Abstract
Lectin receptor-like kinases (LecRK) are widespread in higher plants; however, little is known about their physiological roles. In this study, At1g70130 (designated LecRK-b2), an Arabidopsis LecRK gene, has been investigated. LecRK-b2 was predominantly expressed during seed germination, and its expression was ceased following germination. The expression of LecRK-b2 was induced by abscisic acid (ABA), salt, and osmotic stress. LecRK-b2 loss-of-function mutation slightly reduced the ABA sensitivity during seed germination, and this reduced sensitivity was demonstrated not due to lower ABA accumulation level in the seeds. Dual-luciferase transient expression assay confirmed that the transcription factor ABSCISIC ACID INSENSITIVE3 (ABI3) could activate the luciferase under driving of LecRK-b2 promoter. LecRK-b2 transcription level was found to be down-regulated in abi3 during seed germination. Furthermore, LecRK-b2 loss-of-function mutation reduced the salt and osmotic sensitivity during early development stage of Arabidopsis. Taken together, these results suggest that LecRK-b2 functions as a positive regulator of the ABA response during the seed germination and is involved in salt and osmotic stress response in the early development stage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lectin receptor-like kinases (LecRK) are a class of proteins, which include an extracellular legume lectin-like domain, a transmembrane domain, and an intracellular kinase domain (Herv et al. 1996). Despite their unique structures and wide spread in higher plants, little is known about the precise function of LecRK gene family. In the past decades, some studies suggested a variety of potential roles for these LecRK. Firstly, LecRK might take part in biotic and abiotic stress signal transduction. Poplar LecRK gene PnLPK and Arabidopsis LecRK gene lecRK-a1 could be induced by wounding and senescence, and Arabidopsis AtLecRK2 could be induced by salt stress (Nishiguchi et al. 2002; Riou et al. 2002; He et al. 2004). Furthermore, the extracellular legume lectin-like domain of LecRKs has led to suggestions that LecRK could be involved in legume-rhizobia symbiosis and pathogen resistance (Herv et al. 1996; Hirsch 1999), and recent studies have confirmed this hypothesis (Gouget et al. 2006; Chen et al. 2006). Moreover, LecRK also functions in plant development. SGC, an Arabidopsis LecRK gene, has been found to play an important role in pollen development (Wan et al. 2008).
Sequence analysis and molecular modeling of Arabidopsis LecRKs have revealed that some conserved residues are involved in binding hydrophobic molecular (Hervé et al. 1999; Barre et al. 2002). Thus they may be involved in the recognition of small hydrophobic hormones. Most recently, an Arabidopsis A4 subfamily of lectin receptor kinase has been found to negatively regulate ABA response in seed germination (Xin et al. 2009).
The phytohormone abscisic acid (ABA) regulates various aspects of physiological and developmental processes of plants, such as seed maturation, dormancy, and germination (Leung and Giraudat 1998; Kuhn et al. 2006; Yoshida et al. 2006). ABA also plays a critical role in response to biotic and abiotic stress, such as wounding, pathogen attack, drought, salt, and cold (Hetherington 2001; Finkelstein et al. 2002; Hetherington and Woodward 2003; Fan et al. 2004). ABSCISIC ACID INSENSITIVE3 (ABI3) is a transcription factor that mediates ABA responses in seeds (Parcy et al. 1994; Jones et al. 1997; Zeng et al. 2003). ABI3 acts in promoting seed maturation during the latter stages of seed development, and it may also play a role during seed germination (McCarty 1995; Li and Foley 1997; Bassel et al. 2006). ABI3 loss-of-function mutant abi3 reduces embryo dormancy and exhibits precocious germination (Raz et al. 2001).
Here, we characterized the expression patterns of LecRK-b2 gene in wild type and abi3 mutant. More importantly, LecRK-b2 was confirmed to play a role in the positive regulation of ABA response in seed germination. In addition, LecRK-b2 loss-of-function mutation reduced the salt and osmotic sensitivity during the early development stage of Arabidopsis.
Materials and Methods
Plant Materials, Growth Conditions, and Stress Treatments
Columbia-0 ecotype Arabidopsis was used as wild type (WT). The LecRK-b2 T-DNA insertion mutant line (SALK_020262) was obtained from Arabidopsis Biological Resource Center (http://www.arabidopsis.org). The homozygous was isolated and designate lecrk-b2. Seeds were cold-treated at 4°C for 3 days and then germinated and grown in the growth chamber for harvesting seeds. For stress treatment, the seedlings were grown on Murashige and Skoog (MS) agar for 10 days then transferred to MS liquid or MS liquid containing 100 μM ABA for 1, 2, 4,6, 12 h, or 100 mM NaCl for 1, 2, 4,6, 12 h, or 200 mM mannitol for 1, 2, 4, 6, 12 h, respectively.
Germination and Root Growth Assay
About 50 surface-sterilized seeds were sowed on 0.5× MS medium with various concentrations of ABA, NaCl, and mannitol. After being cold-treated at 4°C for 3 days, plates were transferred to a growth chamber (22°C, 80 μmol m−2 s−1 continuous white light). Germination (a clear appearance of radicle) rates and greening cotyledons rates were determined in three independent experiments (40 seeds per genotype and experiment).
Root growth analyses were performed by transferring 5-day-old seedlings onto 0.8% agar medium (0.5× MS, no sucrose) supplemented with the indicated ABA, NaCl, and mannitol, separately. Root growth was measured 6 days after the transfer in three independent experiments with 30 individuals per genotype and experiment.
Semi-quantitative RT-PCR and Real-Time RT-PCR
Total RNAs were isolated from the treated or untreated Arabidopsis materials using a Qiagen RNeasy plant mini kit. The 3 μg RNA was used for first-strand cDNA synthesis by SuperScript RT-PCR system (Invitrogen). For semi-quantitative RT-PCR, the primers were 5′-ACTAGACTGTTGGGACAGTG GAGAC and 5′-CACTAGAG AGAAACGATTCCGTCA. Actin2 gene was used as an internal standard. The amplification primers were 5′-CACTGTGCCAATCTACGAGGGT and 5′-CACAAACGAGGGCTGGAACAAG. PCR was performed with a 30-s denaturation at 95°C followed by 25 cycles with each cycle composed of 95°C for 30 s, 59°C for 30 s, and 72°C for 30 s. Real-time RT-PCR analyses were performed using Sybr Green PCR Master mix (Applied Biosystems) in Mx3000P thermal cycler (Stratagene), and data were analyzed with MxPro software (Stratagene). The primers used were 5′-ACACAAGGAGGAGCTGGTCAAGTT and 5′-TGGAGAAGGATGAAACAGTGC CGT. Actin2 was used as an internal control to normalize. The primers used for amplifying Actin2 were 5-CACTGTGCCAATCTACGAGGGT and 5′-CACAAACG AGGGCTGGAACAAG. RT-PCR reactions for each experiment were repeated at least three times, and the representative gel images were shown.
Measurement of Endogenous ABA Content
ABA content measurement was performed by using Plant hormone abscisic acid, ABA ELISA Kit (Uscnlife).
Construction of P LecRK-b2 :GUS and 2× 35S: LecRK-b2
LecRK-b2 promoter (from −904 to +19 bp relative to the translation start site) was amplified from genomic DNA template by PCR with forward 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGTCGGATTCTGATATGTGAGAGTC and reverse 5′-GGGGACCACTTTGTACAAGAAAGCTGGGTGA TCTTTAGAA GCAGAGACATGATTC; LecRK-b2 cDNA was amplified with forward 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGTCTCTGCTTCTAAAGATGTTATTA and reverse 5′-GGG GACCACTTTGTACAAGAAAGCTGGGTGTTAGCGT CCACTAGAGAGAAACGA; italic sequences are enzyme sites or BP transferase recognition sites. The PCR fragment was cloned into entry vector by BP reaction and confirmed by sequencing analysis then subcloned into destination vector GW-GUS for P LecRK-b2 :GUS construction and PLeela for 2× 35S: LecRK-b2 construction through LR reaction. Gateway clone system was ordered from Invitrogen.
P LecRK-b2 :GUS and 2× 35S:LecRK-b2 were transformed into the wild-type Arabidopsis and the LecRK-b2 knockout mutant (lecrk-b2), respectively, using floral dip method (Steven and Clough 1998). Independently, P LecRK-b2 :GUS transformed lines were used for GUS activity assay a. Three representative 2× 35S: LecRK-b2 lines, 3–1, 6–4, and 14–6 were chosen for ABA response assays.
Transient Transcription Dal-luciferase (Dual-LUC) Assays
Dual-LUC assays were performed as previously described (Liu et al. 2008). ABI3 cDNA was amplified with forward 5′-AAGGAAAAAAGCGGCCGCATGAAAAGC TTGCATGTGGC and reverse 5′-ACGCGTCGACTCATTTAACAGTTTGAGAAGT TGG; the effector plasmid, pGreenII62-K-ABI3, was constructed by inserting the ABI3 cDNA to pGreenII62-SK between the multiclone sites NotI/SalI. For the reporter plasmid construction, the promoter region of LecRK-b from −886 to +8 bp relative to the translation start site was cloned by PCR and inserted into the SalI/PstI sites of the vector pGreen-0800-LUC. The primer, forward 5′-ACGCGTC GACGAGTCAAGATTTGTTATATGTAAAT and reverse 5′-AAAACTGCAGCAGA GACATGATTCATTAAATTTTG, was used for reporter plasmid construction. Italic sequences are enzyme site. Three biological repeats were measured for each sample.
Results and Discussion
LecRK-b2 Expression Patterns
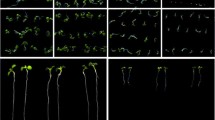
To determine the expression patterns of LecRK-b2, both semi-quantitative RT-PCR and promoter-GUS reporter analysis were performed. Semi-quantitative RT-PCR analysis showed that LecRK-b2 is weakly expressed in all organs of adult Arabidopsis (Fig. 1a), while its transcription level is relatively high in the dry seeds, especially in the seeds during germinating (Fig. 1b). Consistent with the semi-quantitative RT-PCR result, a 904-bp fragment of LecRK-b2 promoter directed GUS reporter also express in the germinating seeds but not in the adult leaves or flowers (Fig. 1c–f). It indicates that the 904 bp fragment of LecRK-b2 promoter likely contains all of the necessary cis elements for proper expression of the LecRK-b2 gene. Four homozygous P LecRK-b2 :GUS lines were used for GUS staining assay. We found that the promoter is very active during the seed germinating (Fig. 1), while after the cotyledon turned green and opened, the GUS activity fades away (Fig. 1). These results reveal that LecRK-b2 expression is activated during germination, but declines following the completion of germination.
Expression patterns of LecRK-b2 in wild type. a Semi-quantitative RT-PCR analysis of LecRK-b2 mRNA transcription levels in different organs. Shown are roots (R), shoots (Sh), stems (St), leaves (L), and flowers (F). Actin2 (ACT2) gene was used as internal control. b Dry seeds or embryos dissected from intact seeds at 6–48 h after imbibition or germination. c–h Expression patterns of LecRK-b2 revealed by P LecRK-b2 :GUS. Shown are germinating seed (c and d), germinating seed without seed coat (e), before cotyledon opening (f), germinated seedling (g), and flower (h)
LecRK-b2 Transcription Is Induced by ABA and Osmotic Stress
To analyze the gene transcription of LecRK-b2 after ABA, salt, and osmotic treatment, real-time PCR was employed. The results showed that the LecRK-b2 transcripts quickly accumulated to high levels in the Arabidopsis seedlings after 1-h exposure to 50 μM ABA, but the transcripts came back to the original level 6 h later (Fig. 2). In contrast, treatment with 100 mM NaCl or 200 mM mannitol resulted in an accumulation of LecRK-b2 transcripts between 2 and 12 h after treatment (Fig. 2). These results indicate that gene transcription of LecRK-b2 can be transiently induced by ABA treatment, while it can be strongly induced after NaCl or mannitol treatment, and its transcription can preserve on a high level. This differential expression patterns imply that LecRK-b2 plays different roles in ABA signaling and NaCl or osmotic stress response. Furthermore, three ACGT-core motifs (Fig. S1A, D, and E), which were related to ABA inducibility (Kamisugi and Cuming 2005), had been characterized in the promoter sequence of LecRK-b2. These ACGT-core motifs may be responsible for the ABA-inducible gene expression of LecRK-b2.
Real-time PCR analysis of LecRK-b2 gene expression in response to ABA, NaCl, and mannitol. Seven-day-old seedlings grown in MS agar medium were treated with 100 μM ABA, 100 mM NaCl, or 100 mM mannitol for 0, 1, 2, 4, 6, and 12 h, respectively. The mRNA levels were normalized with ACT2. Relative mRNA expression rate in untreated seedlings were set at 1. Data represent the means (±SD) of three independent assays
LecRK-b2 Mutant Slightly Reduced ABA Response in Seed Germination Inhibition
To elucidate the function of LecRK-b2 in the ABA response, we characterized a T-DNA line of LecRK-b2 (SALK_020262), which was designated lecrk-b2 (Fig. 3a). RT-PCR analysis showed that LecRK-b2 transcript was absent in this SALK line (Fig. 3c). It indicates that lecrk-b2 is a LecRK-b2 knockout mutant. Then, we investigated the efficiency of radicle emergence and early growth of lecrk-b2 in the presence of various concentrations of ABA. The germination kinetic profile in the presence of 0.6 μM ABA showed that more lecrk-b2 seeds germinated than wild-type seeds before 5 days after cold treatment, and after 5 days, they all germinated (Fig. 3d). A dose response curve showed that after 4 days at both 0.6 and 0.8 μM ABA, lecrk-b2 had higher germination rates than the wild type (Fig. 3f). We also investigated the postgerminative seedling growth by scoring the cotyledon-greening phenotype. The results showed that lecrk-b2 had 30% more seedling showing green cotyledons than that of lecrk-b2 after 6 days in the presence of 0.6 μM ABA (Fig. 3e) or after 7 days in the presence of 0.8 μM ABA (Fig. 3g).
ABA insensitive phenotype of LecRK-b2. a T-NDA insertion site of SALK_020232, b endogenous ABA content of dry seeds of wild type (white bar) and lecrk-b2 (black bar). c Disrupted mRNA expression of LecRK-b2 in the seedlings of lecrk-b2. d and f Germination rates (radicle emergence); e and g greening cotyledon rates of wild-type (black circles) and lecrk-b2 (black squares); seeds were in the presence of 0.6 μM ABA for 7 days after cold treatment (d and e) or in the presence of various concentrations of ABA at 7 days after cold treatment (f and g). h, i, and j Functional complementation of lecrk-b2 by PLeela-LecRK-b2.The seeds of transgenic lines 3–1, 6–4, 14–6, and wild-type lecrk-b2 were germinated in the 0 or 0.6 μM ABA for 3 days after cold treatment (h) or 7 days (j). i RT-PCR analysis of LecRK-b2 gene expression levels in PLeela-LecRK-b2 transgenic lines. In b and d–h, data represent the means (±SD) of three independent assays
To confirm that this ABA insensitive germination phenotype of LecRK-b2-1 is due to LecRK-b2 loss-of-function mutation, a functional complementation test was performed. The full-length LecRK-b2 CDS was cloned to PLeela vector for overexpression. Then PLeela-LecRK-b2 was transformed into lecrk-b2, and three independently transformed lines, 3–1, 6–4, and 14–6, which have high levels of LecRK-b2 transcript (Fig. 3i) were used for germination assay. The result showed that three LecRK-b2 overexpression lines had 100% germination rate, similar to LecRK-b2 and wild type (Fig. 3h, j). In the presence of 0.6 μM ABA, overexpression lines had a reduced germination rate close to that for the WT compared with a relative higher germination rate for lecrk-b2 (Fig. 3i, j). These results confirm that LecRK-b2 loss-of-function mutation is responsible for the ABA insensitive germination phenotype.
To clarify that whether this ABA insensitive germination phenotype of LecRK-b2 was caused by the lower endogenous ABA accumulation, we examined the ABA levels in maturated dry seeds. The result shows that there is no significant difference between LecRK-b2 and wild type on the ABA accumulation level in the maturated dry seeds (Fig. 3b).
To further investigate whether the faster cotyledon greening is caused by reduced ABA sensitivity, we tested the cotyledon-greening rate and root-elongation rate of germinated seeds in different ABA concentration. However, we did not observe any difference in cotyledon-greening rate and root-elongation rate of germinated seeds between lecrk-b2 and wild type (data not shown). Therefore, the postgerminate growth enhancement of lecrk-b2 is not due to altered ABA sensitivity in later growth stages but presumably due to faster germination.
Taken together, these results suggest that LecRK-b2 loss-of-function mutation reduces the ABA sensitivity during seed germination and then retards the cotyledon greening, and this reduced sensitivity during seed germination is not due to lower ABA accumulation level in the seeds; it more likely relates to ABA or other signaling pathway.
To date, approximately 42 LecRK genes have been identified in the Arabidopsis thaliana genome (Barre et al. 2002). These LecRK genes share highly amino acid sequence identity. Redundant gene functions may result in the weak phenotype of LecRK-b2 loss-of-function mutation.
P LecRK-b2 :LUC Can Be Activated by ABI3
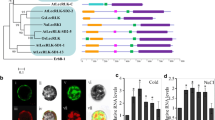
To clarify the relationship between LecRK-b2 and ABA signaling, the promoter sequence of LecRK-b2 was analyzed with Softberry-NSITEP online program (http://linux1.softberry.com/berry.phtml). The result showed that a very similar RY/G motif, a binding site of ABI3 (Ezcurra et al. 2000), existed between −121 and −104 bp relative to the translation start site (Fig. S1A, B). In order to determine the transactivation activity of ABI3 to the P LecRK-b2 , a Dual-LUC transient expression system (Hellens et al. 2005) was used. We examined the effects of ABI3 transiently expressed on the activity of the recombinant P LecRK-b2 :LUC (Fig. 4a). As shown in Fig. 4b, transiently expressed effector plasmid or P LecRK-b2 :LUC solely did not alter the background LUC value, which was detected in the untreated leaves, while in transiently expressed effector plasmid and P LecRK-b2 :LUC together, the LUC value was elevated dramatically. It implies that ABI3 acts as the stimulator of the recombinant P LecRK-b2 :LUC. We further deduce that ABI3 as a transcript factor may also regulate the expression of LecRK-b2 in the Arabidopsis. To confirm this hypothesis, the expression patterns of LecRK-b2 in abi3 were analyzed. The results showed that during the seed germination, the LecRK-b2 transcription level was down-regulated in abi3 compared with wild type (Fig. 4c). We also analyzed the expression pattern of LecRK-b2 in abi3 seedlings under the treatment of ABA and NaCl, but we found no significant changes (data not shown). These results suggest that ABI3 regulates the transcription of LecRK-b2 during the seed germination, while in the adult seedlings, the expression of LecRK-b2 may be controlled by other factors.
ABI3 regulates the transcription of LecRK-b2. a Structure of the P LecRK-b2 :LUC. 35S promoter (black arrow), Renilla luciferase (REN), and firefly luciferase (LUC), LecRK-b2 promoter (white arrow), T-DNA left border (LB), and right border (RB) were indicated. b The effects of ABI3 on the activity of P LecRK-b2 :LUC reporter; leaves of wild type were infiltrated with Agrobacteria harboring the P LecRK-b2 :LUC reporter with or without the effector (pGreenII62-SK-ABI3); (1) uninfilitrated leaves, (2) pGreenII62-SK-ABI3 with empty reporter vector (pGreen-0800-LUC), (3) P LecRK-b2 :LUC reporter only, (4) P LecRK-b2 :LUC reporter with pGreenII62-SK-ABI3 effector. Leaves transfected with Arabidopsis were kept in white light for 3 days, and dual-luciferase assay was used to evaluate the P LecRK-b2 :LUC reporter activity (Liu et al. 2008).The relative LUC activities normalized to the REN activity are shown (LUC/REN, n = 3). c LecRK-b2 mRNA expression levels in wild type and abi3 during germination; data represent the means (±SD) of three independent assays
Disruption of LecRK-b2 causes NaCl and mannitol insensitive root elongation. a and b Comparison of root elongation of wild type and lecrk-b2; 5-day-old seedlings were transferred to 0.5× MS plate with 0, 50, 100, 150, and 200 mM NaCl or with 0, 100, 200, and 300 mM mannitol. Primary root elongation was measured 6 days after the transfer; data represent the means (±SD) of three independent assays. c Growth of wild type and lecrk-b2 on 0.5× MS medium supplemented with 100 mM NaCl or 200 mM mannitol; 5-day-old seedlings were transferred to 0.5× MS plate with 100 mM NaCl or with 200 mM mannitol. Photographs were taken 6 days later
T-DNA Knockout Mutant of LecRK-b2 Reduced Sensitivity to Osmotic Stress in the Early Development Stage of Arabidopsis
RT-PCR analysis has revealed that the gene expression of LecRK-b2 was strongly induced by NaCl and mannitol. To determine whether LecRK-b2 plays a role during salt or osmotic stress response, the root growth assay was performed by transferring 5-day-old seedlings on 0.5× MS plate with 0, 50, 100, 150, and 200 mM NaCl or with 0, 100, 200, and 300 mM mannitol. Primary root elongation was measured 6 days after the transfer in three independent experiments (Fig. 4a, b). Disruption of LecRK-b2 in lecrk-b2 plant exhibits a moderate decline in NaCl stress sensitivity compared to wild type during root growth on 0.5 MS media supplemented with NaCl (Fig. 4a, c); by contrast, LecRK-b2 loss-of-function mutant exhibited significant mannitol stress insensitivity compared with wild type during root growth (Fig. 4b, c). These results indicate that T-DNA knockout mutation of LecRK-b2 reduced sensitivity to salt and osmotic stress in the early developmental stage of Arabidopsis (Fig. 5).
Conclusion
LecRK-b2 is predominantly expressed during seed germination, and its expression was ceased following germination. Its transcription could be transiently induced by ABA, while the transcription could be constitutively induced under NaCl and mannitol treatment. Its transcript level was significantly declined in abi3 during the seed germination. Loss-of-function mutation of LecRK-b2 slightly reduced the ABA sensitivity during seed germination. The reduced sensitivity was demonstrated not due to lower ABA accumulation level in seeds. The mutation reduced salt and osmotic sensitivity during the early development stage of Arabidopsis, which was similar to the stress-induced expression pattern in adult plant. ABI3 could activate the luciferase under driving of the LecRK-b2 promoter. Considering all these, these results offer conclusive evidence that LecRK-b2 functions as a positive regulator of the ABA response during the seed germination and is involved in salt and osmotic stress response in the adult plant.
References
Barre A, Herve C, Lescure B, Rouge P (2002) Lectin receptor kinases in plants. Crit Rev Plant Sci 21:379–399
Bassel GW, Mullen RT, Bewley JD (2006) ABI3 expression ceases following, but not during, germination of tomato and Arabidopsis seeds. J Exp Bot 57:1291–1297
Chen XW, Shang JJ, Chen DX (2006) A B-lectin receptor kinase gene conferring rice blast resistance. Plant J 46:794–804
Ezcurra I, Wycliffe P, Nehlin L, Ellerström M, Rask L (2000) Transactivation of the Brassica napus napin promoter by ABI3 requires interaction of the conserved B2 and B3 domains of ABI3 with different cis-elements: B2 mediates activation through an ABRE, whereas B3 interacts with an RY/G-box. Plant J 24:57–66
Fan L-M, Zhao Z, Assmann SM (2004) Guard cells: a dynamic signaling model. Curr Opin Plant Biol 7:537–546
Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14:S15–45
Gouget A, Senchou V, Govers F, Sanson A, Barre A, Rouge P, Pont-Lezica R, Canut H (2006) Lectin receptor kinases participate in protein–protein interactions to mediate plasma membrane-cell wall adhesions in arabidopsis. Plant Physiol 140:81–90
He XJ, Zhang ZG, Yan DQ, Zhang JS, Chen SY (2004) A salt-responsive receptor-like kinase gene regulated by the ethylene signaling pathway encodes a plasma membrane serine/threonine kinase. Theor. Appl. Genet 109:377–383
Hellens R, Allan A, Friel E, Bolitho K, Grafton K, Templeton M, Karunairetnam S, Gleave A, Laing W (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1:13
Herv C, Dabos P, Galaud JP, Roug P, Lescure B (1996) Characterization of an Arabidopsis thaliana gene that defines a new class of putative plant receptor kinases with an extracellular lectin-like domain. J Mol Biol 258:778–788
Hervé C, Serres J, Dabos P, Canut H, Barre A, Rougé P, Lescure B (1999) Characterization of the Arabidopsis lecRK-a genes: members of a superfamily encoding putative receptors with an extracellular domain homologous to legume lectins. Plant Mol Biol 39:671–682
Hetherington AM (2001) Guard cell signaling. Cell 107:711–714
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Hirsch AM (1999) Role of lectins (and rhizobial exopolysaccharides) in legume nodulation. Curr Opin Plant Biol 2:320–326
Jones HD, Peters NC, Holdsworth MJ (1997) Genotype and environment interact to control dormancy and differential expression of the VIVIPAROUS1 homologue in embryos of Avena fatua. Plant J 12:911–920
Kamisugi Y, Cuming A (2005) The evolution of the abscisic acid-response in land plants: comparative analysis of group 1 LEA gene expression in moss and cereals. Plant Mol Biol 59:723–737
Kuhn JM, Boisson-Dernier A, Dizon MB, Maktabi MH, Schroeder JI (2006) The protein phosphatase AtPP2CA negatively regulates abscisic acid signal transduction in arabidopsis, and effects of abh1 on AtPP2CA mRNA. Plant Physiol 140:127–139
Leung J, Giraudat J (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49:199
Li B, Foley ME (1997) Genetic and molecular control of seed dormancy. Trends Plant Sci 2:384–389
Liu H, Yu X, Li K, Klejnot J, Yang H, Lisiero D, Lin C (2008) Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in arabidopsis. Science 322:1535–1539
McCarty DR (1995) Genetic control and integration of maturation and germination pathways in seed development. Annu Rev Plant Physiol Plant Mol Biol 46:71–93
Nishiguchi NM, Yoshida YK, Sumizono ST, Tazaki TK (2002) A receptor-like protein kinase with a lectin-like domain from lombardy poplar: gene expression in response to wounding and characterization of phosphorylation activity. Mol Gen Genomics 267:506–514
Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J (1994) Regulation of gene expression programs during arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6:1567–1582
Raz V, Bergervoet JH, Koornneef M (2001) Sequential steps for developmental arrest in Arabidopsis seeds. Development 128:243–252
Riou C, Herv C, Pacquit V, Dabos P, Lescure B (2002) Expression of an Arabidopsis lectin kinase receptor gene, lecRK-a1, is induced during senescence, wounding and in response to oligogalacturonic acids. Plant Physiol. Biochem. 40:431–438
Steven J, Clough AFB (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Wan J, Patel A, Mathieu M, Kim SY, Xu D, Stacey G (2008) A lectin receptor-like kinase is required for pollen development in Arabidopsis. Plant Mol Biol 67:469–482
Xin Z, Wang A, Yang G, Gao P, Zheng ZL (2009) The Arabidopsis A4 subfamily of Lectin receptor kinases negatively regulates abscisic acid response in seed germination. Plant Physiol 149:434–444
Yoshida T, Nishimura N, Kitahata N, Kuromori T, Ito T, Asami T, Shinozaki K, Hirayama T (2006) ABA-hypersensitive germination3 encodes a protein phosphatase 2C (AtPP2CA) that strongly regulates abscisic acid signaling during germination among arabidopsis protein phosphatase 2Cs. Plant Physiol 140:115–126
Zeng Y, Raimondi N, Kermode AR (2003) Role of an ABI3 homologue in dormancy maintenance of yellow-cedar seeds and in the activation of storage protein and Em gene promoters. Plant Mol Biol 51:39–49
Acknowledgment
This research was supported by grants from the National Natural Science Foundation of China (30600368, 30770200, and 30871325).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Fig. S1
Putative cis elements involved in the regulation of the LecRK-b2 gene. (A and B) RY/G-like element, (C) start codon, (D and E) ACGT-core motif. (DOC 395 kb)
Rights and permissions
About this article
Cite this article
Deng, K., Wang, Q., Zeng, J. et al. A Lectin Receptor Kinase Positively Regulates ABA Response During Seed Germination and Is Involved in Salt and Osmotic Stress Response. J. Plant Biol. 52, 493–500 (2009). https://doi.org/10.1007/s12374-009-9063-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-009-9063-5