectively. Homma and Fujimaki [75] investigated the metabolic fate of a 15 N-labelled non-dialysable, high molecular weight (HMW) melanoidin fraction in rats. Balance studies on free advanced MRPs are still lacking, but protein-bound LMW premelanoidins and HMW melanoidins have already been investigated in animal experiments using 14C-tracer isotopes.
The amount of ingested radioactivity absorbed and excreted in the urine was found at levels ranging from 16 to 30 % and from 1 to 5 % for premelanoidins and melanoidins, respectively [51]. These different metabolic transit data of premelanoidins and melanoidins can be explained by the following mechanisms involved: (a) intestinal degradation by digestive and microbial enzymes; (b) absorption of these compounds or their degradates, and (c) tissue retention. Structure-specific in vivo effects have been identified for protein-bound premelanoidins on intestinal microbial activity, xenobiotic biotransformation enzymes and further glycation reactions. The latter are hypothesized to be involved in the ageing process and in the course of different diseases. Further investigations are needed to clarify synergistic in vivo effects of dietary ingested melanoidins and endogenously formed glycation products.
Most studies have been developed in rats using model systems that have exaggerated processed conditions in order to amplify the significant effects. Thus, after administration of doses of 110 or 310 mg per kg body weight of the glycation end-products (AGEs) N-(carboxymethyl)lysine (CML), in the form of heated casein to rats, animals eliminated in urine a 26 % and a 29 % of the dose and in faeces about 15 and 22 %, respectively. Between 1.5 and 1.7 % was accumulated in the circulation, liver, and kidney [54]. Other studies have shown urinary excretion of CML in the range of 4–19 % of that which has been ingested [107]. It has to be mentioned that there are very few human assays and those that exist are usually not consistent with data obtained in rats. Among the few that there are, most are focused on the effects of the ingestion of a concrete compound generated in conditions that are not very common in a diet. It has been well-established that complex foods used in daily life are more heavily modified than the single-protein preparations used in many studies [171]. Moreover, the MRPs absorption and metabolism seem to depend on the individual chemical structure and the way they are bound to proteins [161].
N-(carboxymethyl)lysine (CML) as one of the most biologically active MRPs and its faecal and urinary excretion in a sample of healthy male adolescents consuming the kind of a MRPs high diet, which is usually ingested by this section of the population, compared with the consumption of a MRPs-low diet. Delgado-Andrade et al. [40] studied the effects of a high and low MRPs diet on N-(carboxymethyl) lysine (CML) intake and excretion in 11–14 years adolescent males. They founded that CML absorption and faecal excretion were highly influenced by dietary CML levels. Since the compound has long-term effects on health, an excessive intake deserves attention, especially in a population nutritionally at risk as adolescents. The consumption of the MRP-high diet led to a higher CML input (P > 0.05) (11.28 vs. 5.36 mg/day CML for MRP-high and MRP-low diet, respectively). In parallel, the faecal excretion was also greater (P > 0.05) (3.52 vs. 1.23 mg/day CML, respectively) and proportional to the dietary intake. The urinary elimination of CML was not increased significantly when the MRP-high diet was consumed compared with consumption of the MRPs-low diet and was not proportional to the dietary exposure of CML. They sowed that CML absorption and faecal excretion were highly influenced by dietary CML levels. Since the compound has long-term effects on health, an excessive intake deserves attention, especially in a population nutritionally at risk as adolescents.
Studies using rats indicated that only a small proportion of melanoidins prepared from glucose and glycine (or lysine) were absorbed through the gut wall, most being excreted in faeces [4]. However, 90 day toxicity studies of Class I and Class IV caramels using rats resulted in discolouration of the mesenteric lymph nodes that was attributed to the intestinal absorption of high molecular mass caramel components [133].
Investigations involving melanoidins and micro-organisms are focused on (a) the potential of melanoidins to affect the growth of bacteria, including the possible extension of food shelf-life, (b) the ability of micro-organisms to degrade or decolorize melanoidins and, (c) the effect of melanoidins on gut microflora composition [4]. There is very little information on microbial interactions between human colonic bacteria and Maillard reaction products.
The colon is the most metabolically active site in the human body. This is because of the resident microbiota, which comprises about 1014 prokaryote cells in total [154]. The nature of the gut fermentation may impact heavily on host health and welfare and the gut flora is thought to play a central role in homoeostasis, digestion and the prevention of diseases, such as acute gastroenteritis and bowel cancer [58].
Melanoidins as Chelating Agents and Their Antioxidant Activity
Melanoidins are likely to play an important role in the binding of nutritionally important metals. The chelating properties towards metal ions also contribute to the antioxidant and antimicrobial properties of melanoidins in foods [150] and in vivo [166]. Also, the chelating ability of melanoidins is relevant from technological, nutritional and physiological points of view [127, 182].
Literature data relevant that the formation of compounds with antioxidant activity mainly concern the development of the Maillard reaction. In the last years great efforts have been made to detect Maillard reaction rates and the formation of compounds with antioxidant capacity. The majority of these studies was carried out on model systems. Melanoidins are one of the major components of coffee beverages, accounting for up to 25 % of dry matter [16]. Morales and Babbel [125] indicated that the antioxidant capacity is a result of intermediate and low molecular weight (LMW) MRPs and these effects are the subject of considerable research from the view point of preservation of foodstuffs. In any case, the mechanism of the antioxidant effect of melanoidins is still unclear because the chemical structure of melanoidins is unknown. It is assumed that the mechanism is based on the ability of melanoidins to trap positively charged electrophilic species, scavenge oxygen radicals, or carry out metal chelation to form inactive complexes [39]. The formation of d-amino acids in many foods of plant and animal origin are the results of non-enzymatic browning since the presence of amino acids together. As for the racemization mechanism, it is postulated that the reaction of amino acids with glucose or fructose starts with the reversible formation of Schiff bases. The degree of racemization depends in particular on steric and electronic properties of the amino acid side chains. It should be noted that the early stages of the MR proceeds already under mild conditions [19] and do not require alkaline or acidic condition. This new racemization mechanism based on the relatively stable Amadori compounds has been used to explain the generation of free d-amino acid in foods such as dried fruits, concentrated fruit juices and fortified wines [141]. Recently, heating experiments of synthetic Amadori compounds proved that they are sources of amino acid-enantiomers [100].
Several methods have been developed to evaluate global antioxidant capacity; they are usually based on the evaluation of the free radical scavenging capacity. The most commonly used are based on molecular absorption spectrophotometry UV–VIS (MAS) due to its simplicity to handle and low cost. They are indirect methods, based on the pre-formation of a free radical from an aromatic organic compound, such as 2,20 -azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS), the free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and the free radical N, N-dimethyl-p-phenylenediamine (DMPD) Other methods based on electrochemical techniques [131] and on the determination of the lag time by electron spin resonance (ESR) [11] have been used.
Wang et al. [174] found that the fructose-amino acid model MRPs showed higher DPPH and ABTS radical scavenging and ACE inhibitory activities than the glucose–amino acid model MRPs. The a-glucosidase inhibitory effect of MRPs derived from fructose and glucose/tyrosine showed higher a-glucosidase inhibitory activity than that of other MRPs. Sugar-amino acid model MRPs inhibited the growth of HCT116 colon cancer cell in a dose-dependent manner (from 0.5 to 1.5 mg/ml). Glucose MRPs showed slightly higher antiproliferative activity than fructose MRPs. In particular, sugar tryptophan and tyrosine MRPs exerted higher biological activities than the other MRPs.
Antimicrobial
The antioxidant activity of melanoidins results especially interesting since these products are naturally formed during the food most investigations focused on the effect of MRPs on microorganisms have been done in specific microbial growth media, which show that MRPs can stimulate microbial growth or inhibit it [47]. The most extensive study on the influence of MRPs on microbial growth was performed by Einarsson et al. [46]. These authors found that MRPs have a bacteriostatic activity that depends on the type and concentration of MRPs, culture media pH and temperature, and on the molecular weight (MW) of the MRPs. In this way, the products with an MW higher than 1,000 Da exert higher activity than those with LMW. In addition, the studies mentioned found that the antimicrobial action of the MRPs was mediated by lowering iron solubility so that there is a decrease in glucose and oxygen uptake. Rufián-Henares and Morales [150] studied the antimicrobial activity of different melanoidins obtained from glucose-amino acid model systems. Found that glucose-lysine melanoidin exerted the highest antimicrobial activity, being at a concentration of 1 mg/mL, equivalent to an oxytetracyclin solution of 170 mg/L.
The results in the literature seem to indicate that the antimicrobial activity of melanoidins could be related to their anionic charge and their ability to chelate some cations such as Fe, Zn, and Cu, which are essential for the growth and survival of pathogenic bacteria. Also that the anionic behaviour of melanoidins allows the disruption of Escherichia coli membrane by chelating the stabilizing cation Mg2+ [175].
Antimicrobial activity was determined to be bacteriostatic at low concentrations of melanoidins, but bactericidal at higher concentrations [150]. The dependence of the microbicidal mechanism on the melanoidin concentration could be related to their metal-chelating properties. Three different mechanisms for the antimicrobial activity of melanoidins have been proposed. At low concentrations, melanoidins exert a bacteriostatic activity mainly mediated by the chelation of iron from the culture medium. In bacterial strains able to produce siderophores for iron acquisition, the chelation of the siderophore-Fe3+ complex by melanoidins has been observed, which could decrease the virulence of pathogenic bacteria. Finally, melanoidins at high concentrations can disrupt both the outer and inner membranes by chelating Mg2+ ions from the outer membrane, leading to a destabilization of the inner membrane [149]. The higher antimicrobial activity exhibited towards gram-positive microorganisms was ascribed to the absence of a protective outer membrane, which makes them more susceptible to antimicrobial substances [150]. Antimicrobial activity of Maillard compounds exerts its biological activity by decreasing the microbial growth (bacteriostatic activity) [153]. In addition, it has been found that whereas the main part of the antimicrobial activity of food-derived melanoidins is a direct action of the melanoidin core, in the case of model system melanoidins the antimicrobial action falls on the low molecular weigh (LMW) Maillard-derived compounds linked with the melanoidin core. The high antimicrobial activity of food-derived melanoidins could be explained in part tanking into account that these melanoidins are produced within a complex reaction media with a great mixture of amino acids, sugar and proteins, giving rise to polymers with higher complexity. Similar results have been obtained by Del Castillo et al. [38] in gluten-glucose model systems where the antimicrobial activity of melanoidins was higher than that of LMW compounds [152].
Antibacterial Activity and Prebiotic Activity
The first study showing the inhibitory mechanism of antibacterial MRPs was run by Einarsson et al. [47] using the Salmonella mutagenic test system. They showed that the MRPs tested had no mutagenic effect and that their antibacterial effect was primarily due to the interaction between MRPs and iron, resulting in reduced oxygen uptake. Recently, Rufian-Henares and De la Cueva [150]. tested the ability of coffee melanoidins against the growth of different pathogenic bacteria. They found that at low concentrations melanoidins exerted a bacteriostatic activity mediated by iron chelation from the culture medium, whereas at high concentrations they exerted a bactericidal activity by removing Mg2+ cations from the outer membrane, promoting the disruption of the cell membrane and allowing the release of intracellular molecules. Also different molecular weight fractions isolated from roasted cocoa have been tested for their antibacterial effects [165]. All fractions reduced the growth of pathogenic bacteria; however, the authors highlighted that the growth of bifidobacteria was also inhibited. Hiramoto et al. [71] determined the inhibitory activity of a variety of melanoidins on urease-gastric mucin adhesion. H. pylori infection is the primary cause for peptic ulcer and gastric cancer, and its colonization in the stomach is suppressed by anionic polymers such as food melanoidins. Extracellular urease proteins located on the surface of Helicobacter pylori are gastric mucin-targeted adhesins, which play an important role in infection and colonization to the host. In addition, they have determined the anti-colonization effect of melanoidins prepared by heating casein and lactose, in an animal model and in human subjects infected with this bacterium. They concluded that melanoidins might be an alternative to antibiotic-based therapy to prevent H. pylori growth that combines safety, ease of administration and efficacy. Data demonstrated that the more severe the thermal treatment the higher the antibacterial activity, suggesting that melanoidins play an important role.
Coffee melanoidins behave as a soluble dietary fibre since they are fermented by the gut microflora [145]. High amounts of acetate and propionate were produced after microbial degradation of HMW components from coffee. It can be hypothesized that polysaccharide-rich melanoidins such as those present in coffee are preferentially metabolized by bifidobacteria, whereas protein-rich melanoidins, as obtained by protein glucose mixtures or milk-like systems, are good substrates for protein metabolizing bacteria predominantly present in the descending tract of the colon [64]. The Bread melanoidins are constituted by LMW, coloured compounds linked with the gluten polymer. Borrelli and Fogliano [17] studied melanoidins from different bread types were investigated for their potential prebiotic activity by a static batch culture. Results showed that anaerobic bacteria, particularly bifidobacteria strains, are able to use bread melanoidins as carbon source. The bacterial growth is different for the various types of melanoidins samples indicating that starting materials and processing conditions have a strong influence on the prebiotic potential of bread melanoidins. In all cases the bacterial growth obtained using bread melanoidins is lower than that previously observed using melanoidins from other sources, such as coffee silverskin. However, an in vitro study [4, 174] investigating the effects of the melanoidin fractions from glucose-lysine MRPs (refluxing at pH 5 for 2 h) on colonic bacteria demonstrated that melanoidins could cause a non-specific increase in all anaerobic bacteria of the human large bowel, including bacteroides, clostridia, bifidobacteria and lactobacilli. Thus, it may be difficult to ascribe prebiotic effects to melanoidins.
Antihypertensive Activity
The potential antihypertensive activity of pure melanoidins isolated from coffee, beer, sweet wine [150], and purified model melanoidins [149] has been evaluated by investigating the in vitro ACE inhibitory activity. The in vitro ACE inhibitory activity of food melanoidins was shown to be similar to that reported for wellknown antihypertensive peptides [152]. The ACE inhibitory activity of coffee melanoidins was significantly higher with coffee that had been heated for prolonged periods of time, which indicated that the melanoidins are likely responsible for this activity, though the ACE inhibitory activity has also been linked with the presence of bounded melanoidin compounds [151]. Rufian-Henares and Morales [153] also demonstrated that the in vitro ACE inhibitory activity of pure melanoidins extracted from aqueous glucose-amino acids systems (100 °C, 24 h, pH 7) was related to melanoidin structure, and the chromophores of melanoidins were not involved.
Genotoxicity of Maillard Reaction Products
The methods to identify and quantify mutagenic cooked food mutagens face many problems such as time-consuming analytical procedures, requirements of large amounts of samples and difficulties quantitation [132]. Isolation and characterization of mutagens in food systems were initiated in the late 1970s when Kasai et al. [98] identified three potent mutagens from broiled sardines and beef. Advances in column and detection technology and high-performance liquid chromatography/mass spectrometry (HPLC/MS) in conjunction with nuclear magnetic resonance (NMR) and (UV) methods stimulated the isolation and identification of mutagens, such as heterocyclic aromatic amines from model and real food samples.
Heterocyclic amines (HCA) are produced during high-temperature cooking of foods, as part of the Maillard reaction. These compounds pose relative high carcinogenic risks for the human digestive system and respiratory tract [93, 101]. HCA can be classified into IQ-type and non-IQ-type compounds by their structure. IQ-type HCA has a 2-aminoimidazole moiety as a common structure and includes aminoimidazoquinoline, aminoimidazoquinoxaline and aminoimidazopyridine compounds. The imidazole moiety is derived from creatinine in raw meat and fish, and other parts of IQ-type compounds are from amino acids and sugars. Non-IQ-type HCA have a 2-aminopyridine moiety as a common structure, and include aminopyridoindole and aminopyridoimidazole compounds. The aminopyridoindole is formed from the interaction of Maillard reaction products (MRP) with intermediates from the Strecker degradation products of tryptophan, while the aminopyridoimidazoles are produced by glutamic acid at temperatures above 250 °C [126]. A mixtures of creatinine, glucose, and various single amino acids as an aqueous model system to simulate browning meats. Heating of amino acids and glucose in a water solvent system produces substantial amounts of mutagens only at alkaline pH (10.5). Positive response was demonstrated with the Salmonella tester. When creatine was added to this system prior to heating, mutagens were formed at all pHs tested (pH 4, 7, 9 and 10.5) and highest mutagenic activity was recorded at pH 9.0 and pH 7.0. The amount of mutagen produced was dependent on the creatine concentrations [95].
Wines and/or liquors have been widely used to prepare meals, but there is limited information about whether the Maillard reaction in the ethanolic solution occurs to the same extent as in an aqueous solution. Wu et al. [177] studied the formation of mutagenic IQ-type compounds using a creatinine/glucose/threonine ethanolic model system. The carcinogenic IQ and IQx compounds were determined in ethanolic solution and compared with those generated from aqueous solution. They showed that ethanol accelerates Maillard reaction in the model solution. The results suggest an increase in ethanol concentration favours the formation of IQ and IQx mutagens when cooking meat, fish, or chicken with potable alcohol or distilled spirit. IQ-type compounds have been categorized as probably carcinogenic to humans. They can reduce intake of HCA in our diet by decreasing the extent of the Maillard reaction in daily cooking, and avoiding high concentrations of ethanol as an ingredient in cooked food.
Gazzani et al. [62] tested mutagenic activity of Maillard browning products from ribose-lysine and glucose-lysine mixtures, heated at l00 °C, was evaluated with the Salmonella assay. For both mixtures optimal conditions for producing mutagenic derivatives they observed at equimolar concentrations of reactants. Ribose-lysine browning products showed a higher mutagenic activity than the glucose-lysine mixture and were more active in inducing gene conversion and mitotic crossing-over in Saccharornyces cerevisiae. The higher mutagenic activity of the browning products of ribose was also demonstrated with several amino acids: alanine, aspargine, aspartic acid, cysteine, cystine, leucine, methionine, phen-ylalanine, proline, hydroxyproline, serine, valine. A DNA-damaging activity of the browning mixtures of ribose with histidine, proline, hydroxyproline, valine, was also detected in the Bacillus subtilis rec-assay.
Genotoxicity Testing
The mutagenic activity of foods and food components as well as other mutagens have been studied in a number of test systems using bacteria (Salmonella typhimurium, Escherichia coli), fungi (Saccharomyces cerevisiae), insects (Drosophila melanogaster) and mammalian species in vitro (Chinese hamster ovary cells) and in vivo (mouse). The three mayor mutagenicity test systems are gene mutations, chromosomal damage and DNA damage/repair [5] All three test system have been used in evaluating MRPs, but the Salmonella/mutagenicity assay has been the commonly used method to determine gene mutation [4].
Mutagenics Studies on Fruit Juices
Ekasari et al. [49] studied and found mutagenic activity in fresh orange juice heated at 93 °C for 30 min in S. typhimurium strain TA100. Fresh-pressed orange juice possessed no mutagenic properties, the mutagenicity being induced by the heat treatment. These investigators proposed that the mutagenic response is due to Maillard browning. In later studies, Eksaki et al. [48] attempted to characterize the mutagenic compounds in the heated orange juice using ion-exchange chromatography and solvent extraction. Their results showed that these mutagens were neutral, polar and non-volatile compounds of molecular weights less than 700 Da. Analysis by fast atom bombardment mass spectrometry was used to determine the molecular weights of the mutagens at 162, 180, 254, 288, 342 and 540 Da [50] the elemental compositions of some of these compounds were determined to be C6H12O6, C9H18O11, C11H20O13, and C11H22O15. In another study, Friedman et al. [59] studied the mutagenic responses of fresh and heated orange, apple, grape and pear juices in S. typhimurium strains TA98, TA100, TA102 and TA2637 with and without S9 activation. Results show that fresh orange juice yielded revertant frequencies 2–3 times greater than the water controls, but the mutagenic levels of the heated juices were the same as the fresh juices.
Inhibitory Effect of Melanoidins
Trypsin is an enzyme with an activity influenced negatively by physical parameters (temperature and pH), by conformational changes (denaturalization), by chemical modifications (substitution of amino acid residues and reduction of disulphide bridges) or due to specific interactions with polypeptide inhibitors. These molecules, by the formation of complex enzyme inhibitor, allow the interactions enzyme-substrate during the catalysis and its mechanism to be known [140].
Hirano et al. [72] studied the effect of the melanoidin obtained from d-glucose and lysine using Na-benzoyl-d-l-argininep-nitroanilide (BANA) as a substrate and it was observed that the concentration necessary to reduce the enzymatic activity by 50 % was lower than 1 mg/mL. However, the same authors observed that the melanoidin did not affect the chymotrypsin activity using Na-benzoyl-d-l-tyrosin-p-nitroanilide (BTNA) as a substrate. Enzymes and hormones of peptidic origin lose their properties in the presence of melanoidins and modify their immunologic behaviour [119].
The melanoidins resulting from the Maillard reaction between d-glucose and lysine affect the activity of different proteolytic enzymes in the gastro-intestinal tract [138]. The authors used Na-benzoyl-l-arginine ethyl ester (BAEE) and casein to study the inhibitor effect of melanoidin on trypsin activity. When using BAEE as a substrate, they observed that the addition of 0.5 mg/mL did not affect the value of Michaelis–Menten constant, while the maximum reaction rate reduced its value from 0.07 to 0.05 mM/min. In the case of using casein as a substrate, the addition of 1 mg/mL of melanoidin caused a decrease of approximately 15 %.
Ibarz et al. [80] showed the effect of the presence of the melanoidins from glucose-asparagine on the enzymatic activity of trypsin. It was observed that an excess of Na-benzoyl-l-arginine ethyl ester (BAEE) has an inhibiting effect on this enzyme activity. The maximum reaction rate was obtained for a 0.06 mN substrate concentration. It is also observed that the presence of melanoidin inhibits the enzymatic activity of trypsin. This inhibition can be described as a linear mixed type where the inhibition constant αKi of the substrate inhibitor complex is higher than the inhibition constant Ki of the complex enzyme inhibitor with a value of 1.88. Moreover, glucose was shown to be the most active sugar in producing inhibitory derivatives in the browning reaction with l-cysteine. To add further support to the possible involvement of MRPs derived from a thiol precursor, Billaud et al. [14] showed that the inhibitory potency of various amounts of MRPs derived from hexose/glutathione, heated at various temperatures (80–110 °C) for different times (2–39 h), on enzymatic browning and apple PPO activity in order to optimize organoleptic properties of fruit and vegetables. Compared with MRPs prepared from hexose with cysteine, those from GSH exhibited a more potent inhibitory effect, due to the presence of an additional carboxylic function on the tripeptide. Therefore, these MRPs could be considered as potential natural inhibitors effect on apple PPO for use with minimally processed fruits.
Hansen and Millington [67] observed a blockage in the protein digestion catalysed by carboxypeptidase-B (CPB) due to the products obtained from the browning reaction between glucose and lysine. As the reaction temperature increased, which is for higher melanoidin content, the activity of the enzyme decreased, so that the products formed could not degrade in the intestine, that is, they could not be absorbed. As a reaction to this, the organism puts the immune system to work, synthesizing immunoglobulin A and M. The low molecular mass constituents in the browning reaction between glucose and lysine affected the daily protein ingestion in rats [138, 139] studying the effect of these products on various pancreatic enzymes, concluding that their inhibitor effect on trypsin was non-competitive. However, their action on CPA and CPB does not follow the established classic inhibition models. Both enzymes are completely inhibited with 0.5 mg/mL of the low molecular fraction of formed melanoidin.
Ibarz et al. [83] study the effect of melanodins obtained from a glucose/asparagine system and also the effect of excess of substrate on the activity of the carboxypeptidase A (CPA) and carboxypeptidase-B (CPB) enzymes. The presence of melanoidins has an inhibitory effect on both the enzymes, with the value of the maximum reaction rate of the enzymatic reaction decreasing with the increase in melanoidins, so that the maximum activity is reached for substrate concentrations of 1 mM.
Final Remarks
The Maillard reaction (MR) is a chemical reaction between an amino acid and a reducing sugar, usually requiring the addition of heat. The sugar interacts with the amino acid, producing a variety of odours and flavours. The MR is the basis of the flavouring industry. On the other hand in the manufacture of fruit juices and pulps, is of paramount importance to refer to non-enzymatic browning as an undesirable process. Non-enzymatic browning is the last step of the MR where the presence of brown polymers called melanoidins produces darkening, especially in liquid foods. Modelling studies of colour formation and definition of rheological behaviour, considered in this review lead to the conclusion that models of zero order and first order are giving better data fittings in kinetic studies of non-enzymatic browning. The most influential factors with the reactions are mainly: temperature, amino acids presence, reducing sugars, water activity and pH.
Melanoidins produced in the Maillard reactions between proteins and sugars in real foods are predominantly high molecular weight compounds. Considering that the molecular weight of melanoidins produced from MRs is highly dependant on the heating intensity, and HMW melanoidins are produced at longer reaction times. To better understand the benefits of melanoidins in healthy foods, these beneficial compounds need to be chemically characterized and categorized according to their structure-specific health effects. Absorbed melanoidins may have beneficial in vivo effects, such as antioxidant activity. Also, the indigestible melanoidins are more likely to be beneficial to human health, displaying in vivo antioxidant, antimicrobial and prebiotic activity in the intestine.
References
Adams A, Borrelli R, Fogliano V, De Kimpe N (2005) Thermal degradation studies of food melanoidins. J Agric Food Chem 53:4136–4142
Ajandouz E, Tchiakpe F, Dalle Ore F, Benajiba A, Puigserver A (2001) Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems. J Food Sci 66:7–200
Ames J (1998) Applications of the Maillard food industry. Food Chem 62(4):431–439
Ames J, Wynne A, Hofmann A, Plos S, Gibson G (1999) The effect of a model melanoidin mixture on faecal bacterial populations in vitro. BJN 82:489–495
Anderson D, Yu TW, McGregor DB (1988) Comet assay responses as indicators of carcinogen exposure. Mutagenesis 13(6):539–555
Baltes W (1982) Chemical changes in food by the Maillard reaction. Food Chem 9:59–73
Bartz J, Brecht J (2003) Posthatvest physiology and pathology of vegetables Second Edic. Marcel Dekker, Inc., New York, pp 464–465
Bekedam E, Schols H, Van Boekel M, Smit G (2006) High molecular weight melanoidins from coffee brew. J Agric Food Chem 54:7658–7666
Bekedam E, Roos E, Schols H, Van Boekel M, Gerrit S (2008) Low molecular weight melanoidins in coffee brew. J Agric Food Chem 56:4060–4067
Bekedam K, De Laat M, Schols H, Van Boekel M, Smit G (2007) Arabinogalactan proteins are incorporated in negatively charged coffee brew melanoidins. J Agric Food Chem 55(3):761–768
Beker K, Güçlü K, Tor I, Demirata B, Apak R (2009) Total antioxidant capacity assay using optimized ferricyanide/Prussian blue method. Food Anal Method 2(1):154–168
Benjakul S, Visessanguan W, Phongkanpai V, Tanaka M (2005) Antioxidative activity of caramelisation products and their preventive effect on lipid oxidation in fish mince. Food Chem 90:231–239
Berger RG (ed) (2007) Flavours and fragrances: chemistry, bioprocessing and sustainability. Springer, Berlin, pp 694–695
Billaud C, Brun-Mérimeée S, Louarme L, Nicolas J (2004) Effect of glutathione and Maillard reaction products prepared from glucose or fructose with glutathione on polyphenoloxidase from apple-I: enzymatic browning and enzyme activity inhibition. Food Chem 84:223–233
Borneman Z, Gökmen V, Nijhuis H (2001) Selective removal of polyphenols and brown colour in apple juices using PES/PVP membranes in a single-ultrafiltration process. Separ Purif Tech 22(23):53–61
Borrelli R, Visconti A, Mennella C, Anese M, Fogliano V (2002) Chemical characterization and antioxidant properties of coffee melanoidins. J Agric Food Chem 50:6527–6533
Borrelli RC, Fogliano V (2005) Bread crust melanoidins as potential prebiotic ingredients. Mol Nutr Food Res 49:673–678
Lj Bozidar, Djilas M, Jasna M, Canadanovig-Brunet (1993) Synthesis of some heterocyclic amino-imidazoazarenes. Food Chem 46:273–276
Brückner H, Justus J, Kirschbaum J (2001) Saccharide induced racemization of amino acids in the course of the Maillard reaction. Amino Acids 21:429–433
Brudzynski K, Miotto D (2011) Honey melanoidins: analysis of the compositions of the high molecular weight melanoidins exhibiting radical-scavenging activity. Food Chem 127:1023–1030
Buedo A, Elustondo M, Urbican M (2001) Non-enzymatic browning of peach juice concentrate during storage. Innov Food Sci Emerg Technol 1:255–260
Cämmerer B, Jalyschro W, Kroh L (2002) Intact carbohydrate structure as part of the melanoidin skeleton. J Agric Chem 50:2083–2087
Cämmerer B, Kroh LW (1995) Investigation of the influence of reaction conditions on the elementary composition of melanoidins. Food Chem 53:55–59
Capuano E, Ferrigno Antonella, Acampa I, Serpen A, Açar Ö, Gökmen V, Fogliano V (2009) Effect of flour type on Maillard reaction and acrylamide formation during toasting of bread crisp model systems and mitigation strategies. Food Res Int 42:1295–1302
Carabasa M, Ibarz A, Garza S, Barbosa-Cánovas (1998) Removal of dark compounds from clarified fruit juices by adsorption processes. J Food Eng 37:25–41
Carabasa-Giribet M, Ibarz-Ribas A (2000) Kinetics of colour development in aqueous fructose systems at high temperatures. J Sci Agric 80:2105–2113
Carabasa-Giribet M, Ibarz-Ribas A (2000) Kinetics of colour development in aqueous glucose systems at high temperaturas. J Food Eng 44:181–189
Carrín M, Buglione M, Lozano J (2007) Removal of dark compounds from fruit juices by membrane separation proceedings of European congress of chemical engineering (ECCE-6), Copenhagen, pp 16–20
Cerami C, Founds H, Nicholl I, Mitsuhashi T, Giordano D, Vanpatten S, Lee A, Al-Abed Y, Vlassara H, Bucal R, Cerami A (1997) Tobacco smoke is a source of toxic reactive glycation products. Proc Natl Acad Sci USA 94(25):13915–13920
Chandra R, Naresh Bharagava R, Bioresource V (2008) Melanoidins as major colourant in sugarcane molasses based distillery effluent and its degradation. Technology 99:4648–4660
Charles-Bernard M, Kraehenbuehl K, Rytz A, Roberts D (2005) Interactions between volatile and nonvolatile coffee components. 1. Screening of nonvolatile components. J Agric Food Chem 53(11):4417–4425
Charles-Bernard M, Roberts D, Kraehenbuehl K (2005) Interactions between volatile and nonvolatile coffee components. 2. Mechanistic study focused on volatile thiols. J Agric Food Chem 53(11):4426–4433
Coca M, García M, Gonzalez G, Peña M, García J (2004) Study of coloured components formed in sugar beet processing. Food Chem 86:421–433
Danehy JP (1986) Maillard reactions: nonenzymatic browning in food systems with special reference to the development of flavor. Adv Food Res 30:77–138
Dattatreya A, Rankin (2006) Moderately acidic pH potentiates browning of sweet whey poder. Int Dairy J 16:822–828
Davidek T, Clety N, Devaud, Robert F, Blank (2003) Simultaneous quantitative analysis of Maillard reaction precursors and products by high-performance anion exchange chromatography. J Agric Food Chem 51:7259–7726
Davis A (1995) Functionality of sugars: physicochemical interactions in foods. Am J Clin Nutr 62:170–177
Del Castillo M, Ferrigno A, Acampa I, Borrelli R, Olano A, Martínez-Rodríguez, Fogliano V (2007) In vitro release of angiotensin-converting enzyme inhibitors, peroxyl-radical scavengers and antibacterial compounds by enzymatic hydrolysis of glycated gluten. J Cereal Sci 45:327–334
Delgado-Andrade C, Morales F (2005) Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J Agric Food Chem 53:1403–1407
Delgado-Andrade C, Morales F, Seiquer I, Navarro M (2010) Maillard reaction products profile and intake from Spanish typical dishes. Food Res Int 43:1304–1311
Delgado-Andrade C, Tessier F, Niquet-Leridon C, Seiquer I, Navarro MP (2011) Study of the urinary and faecal excretion of N (ε)-carboxymethyllysine in young human volunteers. Amino Acids. doi:10.1007/s00726-011-1107-8
Dell’Aquila C, Ames JM, Gibson GR, Wynne AG (2003) Microbial degradation of heated gluten-glucose systems. Implications for gut health. In: Vegarud G, Morales F (eds) Melanoidins in food and health, vol 4. Office for Official Publications of the European Communities, Luxembourg, pp 177–179
Echavarría AP, Torras C, Pagán J (2011) Fruit juice processing and membrane technology application. Food Eng Rev 3(3–4):136–158
Echavarría A, Pagán J, Ibarz A (2011) Effect of previous enzymatic recirculation treatment through a tubular ceramic membrane on ultrafiltration of model solution and apple juice. J Food Eng 102:334
Echavarría AP, Falguera V, Torras C, Berdún C, Pagán J, Ibarz A (2012) Ultrafiltration and reverse osmosis for clarification and concentration of fruit juices at pilot plant scale. LWT 46:189–195
Einarsson H (1987) The effect of time, temperature, pH and reactants on the formation of antibacterial compounds in the Maillard reaction. LWT 20:56–58
Einarsson H, Snygg B, Eriksson C (1983) Inhibition of bacterial growth by Maillard reaction products. J Agric Food Chem 83(31):1043–1047
Ekasari I, Berg H, Jongen M, Pilnik W (1990) Characterization of mutagenic compound(s) in heated orange juice. Food Chem 36:11–18
Ekasari I, Bonestroo M, Jongen M, Pilnik W (1989) Mutagenicity and possible occurrence of flavonol aglycones in heated orange juice. Food Chem 31:289–294
Ekasari I, Fokkes R, Bonestroo MH, Schols HA, Nibbering NM, Pilnik W (1993) Characterization of mutagenic compounds in heated orange juice by UV and mass spectra. Food Chem 46(1):77–79
Eriksson C (1981) Maillard reactions in food. In: Eiksson C (ed) Progress in food and nutrition science. Pergamon Press, Oxford, vol 5, pp 1–6
Faist V, Erbersdobler HF (2001) Metabolic transit and in vivo effects of melanoidins and precursor compounds deriving from the Maillard reaction. Ann Nutr Metab 45:1–12
Faist V, Lindenmeier M, Geisler C, Erbersdobler H, Hofmann T (2002) Influence of molecular weight fractions isolated from roasted malt on the enzyme activities of NADPH-cytochrome C-reductase and glutathione-Stransferase in Caco-2 cells. J Agric Food Chem 50:602–606
Faist V, Wenzel E, Randel G, Löwer C, Münch G (2000) In vitro and in vivo studies on the metabolic transit of N (ε) -Carboxymethyllysine. Czech J Food Sci 18:116–119
Falguera V, Pagán J, Garza S, Garvín A, Ibarz A (2011) Ultraviolet processing of liquid food: a review part 2: effects on microorganisms and on food components and properties. Food Res Int 44:1580
Ferreira A, de Pinho Guedes (2003) Analytical method for determination of some aroma compounds on white wines by solid phase microextraction and gas chromatography. J Food Sci 68:9
Fogliano V, Morales F (2011) Estimation of dietary intake of melanoidins from coffee and bread. Food Funct 2(11):93–144
Freter R (1983) Mechanisms that control the microflora. In: Hentges DJ (ed) Human intestinal microflora in health and disease. Academic Press, London, pp 35–54
Friedman R, Wilson I, Ziderman I (1990) Effect of heating on mutagenicity of fruit juices in the Ames test Mendel. J Agric Food Chem 38(3):740–743
Ganjloo A, Rahman R, Bakar J, Osman A, Bimakr M (2009) Modelling the kinetics of peroxidase inactivation and colour changes of seedless guava (Psidium guajava L) during thermal treatments. WASJ 7(1):105–112
Garza S, Ibarz A, Pagán J, Giner J (1999) Non-enzymatic browning in peach puree during heating. Food Res Int 32:335
Gazzini G, Vagnarelli P, Cuzzoni M, Azzani G, Mazza (1987) Mutagenic activity of the Maillard reaction products of ribose with different amino acids. JFS 52:757–760
Giardino I, Edelstein D, Brownlee M (1994) Nonenzymatic glycosylation in vitro and in bovine endothelial cells alters basic fibroblast growth factor activity: a model for intracellular glycosylation in diabetes. J Clin Invest 94:110–117
Gniechwitz D, Reichardt N, Ralph J, Blaut M, Steinhart H, Bunzel M (2008) Isolation and characterisation of a coffee melanoidin fraction. J Sci Food Agric 88:2153–2160
Gu F, Kim J, Hayat K, Xia S, Feng B, Zhan X (2009) Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a casein-glucose model system. Food Chem 117:48–54
Hadiyanto C, Esveld D, Boom M, van Straten G, van Boxte A (2008) Product quality driven design of bakery operations using dynamic optimization. J Food Eng 86:399–413
Hansen LP, Millington RJ (1979) Blockage of protein digestion (Carboxypeptidase-B) by heat-induced sugar-lysine reactions. J Food Sci 44:1173–1177
Hayashi T, Namiki M (1980) Formation of two-carbon sugar. Fragment at an early stage of the browning reaction of sugar with amine. Agric Biol Chem 44:2575–2580
Hellwig M, Henle T (2010) Formyline, a new glycation compound from the reaction of lysine and 3-deoxypentosone. Eur Food Res Technol 230:903–914
Hidalgo F, Zamora R (2000) The role of lipids in nonenzymatic browning. Grasas Aceites 51:35–49
Hiramoto K, Shizuuchi S, Kawachi Y, Morishita Y, Nagase M (2004) Melanoidin, a food protein-derived advanced Maillard reaction product, suppresses Helicobacter pylori in vitro and in vivo. Helicobacter 9(5):429–435
Hirano M, Miura M, Gomyo T (1994) Melanoidin as a novel trypsin inhibitor. Biosci Biotech 58:940–941
Hodge JE (1953) Dehydrated foods. Chemistry of browning reactions in model systems. J Agric Food Chem 1:928–943
Hofmann T (1998) Studies on the relationship between molecular weight and the color potency of fractions obtained by thermal treatment of glucose amino acid and glucose/protein solutions by using ultracentrifugation and color dilution techniques. J Agric Food Chem 46(10):3891–3895
Homma S, Fujimaki M (1981) Growth response of rats fed a diet containing nondialyzable melanoidin. Prog Fd Nutr Sci 5:209–216
Hwang I, Young H, Woo K, Lee J, Sang H (2011) Biological activities of Maillard reaction products (MRPs) in a sugar-amino acid model system. Food Chem 126:221–227
Ibarz A, Naves J (1995) Efecto de la temperatura y contenido en sólidos solubles sobre la cinética de pardeamiento no enzimático de zumos clarificados de manzana. Food Sci Tech Int 1:29–34
Ibarz A, Casero T, Miguelsanz R, Pagán J (1989) Efecto de la temperatura en la cinética de pardeamiento no enzimático en zumos clarificados de pera con diferente contenido de sólidos solubles. Revista de Agroquímica y Tecnología de Alimentos 29:530–537
Ibarz A, Falguera V, Garza S, Garvín A (2012) Discoloration kinetics of clarified apple juice treated with Lewatit® S 4528 adsorbent resin during processing. Food Bioprocess Technol. doi:10.1007/s11947-011-0649-9
Ibarz A, Garvín A, Garza S, Pagán J (2009) Toxic effect of melanoidins from glucose-asparagine on trypsin activity. Food Chem Toxicol 47:2071–2075
Ibarz A, Garza S, Garvín A, Pagán J (2008) Kinetics of peach clarified juice discoloration process with an adsorbent resin. Food Sci Tech Int 14(5):057–062
Ibarz A, Garza S, Garvín A, Pagán J (2011) Degradation of mandarin juice concentrates treated at high temperatures. J Food Process Eng 34(3):682–696
Ibarz A, Garza S, Pagán J (2008) Inhibitory effect of melanoidins from glucose-asparagine on carboxypeptidases activity. Eur Food Res Technol 226:1277–1282
Ibarz A, Garza S, Pagán J (2008) Nonenzymatic browning of selected fruit juices affected by D-galacturonic acid International. JFST 43:908–914
Ibarz A, González C, Esplugas S (1994) Rheology of clarified fruit juices. III: Orange juices. J Food Eng 21:485–494
Ibarz A, González C, Esplugas S, Miguelsanz R (1990) Non-enzimatyc browning kinetics of clarified peach juices at different temperatures. Confructa (Flüssiges Obst GmbH) 34:152–159
Ibarz A, Pagán J, Garza S (2000) Kinetic models of non-enzymatic browning in apple puree. J Sci Food Agric 80:1162–1168
Ibarz A, Pagan J, Garza S (1999) Kinetic models for colour changes in pear puree during heating at relatively high temperatures. J Food Eng 39:415–422
Ibarz A, Pagán J, Garza S (2005) Photochemical destruction of color compounds in fruit juices. J Food Eng 69:155–160
Ibarz-Martínez R, Pagán J, Garza S, Ibarz A (2010) Browning of clarified lemon juices treated at high temperatures. Sciencia Agropecuaria 1:07–20
IFFJP (1985) International federation of fruit juice producers methods, analysen-analyses. Zug. Swizerland: fruit-Union Suisse Assoc. Suizzera Frutta 12:1–2
Jakas A, Horvat S (2008) Reactivity and oxidative potential of fructose and glucose in enkephalin-sugar model systems. Amino Acids 34:329–332
Jakszyn P, Agudo A, Ibáñez R, García-Closas R, Pera G, Amiano P, Gonzales CA (2004) Development of a food database of nitrosamines, heterocyclic amines, and polycyclic aromatic hydrocarbons. J Nutr 134:2011–2014
Jiménez N, Bohuon P, Dornier M, Bonazzi C, Pérez A, Vaillant F (2012) Effect of water activity on anthocyanin degradation and browning kinetics at high temperatures (100–140 °C). Food Res Int 47:106–115
Johansson M, Laurent B, Gian A, Kjell O, Jagerstad M (1995) Influence of amino acid on the formation of mutagenic/carcinogenic heterocyclic amines in a model system. Carcinogenesis 16:2553–2560
Karel M, Saguy I (1991) Effects of water on diffusion in food systems. In: Levine H, Slade L (eds) Water relationships in foods. Plenum Press, New York, pp 157–174
Karmas R, Buera M, Karel M (1992) Effect of glass transition on rates of nonenzymatic browning in food systems. J Agric Food Chem 40:873–879
Kasai H, Shimoi T, Sugimura T, Nishimura S (1981) Synthesis of 2-amino-3, 8-dimethylimidazol (4,5-f) quinoxaline(Me-IQx), a potent mutagen isolated from fried beef. Chem Lett 10(5):675–678
Kim JS, Lee Y (2009) Activity of melanoidins from different sugar/amino acid model systems: influence of the enantiomer type. Amino Acids 36(3):465–474
Kim JS, Lee YS (2008) Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction. Food Chem 108:582–592
Knol J, Linssen J, Van Boekel M (2010) Unravelling the kinetics of the formation of acrylamide in the Maillard reaction of fructose and asparagine by multiresponse modeling. Food Chem 120:1047–1057
Kuntcheve M, Obretenov T (1996) Isolation and characterization of melanoidins from beer. Zeitschrift für Lebensmitteluntersuchung und-Forschung A 202(3):238–243
Kwak E, Soon L, Murata M, Homma S (2005) Effect of pH control on the intermediates and melanoidins of nonenzymatic browning reaction. LWT 38:1–6
Labuza TP, Saltmarch M (1981) The nonenzymatic browning reaction as affected by water in food. In: Rockland LB, Stewart GF (eds) Water activity: Influences in food quality. Academic Press, London, pp 605–623
Ledl F, Schleicher E (1990) New aspects of the Maillard reaction in foods and in the human body. Angew Chem Int Ed England 29:565–594
Leong LP (1999) Modelling of the Maillard reaction involving more than one amino acid. PhD. Dissertation. University of Leeds, Leeds, UK
Liardon R, De Weck-Gaudard D, Philippossian G, Finot P (1987) Identification of Nepsilon-carboxymethyllysine: a new Maillard reaction product in rat urine. J Agric Food Chem 35(3):427–431
Lozano J (2006) Fruit manufacturing: scientific basis, engineering properties, and deteriorative reactions of technological. Food Engineering series. Springer, Berlin, pp 169–170
Lozano J, Ibarz A (1997) Colour changes in concentrated fruit pulp during heating at high temperatures. J Food Eng 31:365–373
Lozano J, Porras J, Errazu A, Tonelli S (1995) Prediction of 5-HMF formation in an industrial apple juice evaporator. J Food Sci 60:1292–1294
Lyons T, Bailie KE, Dyer DG, Baynes JW, Dunnja (1991) Decrease in skin collagen glucation with glycaemic control in patients with insulin dependent diabetes mellitus. J Clin Invest 87:1910–1915
Manach C, Scalbert A, Morand C, Rëmésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79(5):727–747
Manayay D, Ibarz A (2010) Modeling the kinetics nonenzymatic browning reactions and rheological behavior in the thermal process of fruit juices and pulps. Scientia Agropecuaria 1:155–168
Manzocco L, Calligaris D, Nicoli M, Lerici R (2001) Review of nonenzymatic browning and antioxidant capacity in processed foods. Trends Food Sci Tech 11:340–346
Manzocco L, Nicoli C, Maltini E (1999) DSC analysis of Maillard browning and procedural effects. J Food Process Pres 23:317–328
Martins S, Jongen W, van Boekel M (2001) A review of the Maillard reaction in food and implications to kinetic modeling. Trends Food Sci Tech 11:364–373
Martins S, Van Boekel M (2005) Kinetic model validation for the glucose/glycine Maillard reaction pathways: influence of pH and reactants initial concentration. Food Chem 90:257–269
Matmaroh K, Benjakul S, Tanaka M (2006) Effect of reactant concentrations on the Maillard reaction in a fructose-glycine model system and the inhibition of black tiger shrimp polyphenoloxidase. Food Chem 98:1–8
Matsuda T, Kato Y, Watanabe K, Nakamura R (1985) Direct evaluation of betalactoglobulin lactosylation in early Maillard reaction using an antibody specific protein-bound lactose. J Agric Food Chem 33(119):3–1196
Mesías-García M, Seiquer M, Delgado-Andrade C (2009) Intake of Maillard reaction products reduces iron bioavailability in male adolescents. Mol Nutr Food Res 53:1551–1560
Monnier V, Nagaraj R, Portero-Otin M, Glomb M, Elgawish A, Sell D, Friedlander M (1996) Structure of advanced Maillard reaction products and their pathological role. Nephrol Dial Transplant 11:20–26
Monnier V(2005) Bacterial enzymes that can deglycate glucose and fructose-modified lysine. Biochem J 392:1–3
Montavon P, Mauron A, Duruz E (2003) Changes in green coffee protein profiles during roasting. J Agric Food Chem 51:2335–2343
Morales F (2002) Application of capillary zone electrophoresis to the study of food-model melanoidin. Food Chem 76(3):363–369
Morales F, Babbel M (2002) Melanoidins Exert a weak antiradical activity in watery fluids. J Agric Food Chem 50:4657–4661
Morales F, Jiménez-Pérez S (2004) Peroxyl radical scavenging activity of melanoidins in aqueous systems. Eur Food Res Technol 218:515–520
Morales F, Somoza V, Fogliano V (2010) Physiological relevance of dietary melanoidins. Amino Acids. doi:10.1007/s00726-010-0774-1
Mottram D (1998) Flavour formation in meat and meat products: a review. Food Chem 62(4):415–424
Mundt S, Wedzicha B (2004) Comparative Study of the composition of melanoidins from glucose and maltose. J Agric Food Chem 52:4256–4426
Nessar A (2005) Advanced glycation endproducts-role in pathologym of diabetic complications. Diabetes Res Clin Pract 67:3–21
Niko E (2010) Assessment of antioxidant capacity in vitro and in vivo. FRBM 49:503–515
Nishimura S (1986) Chemistry of mutagens and carcinogens in broiled food. EHP 67:11–16
Noltes AW, Chappel CA (1985) Toxicology of caramel colours: current status. In: Gibson, Walker R (ed) Food toxicology-real or imaginary problems? London, pp 214–228
Nunes F, Coimbra M (2010) Role of hydroxycinnamates in coffee melanoidin formation. Phytochem Rev 9:171–185
O’Brien J, Nursten HE, Crabbe M, Ames JM (eds) (1998) The Maillard reaction in foods and medicine. The Royal Society of Chemistry, Cambridge, pp 173–184
Obretenov T, Snezhana D, Kuntcheva M, Ivanova S, Somovi G (1993) Melanoidin formation in cooked meat product. J Agric Food Chem 41(4):653–656
Oh SH, Lee YS, Lee JW, Kim MR, Yook HS, Byun MW (2005) The effect of γ-irradiation on the non-enzymatic browning reaction in the aqueous model solutions. Food Chem 92:357–363
Öste R, Dahlqvist A, Sjöström H, Norén O, Miller R (1986) Effect of Maillard reaction products on protein digestion. In vitro studies. J Agric Food Chem 34:355–358
Öste RE, Miller R, Sjöström H, Norén O (1987) Effect of maillard reaction products on protein digestion. Studies on pure compounds. J Agric Food Chem 35:938–942
Oyama Y, Hori N, Allen C, Carpenter D (1990) Influences of trypsin and collagenase on acetylcholine responses of physically isolated single neurons of Aplysia californica. Cell Mol Neurobiol 10(2):193–205
Pätzold R, Nieto-Rodriguez A, Brückner H (2003) Chiral gas chromatographic analysis of amino acids in fortified wines. Chromatograph Suppl 57:207–212
Portero-Otín PamplonaR, Bellmunt M, Bergua M, Prat J (1997) Glycaemic control and in vivo non-oxidative Maillard reaction: urinary excretion of pyrraline in diabetes patients. Eur J Clin Invest 27:767–773
Pripis-Nicolau L, De Revel G, Bertrand A, Maujean A (2000) Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. J Agric Food Chem 48:9–10
Randhir R, Kwon Y, Shetty K (2008) Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. IFSET 9:355
Reichardt N, Gniechwitz D, Steinhart H, Bunzel M, Blaut M (2009) Characterization of high molecular weight coffee fractions and their fermentation by human intestinal microbiota. Mol Nutr Food Res 53(2):287–299
Rivero-Perez M, Perez-Magarino S, Gonzalez-San J (2002) Role of melanoidins in sweet wines. Anal Chim Acta 458:169–175
Rizzi G (1997) Chemical structure of colored Maillard reaction products. Food Rev Int 13(1):1–28
Roos Y, Joupilla K, Zielasko B (1996) Nonenzymatic browning-induced water plasticization. JTAC 47:1437–1450
Rufian-Henares J, Morales F (2007) Antimicrobial activity of melanoidins antimicrobial. J Food Qual 30:160–168
Rufián-Henares JA, De la Cueva SP (2009) Antimicrobial activity of coffee melanoidins—a study of their metal-chelating properties. J Agric Food Chem 57:432–438
Rufian-Henares JA, Morales FJ (2007) Angiotensin-I converting enzyme inhibitory activity of coffee melanoidins. J Agric Food Chem 55:1480–1485
Rufian-Henares JA, Morales FJ (2007) Effect of in vitro enzymatic digestion on antioxidant activity of coffee melanoidins and fractions. J Agric Food Chem 55:10016–10021
Rufian-Henares JA, Morales FJ (2007) Functional properties of melanoidins: in vitro antioxidant, antimicrobial and antihypertensive activities. Food Res Int 40:995–1002
Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I (1998) Functional food science and gastrointestinal physiology and function. BJN 80:S147–S171
Samaras T, Carburn P, Chandra, Gordon M (2005) Antioxidant properties of kilned and roasted malts. J Agric Food Chem 53:8068–8074
Sebekova K, Eifert T, Klassen A, Heidland A, Amann KR (2007) Effects of S18886 (Terutroban), a TP receptor antagonist, in an experimental model of type 2 diabetes. Diabetes 56(4):968–974
Shen SC, Tseng KC, Wu JS (2007) An analysis of Maillard reaction products in ethanolic glucose-glycine solution. Food Chem 102:281–287
Shen SC, Wu JS (2004) Maillard browning in ethanolic solution. JFS 69:273–279
Sherwin C, Labuza T (2003) Role of moisture in Maillard browning reaction rate in intermediate moisture foods: comparing solvent phase and matrix properties. J Food Sci 68(2):588–593
Silván J, Van de Lagemaat J, Olano A, del Castillo M (2006) Analysis and biological properties of amino acid derivates formed by Maillard reaction in foods. JPBA 41:1543–1551
Singh R, Barden A, Mori T, Beilin L (2001) Advanced glycation end-products. A review. Diabetologia 44:129–146
Slade L, Levine H, Finey J (1989) Protein-water interactions: water as a plasticizer of gluten and other protein polymers. In: Phillips RD, Finlay JW (eds) Protein quality and the effects of processing. Dekker, New York, pp 9–123
Somoza V (2005) Review five years of research on health risks and benefits of Maillard reaction products: an update. Mol Nutr Food Res 49:663–672
Stadtman E, Levine R (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218
Summa C, McCourt J, Cämmerer B, Fiala A, Probst M, Kun S, Anklam E, Wagner KH (2008) Radical scavenging activity, anti-bacterial and mutagenic effects of cocoa bean Maillard reaction products with degree of roasting. Mol Nutr Food Res 52:342–351
Tagliazucchi D, Verzelloni E, Conte A (2010) Effect of dietary melanoidins on lipid peroxidation during simulated gastric digestion: their possible role in the prevention of oxidative damage. J Agric Food Chem 58:2513–2519
Terasawa N, Murata M, Homma S (1991) Separation of model melanoidin into components with copperchelating Sepharose 6B column chromatography and comparison of chelating activity. Agr Biol Chem 55:1507–1514
Thomsen M, Lauridsen L, Skibsted L, Risbo J (2005) Temperature effect on lactose crystallization, Maillard reactions, and lipid oxidation in whole milk powder. J Agric Food Chem 53:7082–7090
Totlani V, Peterson D (2007) Influence of Epicatechin reactions on the mechanisms of Maillard product formation in low moisture model systems. J Agric Food Chem 55:414–420
Vaikousi H, Konstantinos K, Biliaderis C (2008) Kinetic modelling of non-enzymatic browning of apple juice concentrates differing in water activity under isothermal and dynamic heating conditions Journal. Food Chem 107:785–796
Van Nguyen C (2006) Toxicity of AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological AGEs. Mol Nutr Food Res 50:1140–1149
Venir E, Pittia P, Giavon S, Maltini E (2009) Structure and water relations of melanoidins investigated by thermal, rheological, and microscopic analysis. Int J Food Prop 12:819–833
Verzelloni E, Tagliazucchi D, Conte A (2010) From balsamic to healthy: traditional balsamic vinager melanoidins inhibit lipid peroxidation during simulated gastric digestion of meat. Food Chem Toxicol 48(8–9):2097–2102
Wang HY, Qian H, Yao WR (2011) Review melanoidins produced by the Maillard reaction: structure and biological activity. Food Chem 128:573–584
Wen X, Enokizo A, Hattori H, Kobayashi S, Murata M, Homma S (2005) Effect of roasting on properties of the zinc-chelating substance in coffee brews. J Agric Food Chem 53:2684–2689
Wrodnigg M, Eder B (2001) The Amadori and Heyns rearrangements: landmarks in the history of carbohydrate chemistry or unrecognized synthetic opportunities? Top Curr Chem 215:115–152
Wu MC, Ma CY, Yang CC, Kao WC, Shen SC (2011) The formation of IQ type mutagens from Maillard reaction in ethanolic solution. Food Chem 125(2):582–587
Yang C, Chen S, Zou T (2011) Response surface methodology for meat-like odorants from the Maillard reaction with Glutathione II: the tendencies analysis of meat-like donors. J Food Sci 76(9):1267–1277
Yang Z, Han Y, Gu Z, Fan G, Chen Z (2008) Thermal degradation kinetics of aqueous anthocyanins and visual color of purple corn (Zea mays L.) cob. IFSET 9:341–347
Yaylayan V (1990) In search of alternative mechanisms for the Maillard reaction. Trends Food Sci Technol 1:20–22
Yoshida A, Wei D, Nomura W, Izawa S, Inoue Y (2012) Reduction of glucose uptake through inhibition of hexose transporters and enhancement of their endocytosis by methylglyoxal in Saccharomyces cerevisiae. J Biol Chem 287(1):701–711
You J, Luo Y, Shen H, Song Y (2011) Effect of substrate ratios and temperatures on development of Maillard reaction and antioxidant activity of silver carp (Hypophthalmichthys molitrix) protein hydrolysate-glucose system. IJFST 46:2467–2474
Yuan Y, Zhao G, Hu X, Wu J, Liu J, Chen F (2008) High correlation of methylglyoxal with acrylamide formation in glucose/asparagine Maillard reaction model. Eur Food Res Technol 226:1301–1307
Zabala Díaz I, Chalova V, O’Bryan C, Crandall P, Ricke S (2010) Effect of soluble maillard reaction products on cadA expression in Salmonella typhimurium. J Environ Sci Health B 45:162–166
Zamora R, Hidalgo F (2005) Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit Rev Food Sci Nutr 45:49–59
Zhang Q, Ames J, Smith R, Baynes J, Metz T (2009) A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J Proteome Res 8:754–769
Acknowledgments
A.P. Echavarria thanks the Commission for Universities and Research of the DIUE of the Generalitat de Catalunya and the European Social Fund for the predoctoral grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Echavarría, A.P., Pagán, J. & Ibarz, A. Melanoidins Formed by Maillard Reaction in Food and Their Biological Activity. Food Eng Rev 4, 203–223 (2012). https://doi.org/10.1007/s12393-012-9057-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-012-9057-9