Abstract
The worldwide expansion in energy and resource use has resulted in a number of unsustainable innovations, necessitating the development of resource sustainability and a reduction in energy usage. Valorization of industrial waste is centred on reducing the amount of pollutants in the environment as well as increasing the revenue generated by industries. Sugarcane processing generates large amount of by-products, namely cane trash, bagasse, molasses and press mud which can be valorized into various value-added products. In this paper, the authors reviewed the variety of applications of sugar industry by-products that has been physically and chemically transformed. It also observed that the technology for producing power from the by-products has advanced, while the manufacture of value-added chemicals has not. The key technological challenges in this area are downstream separation and purification. The difficulties in putting these waste valorization methods in place are also discussed. The amount of investigation and implementation of various solutions varies a lot. In order to translate research findings into commercial products, both business participation and government encouragement are essential. Economic and technological constraints must be recognized for effective commercialization. Some interesting areas were also highlighted which can become the basis for further investigations and could act as guidance for further research in this domain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
All living creatures require sugar as a source of energy (Iwuozor et al. 2022a). Sugar cane and sugar beet are the primary sources of crystalline sugar for humans (Afiomah and Iwuozor 2020). Every year, roughly 125–130 million tonnes of sugar are produced worldwide, with two-thirds coming from sugarcane and one-third from sugar beet (Casu et al. 2012; Chauhan and Rai 2012). Sugarcane (Saccharum spp.) is a massive, thick perennial grass belonging to the Poaceae family (Iwuozor et al. 2021a; Williams et al. 2016). Sugarcane is a major crop in countries like Brazil, India, Thailand, China and Australia that are tropical or subtropical. Sugarcane, among other crops, has a high sucrose content, a high biomass content and a high production efficiency (Adeniyi et al. 2019). The plant's abundance is undeniable, as Brazil being the largest producer of sugarcane in the world produced about 647 million metric tons of sugarcane in the 2019/20 sugarcane crushing season, the majority of which was used in the ethanol and/or sugar industries (Barros 2020; Bezerra and Ragauskas 2016).
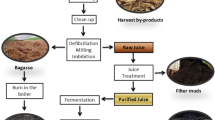
Sugar processing is a lengthy process as shown in Fig. 1 that involves multiple phases in order to produce the final product (crystal sugar) (Akbar and Ali 2017). The sugar industry is regarded as one of the most significant sources of pollution in the environment. The industrial processing of sugar involves a number of physical and chemical techniques. Calcium hydroxide (milk of lime, Ca(OH)2), carbon dioxide (CO2), phosphoric acid (H3PO4), sulphur dioxide (SO2), polyelectrolytes, polyacrylamide flocculent, caustic soda (NaOH), soda ash (Na2CO3), lead sub-acetate and hydrochloric acid are among the most commonly used compounds in the industry. The presence of all of these complicated contents in sugar industrial waste causes environmental pollution, but on the other hand, it provides opportunities for valuable by-products isolation and re-use of polluting elements (Akbar 2006; Iwuozor 2019a; Iwuozor and Gold 2018; Zhul-quarnain et al. 2018).
Environmental awareness and ecological concerns are driving the development of novel eco-friendly materials (Adeniyi et al. 2020a, b; Iwuozor et al. 2021d). Waste and by-products created during the sugar production process are a rich source of high-value chemicals that could be employed as food/chemical additives and/or nutraceuticals as shown in Fig. 2 (Esparza et al. 2020; Gharib-Bibalan 2018). The principal by-products of the sugar industry are bagasse, cane trash, press mud and molasses. Molasses can contain up to 48% sugar. It is widely utilized in livestock feeds and other industrial processes. In the case of untreated effluent discharge, it can also be a major source of contamination in water bodies (Casu et al. 2012). Molasses is a common substrate used in the fermentation industry as a feedstock for the manufacture of ethanol and baker's yeast. It is more appealing for industrial fermentation and other related uses due to its abundance, high sugar content and cheap cost availability (Cueva-Orjuela et al. 2017; Fatoye and Onigbinde 2020).
The worldwide expansion in energy and resource use has resulted in a number of unsustainable developments, necessitating the development of resource sustainability and a reduction in energy usage (Adeniyi et al. 2021; Umenweke et al. 2021). Sugarcane is one of the most widely cultivated crops in the world with great demand. As a result, it would be beneficial to examine and discuss the various valorization strategies, as well as the factors impacting the valorization of its by-products. Lots of studies have reviewed the valorization of these wastes, but none of the works in published literature has comprehensively studied current progresses that has been made in this field irrespective of region with a view to providing a stepping stone for future researches. This study is aimed at reviewing the use of various valorization techniques aimed at reducing hazards and recovering value-added products from sugarcane in order to provide an overview and key insights into current progress in the disposal and valorization of this abundant waste generated in the sugar industry. Some interesting areas were observed by this review which can become the basis for further investigations in the future.
By-products of the Sugar Industry
From the cane and beet harvesting stage, all through to the milling stage and down to the sugar refining stage (which includes numerous separation processes), the sugar industry generates vast amount of waste materials and by-products which are rich in chemicals and have great potential for use in numerous other processes. By-products of sugar industry include cane trash, sugarcane bagasse, press mud, molasses, wastewater, etc.
Generally, for every tone of sugarcane harvested, approximately 270 kg of cane trash is generated. Also, for every tone of sugarcane crushed 0.3 tonne of bagasse is produced. Similarly, for every tone of sugar refined, 0.03 tonne of press-mud and 0.041 tonne of molasses are generated (Meghana and Shastri 2020). This review will focus on the applications of cane trash, sugarcane bagasse, press mud and molasses (shown in Fig. 3).
Cane Trash
Cane trash consists of the leaves and other extraneous matter which are found together with the sugarcane stalk during harvesting of the cane. Per ton of sugarcane harvested, 0.09–0.11 ton of trash is generated (Balakrishnan and Batra 2011; Singh et al. 2008). Traditionally, waste is disposed of by burning in fields. It is left in the field after cane harvesting because the presence of large amount of cane trash with the stalk during the processing of the cane to produce sugar can lead to the following: increased wear of machinery cane knives, mill rollers, etc., extend load on juice clarification and crystallization adversely affecting sugar quality and recovery, higher loss in milling due to unwanted fibrous material, lower juice purity resulting in lower sugar recovery and more molasses formation (Mohan 2021). The cane trash which consists of 12–23% of the total cane harvested is a substantial by-product of the sugarcane industry (Romero et al. 2007). Cane trash has been utilized in various application fields, as shown in Table 1.
Besides being availed as cattle feed (Mahala et al. 2013) and cane field blanketing purposes, studies focusing on utilization of cane trash along with bagasse for heat and electricity required to make sugar mill energy self-sufficient along with surplus electricity generation have been reported (Khatiwada et al. 2016). Cane trash along with bagasse has been extensively studied for power generation using biomass integrated-gasifier/gas turbine combined cycle technology (BIG/GT-CC), especially in Brazil and Cuba (Balakrishnan and Batra 2011). However, BIG/GT-CC technology has not yet been commercialized (Rasche and Del Diego 2020). Utilization of 9 and 27% of the cane trash recovered from the field, in combination with bagasse for electricity production, increased the surplus electricity generation by 22 and 57%, respectively, in Brazilian sugarcane biorefinery scenario (Sampaio et al. 2019). Production of 2G biofuels, such as lignocellulosic ethanol from cane trash in combination with bagasse, has also been explored (Chandel et al. 2012; Krishnan et al. 2010). Another study looked at optimal utilization of cane trash for energy demands in a sugar mill to produce bagasse derived ethanol. This study highlighted the trade-off between the economic benefit of utilizing trash and the environmental cost of the added fertilizer requirement (Vikash et al. 2018). Additional fertilizer is required to meet the necessary nutrient content deficit caused by trash removal from the farm, which in turn increased the environmental burdens due to fertilizer production. Higher content of lignin (36.1%) and silica (6.96%) in sugarcane leaves limits its industrial applications (Chandel et al. 2012).
In India, Brazil and Thailand, cane trash is generally retained in the fields that have adapted green harvesting (Chandel et al. 2012; Rasche and Del Diego 2020). Blanketing replenishes the soil condition by the addition of organic nutrients. Further, it retains the moisture of soil and prevents weed growth and ill effects due to extreme temperatures (Ram et al. 2006; Yadav et al. 1994). Conversion of sugarcane leaves into biochar for cooking applications is another eco-friendly practice of valorization (Bhatnagar et al. 2016). Appropriate Rural Technology Institute of India (ARTI) developed kilns that can produce charcoal briquettes from cane trash. These briquettes can be utilized as fuel in non-pressurized cooker developed by ARTI (Meghana and Shastri 2020). This practice of charcoal briquettes production has been adapted in some regions of South India (Balakrishnan and Batra 2011). Charcoal briquettes production is economically viable and environmental benign practice that also aids in the reduction of deforestation (Bhatnagar et al. 2016).
Molasses
This section discussed the composition and applications of molasses, the by-product of the sugar processing mill. The composition and applications of molasses are clearly presented in the subsections that follows. Sugarcane molasses (SCM) is the viscous liquid produced after sucrose crystals have been removed from the concentrated juice by centrifugation. In the sugar manufacturing process, three vacuum evaporator crystallizers are employed. The juice is fed into the first vacuum which removes the crystalline sucrose and the by-product is called ‘molasses A’. This by-product goes through the second vacuum, and the by-product is ‘molasses B’, and then through the third vacuum to ensure complete extraction of the crystalline sucrose, leaving behind the final by-product ‘molasses C’, a dark viscous liquid also called blackstrap molasses (Bhatti et al. 2019; de Oliveira Lino et al. 2018; Gutiérrez-Rivera et al. 2015). Molasses has a molecular formula of C6H12NNaO3S, a molecular weight of 201.22 g/mol and a density of 1.41 g/cm3 (Jain and Venkatasubramanian 2017). On estimate, 100 tons of sugarcane produces 7 tons of molasses (Boviatsi et al. 2020), with over 160 m ton/year produced globally (Vidra et al. 2017). SCM is mainly used in the production of ethanol, rum and as a supplement in livestock feeds. It has also been utilized in the production of butanol, sorbitol, citric acid, succinic acid, lactic acid, among others.
Composition of SCM
The general composition of SCM differs according to the geographical location of the producing country (Jamir et al. 2021). Molasses has a complex composition with different studies reporting several components and varying ranges of the components. This is attributed to the sugar type and method of refining. SCM contains on average, 62.3% of easily fermentable sugars (Palmonari et al. 2020), proteins, vitamins and trace elements. The sugars in SCM are mostly sucrose (48.8%), glucose (5.29%) and fructose (8.07%), with very little amounts of galactose (0.04%), raffinose (0.03%) and arabinose (0.01%). Besides sugars, SCM also contains, brix, ash, pH, minerals such as calcium, magnesium, sodium, potassium, iron, copper, manganese and zinc. Increase in concentrations of these metal ions results in an increase in inhibition against the activity of the enzyme, invertase, secreted by yeast to convert sucrose to reducing sugar (Raharja et al. 2019). Table 2 presents a summary of the findings of the compositions of SCM (but not restricted to that). The values are not final determinants but on average or simply as reported in the literature.
Applications of Sugarcane Molasses
SCM is widely used in many industries ranging from foods, plastics, to agro industries and many more, for the production of many value-added products. Some of these areas that highly utilize molasses are highlighted below.
Bioethanol Production
Bioethanol is ethanol produced by microbial fermentation. It is one of the most important biofuels proposed as an alternative to the depleting and price fluctuating fossil fuels (Akhabue et al. 2019). Unlike fossil fuels, bioethanol can reduce the amount of carbon dioxide and hydrocarbons released into the atmosphere and lowers environmental degradation because of its high octane number (Mayzuhroh et al. 2016). Molasses is the by-product of sugar manufacturing process primarily used in the production of ethanol because of its low cost, availability and ability to produce fermentable sugars without any prior treatment (El-Gendy et al. 2013). One litre of ethanol can be produced from approximately 4 kg of molasses, though this can vary depending on the extraction method and sugar content of the molasses (Rasmey et al. 2018). In 2013, 60% of total ethanol produced globally came from molasses (Boviatsi et al. 2020). The process of bioethanol production from molasses involves direction fermentation of the already stored substrate (molasses) since the content sugars are easily converted to ethanol be anaerobic fermentation.
Fermentation is normally carried out using microorganisms such as yeast, bacterial or fungal. The yeast saccharomyces cerevisiae (S. cerevisiae) is the most commonly used microorganism in the fermentation of molasses to ethanol, accounting for majority of ethanol production by anaerobic process. The bacteria zymomonas mobilis (Z. mobilis) have also been recently utilized in fermentation of sugar to ethanol (Khoja et al. 2018, 2015), though not yet on a commercial scale. Many factors are considered in the selection of yeast for fermentation which include osmotic stress in molasses caused by the high quantity of salts of non-carbon origin, tolerance to yeast cells, flocculation capacity that is dependent on the process demands and good specific ethanol productivity (Bhatti et al. 2019; Fadel et al. 2013). Bacteria contamination which grows under the same conditions as the yeast and competes with the yeast for the available sugars can also drastically reduce the efficiency of the yeast, resulting in decreased ethanol yield (Inaba et al. 2013). The use of lactatic can effectively remove this contaminant. The anaerobic reaction between molasses and S. cerevisiae produces zymase enzyme which acts as catalyst to convert sugar into ethanol, carbon dioxide and heat according to the following equation (Bhatti et al. 2019; Gasmalla et al. 2012).
The CO2 is captured and liquefied for sale to other industries such as beverages and refrigerator-producing companies (Bhatti et al. 2019). The fermented liquor is then introduced to the distillation column where ethanol is produced. Table 3 presents some of the studies on bioethanol production by SCM, with the yeast and the optimum operating conditions (pH, temperature and fermentation period). Using locally isolated S. Cerevisiae Y-39, El-Gendy et al. (2013) designed and optimized a statistical model for maximum bioethanol production and obtained a maximum ethanol production of 255 g/L in a batch fermentation process. Wu et al. (2020) replaced the fermentation gene, PHO4, from fast-growing S. cerevisiae strain, MC15 to MF01 via homologous recombination to yield an industrially engineered strain, MF01-PHO4. The ethanol yield rose to 114.7 g/L, an increase of 5.30% in ethanol production and 12.5% decrease in fermentation time when compared to that of the original strain, MF01, one of the highest ethanol-producing strains in SCM fermentation.
Production of Lactic Acid
Lactic acid (2-hydroxypropanoic acid) is the most common hydroxycarboxylic acid with the molecular formula CH3CHOHCOOH. It is an organic acid with α-hydroxyl and acid functional group (Alves de Oliveira et al. 2018), used in many industries such as food, textile, pharmaceutical and chemicals, and as a monomer in the production of the biodegradable and biocompatible polymer, polylactic acid (PLA), which has several applications (Chaisu et al. 2014). Pure sugars are often used as raw material for the production of lactic acid. However, the high cost of pure sugars and that of the subsequent lactic acid produced make the process economically disadvantaged (Farooq et al. 2012). The high cost of lactic acid produced from pure sucrose has also limited the production of PLA which has to compete with the traditional petrochemical-based plastics to remain competitive in the market (López-Gómez et al. 2019). Hence, the need for an alternative low-cost substrate like SCM is also readily available and enhances lactic acid yield.
On a commercial scale, lactic acid is produced either by chemical synthesis or by biotechnological fermentation. The chemical synthesis is based on the use of lactonitrile hydrolysed with strong acid (Rodrigues et al. 2017), while the biotechnological fermentation is based on the use of microorganisms to degrade the substrate (molasses) into metabolites such as lactic acid (Komesu et al. 2017). Production of lactic acid by microbial fermentation has many merits over chemical synthesis, such as low temperature, high purity, low concentrations of inhibitors and ability to be stored and use all year round (Alves de Oliveira et al. 2018; Oliveira et al. 2016b, a). In addition, microbial fermentation produces the more desired stereoisomer, optically pure L- or D-lactic acid, while chemical synthesis produces the racemic mixture of DL-lactic acid. Currently, fermentation process accounts for about 80–90% of the global lactic acid production and it is estimated that the demand for this product will rise from 1220 kilotons in 2016 to 1960 kilotons in 2025 (López-Gómez et al. 2019). The commonly used microorganism in lactic acid production is lactobacillus sp., because of its tolerance to acid medium, and also being able to be modified to produce a specific lactic acid optical isomer (Oliveira et al. 2016b, a). The presence of metal ions in SCM may inhibit cell growth and affect the medium pH during fermentation. Therefore, pre-treatment with dilute acid may be required before fermentation (Vidra et al. 2017), though this may increase the production cost. In the polymer industry, lactic acid is dehydrated in the presence of acid catalyst to give lactides according to the equation below. The lactides are then polymerized to obtain the biodegradable thermoplastic polymer, PLA (Komesu et al. 2017).
Table 4 presents some published works on lactic acid yield and productivity using SCM substrate. Sun et al. (2019) used a microbial consortium, CEE-DL15, consisting of Clostridium sensustricto, Escherichia and Enterococcus in batch fermentation process to produce one of the best published lactic acid productivity from molasses (4.49 g/Lh) with a yield of 0.81 g/g. Vidra et al. (2017) produced lactic acid from SCM using two Lactobacillus species: Lactobacillus casei and Lactobacillus sp. MKT87. The concentration of lactic acid produced after 150 h was 83 g/L and 68 g/L, respectively, with a yield of 0.57 g/g and 0.76 g/g. The amount of enzyme utilized or the length of pre-treatment time was not sufficient as a result of lack of invertase to fully hydrolyse the sucrose. This leads to the low lactic acid productivity reported.
Production of Rum
Rum is the alcoholic beverage produced from SCM. It is a fairy tasteless and neutral spirit produced from fermentation and distillation of molasses and sugar juice or syrup, traditionally produced by the West Indies and Central Americans (Pino and Roncal 2016; Pino et al. 2012). Rum is one of the most popular alcoholic beverages with a global consumption of over 1 billion litres per year (Hinojosa-Nogueira et al. 2020). The French-speaking Caribbean commonly uses sugarcane juice for the production of rum, but about 97% of rums are produced from molasses (Medeiros et al. 2017). Several countries produce different types of rums, but the major types include the white, dark, gold, over-proof, spiced and Demerara rums. However, the grade and quality of a rum ultimately depend on the quality of molasses, the production process, the desired product specifications and the countries legislations. The production of rum from SCM commonly follows 3 processes: the fermentation of the molasses, distillation of the ethanol produced, ageing in barrels and finally dilution, to adjust the desired alcohol content (at least 37.5 vol.%) (Franitza et al. 2018).
Fermentation is the process by which the sugars in molasses are converted to ethanol by the action of a microorganism (usually yeast). The major difference between the types of rum produced depends on the type of microbial strain used for fermentation. Typically, strains of S. cerevisiae are employed in the fermentation of molasses for rum production. Water is first added to the must (sugary molasses) before fermentation enhances the growth of the strain. Fermentation can last from 20 h to 4 days for light rums and up to 3 weeks for heavy rums (Medeiros et al. 2017). During this time, more than a hundred different aroma-active compounds are formed (Franitza et al. 2016; Hinojosa-Nogueira et al. 2020). Such compounds like alcohols, ethyl acetate, acetic acid, ethyl esters, benzoic acid, vanillin, vanillic acid, among others, all help to confer on the rum, its flavoury taste. Distillation is carried out after fermentation to separate the volatile components of the fermented products and concentrate the ethanol produced (Mangwanda et al. 2021). Distillation can be carried out in either the pot still or column still (still commonly refers to the distillation unit) with the former preferred industrially. Alcohol in the fermented broth is evaporated during distillation, re-condensed and collected as raw spirit (Mulye 2019).
Rum needs time to mature, and just like other spirits, ageing or maturation is carried out to improve the quality of the final product. Rum ageing is carried out in oak barrels previously used in whisky or brandy production (Franitza et al. 2016). The period of the ageing depends on the producing country. However, rums require a minimum of 1–2 years to be matured, except over-proof rum which can be sold in some countries without ageing (Mangwanda et al. 2021). Over-proof rum, unlike other rums, also contains up to 70–80% alcohol vol% (Mangwanda et al. 2021). Oxidative reactions, condensations, esterifications and other reactions going on in the oak casts give the freshly distilled rum its final character and colour (Pino and Roncal 2016). Blending is carried out on the final rum by the master blender, modifying it to the desired alcohol content. The addition of sugar up to 6 g/L may be allowed depending on the rum type, so did caramel for colouration and activated charcoal for decolouration, and other desired ingredients (Medeiros et al. 2017).
Livestock Feed Supplement
In developing countries, only about 20% of agricultural outputs are attributed to livestock production despite the increasing demand for milk and meat in these countries (Windsor et al. 2020). The main reason for this low output is the poor quality of diets the animals are fed on, especially during the dry season. The major sources of feed for dairy cows are natural grass hay, elephant grass, purchased concentrate feeds (soya bean, grass pea and maize) and brewery grain (Demoz et al. 2018). However, these crop residues and forages are low in nitrogen and high in the crude fibre, lignin, restricting their intake and digestibility (Lawania and Khadda 2017), and continuous feeding of the animals with these poor quality forages with little amount of energy and concentrate ultimately leads to a lower milk production and poor productive performance (Jayawickrama et al. 2013). Different strategies have been employed in a bid to improve the quality of dairy feeding, of which feed treatment and supplementation have been regarded as the best feeding strategy. Including sources of readily fermentable sugars and nitrogen to the feeds of ruminants to ensure their good body conditions has been recommended as they improve the digestibility and bioavailability of nutrients (Assefa and Nurfeta 2013). Urea, a non-protein nitrogen-rich compound, has been widely used to treat livestock feed as its nitrogen content can be used by microbial organisms in the rumen to produce protein. Inclusion of urea in dairy feeds, however, leads to a release of excess nitrogen that far overweighs the energy in the rumen for microbial protein synthesis, and consequently leading to the absorption of this nitrogen as ammonia by the rumen and lost as urea (Kiani et al. 2013). To avoid such occurrence, a readily available sugar source, such as SCM, is supplemented to the feed. Feeding a sugar-based product in diet to livestock can result to a change in ruminal fermentation patterns and decrease ammonia concentration (Martel et al. 2011). Sugars are fermented rapidly in rumen than starch. SCM, in dry or liquid form, is added to dairy feeds to improve microbial growth in rumens which promotes the digestion of fibre and non-protein nitrogen (Rahiman and Pool 2016). In addition, SCM is classified as concentrate, which is a component of feed that provides nutrients such as protein, carbohydrate and fat at higher levels and contains less than 18% crude fibre, and reduced moisture (Trivedi and Shah 2014).
Urea-molasses mineral block (UMMB) has been for years, considered as one of the easiest and effective way of treating and supplementing cattle feed as it provides readily available source of energy and protein in the form of molasses and nitrogen in the form of urea, as well as fibre and minerals (Jayawickrama et al. 2013). Supplementation with UMMB can increase fibrous feed digestibility by up to 20%, feed intake by 25–30% productivity and reproducibility of diary animals (Mengistu and Hassen 2017). Lawania and Khadda (2017) supplemented the diet of zebu lactating cows with UMMB and the result revealed an improved nutrient intake and milk production. Similar result was obtained by Windsor et al. (2020), Kebede et al. (2018), and host of others. An improvement in the productive performance of sheep was also reported (Sheikh et al. 2017). However, Jayawickrama et al. (2013) found no effect no effect on milk production of dairy cows after being fed with UMMB-supplemented basal diet. This was attributed to the fact that the basal diet (rice straw) is a good quality feed with sufficient amount of concentrate and, hence, needs no supplementation.
SCM has also been used exclusively to supplement livestock diets at relatively low concentrations, leading to an enhanced growth, increased milk and meat quality. Osman et al. (2020) reported improved growth performance, protein metabolism and efficient rumen fermentation of goat kids fed with molasses-supplemented diet for 3 weeks without harming their growth and immunity. Assefa and Nurfeta (2013) supplemented molasses in wheat brain in a concentrate mixture to improve the intake of dietary dry matter, organic matter, metabolizable energy and growth performance of female crossbred heifer calves. The ability of sugars to ferment rapidly in rumen leads to production of lactic acid but a decrease in ruminal pH, which could potentially depress fibre digestibility (Martel et al. 2011). Siverson et al. (2014) reported that replacing molasses-based products for corn did not influence productivity and had little impact on milk fatty acid production. Similar results on no effect on milk production and animal performance were also reported by Martel et al. (2011), Baurhoo and Mustafa (2014), Salvador-Loreto et al. (2016), among others. A decrease in milk productivity was even reported by Trivedi and Shah (2014) after introducing molasses to the basic feed plan of dairy farm. Although SCM may be a good source of energy and protein, several others factors like timing of the supplementation, ease of feeding, lactation stage of the ruminants and environmental factors need to be assessed to determine its viability in animal feeds supplement (Trivedi and Shah 2014). The summary of some of the results in open literature on the treatment and supplementation of livestock feeds with urea-molasses mineral block and sugarcane molasses is presented in Table 5.
Therapeutic Potentials and Other Applications
Molasses is categorized as ‘Generally Regarded as Safe’ (GRAS) product, by the US food and drug administrator. SCM is known to contain a high amount of polyphenols, making it a very good antioxidant agent, and a potential agent in the prevention of several chronic diseases that involves oxidative stress (Iwuozor 2019b). The antioxidant effect of SCM has been studied by many groups (Asikin et al. 2013, 2016; Deseo et al. 2020; Valli et al. 2012). SCM was found to be a very god agent that could protect against oxidative DNA damages caused by peroxyl radicals (Asikin et al. 2013) and free radicals (Asikin et al. 2016). This validates its use in bioresource-based products. SCM has been reported to produce lowered peak and global responses to glucose, insulin, amylin and gastric inhibitory polypeptide in healthy rats (St-Pierre et al. 2014), making it a potential save alternative to refined sugars. SCM also decreased mutation and oxidation and inhibits reactive nitrogen species in lipopolysaccharide stimulated macrophages, showing its biological activities in antioxidation, antimutation and anti-inflammation (Wang et al. 2011). This antimutation effect of SCM has been consolidated in its successful inhibition of heterocyclic amine compounds (Cheng et al. 2021), and advanced glycation end-products (Yu et al. 2017). A filtered SCM concentrate has shown potentials to lower blood glucose level and insulin responses, reduces obesity and helps in diabetes treatment (Ellis et al. 2016).
A polyphenol-rich sugarcane extract (PRSE) consisting of sugarcane juice and molasses has been reported as a potential antiageing agent that should be considered in skin care cosmetics industries with more works (Ji et al. 2020b). PRSE also exerts anticancer properties on a range of cancer cells including colon cancer cell lines, human lung cancer, human ovarian cancer, pro-monocytic human leukaemia and mouse melanoma cell lines (Prakash et al. 2021). PRSE was recently reported to halt the uptake of glucose and fructose in the intestinal epithelial barrier, Caco-2, and revive insulin production in dysfunctional β-cells (Ji et al. 2019). Furthermore, the extract also has reportedly shown potentials to exert neurological benefits through promotion of genes that encode neurogenesis-related growth factors and regulate neuronal differentiation while also preserving neuronal DNA oxidative stress damage (Ji et al. 2020a). The antibacterial potential of SCM has also been demonstrated (Shafiqa-Atikah et al. 2020). This medicinal value of SCM is as a result of its phytochemical properties and in particular, its phenolic and flavonol content. The complex nature of SCM, however, makes it difficult to underpin the components responsible for its vastly researched antioxidant property (Deseo et al. 2020).
In the food industry, SCM is commonly used in foods like cookies or pies, barbecue sauces, ginger bread, among others, because of its special characteristics flavour and sweetish aroma (Mulye 2019). SCM extract can reduce hazardous compound formation during meat processing (Cheng et al. 2021). It also has potentials as a natural functional ingredient capable of modifying carbohydrate metabolism and contributing to glycaemic index reduction of processed foods and beverages (Wright et al. 2014). Moreover, SCM can also be used in the production of succinic acid (Chan et al. 2012), citric acid, butanol, sorbitol, among others. It is used in hookah to add flavour to tobacco and shisha (Jamir et al. 2021).
In spite of this numerous potentials of SCM, it is still not fully utilized. Molasses is a by-product that is easily affordable and accessible to livestock farmers in many sugarcane producing countries, yet not utterly utilized in that regard. In order to meet the global projected 19% and 33% increase in meat and milk production, respectively, by 2030 (Windsor et al. 2020), a mechanistic farming method needs to be adopted, and these include improvement of the livestock feeds. Having shown its efficiency, feed supplementation with SCM and other additives should be made a conventional livestock feeding strategy, and awareness created for local farmers to fully take advantage of this. In addition, most of the therapeutic potentials of SCM are pre-studies that requires more works for support. This opens an interesting research area that researchers should be further explored to provide more insights on the medicinal efficacies of this by-product.
Press Mud (PM) or Filter Cake
The dark brownish amorphous residue obtained during the clarification of cane juice is called press mud, and it is generated at about 3% of sugarcane processed (Gupta et al. 2011). The composition of press mud generated is influenced by locality, cane variety, milling process and the clarification process chosen for sugar purification. Main components of press mud are moisture (50–65%), fibre (15–30%), crude wax (5–14%), sugar (5–15%), crude protein (5–15%) and nitrogen (2–2.5%) (Gangavati et al. 2005). Sugarcane industries from all over the world are producing large amounts of PM every year, and the disposal of this by-product is a vital concern. Usually, PM is being dumped as garbage in open fields or sold/given to farmers to use as fertilizer. This disposal method pauses some environmental challenges such as air pollution due to odour, surface and ground water pollution and overall pollutes the environment. Recently, much attention has been focused on better use of PM as shown in Table 6. Ansari and Gaikar (2014) reported PM could be a potential source of hydrocarbons and valuable chemicals on thermochemical conversion.
Several intermediate products such as enzymes, biogas/methane, compost, wax and protein have been produced from sugarcane PM (Sarker et al. 2017). In India, press mud is utilized for field applications usually in combination with the spent wash as fertilizer or sold as compost or burnt in kilns to manufacture bricks (Ansari and Gaikar 2014; Kumar and Chopra 2016). In Brazil and China, press mud is used as fertilizer in combination with spent wash or bagasse ash in sugarcane cultivation (Xu et al. 2019). The decomposition of press mud in such land application practices is detrimental to the environment due to leaching and emission of greenhouse gases (George et al. 2010).
The extraction of residual sugar, wax and protein from sugarcane press mud has been studied (Partha and Sivasubramanian 2006). In particular, it is possible to obtain microcrystalline wax with degree of crystallinity comparable to that of carnauba wax (Phukan and Boruah 1999). Press mud has also been used as a substrate in solid state fermentation for production of citric and lactic acids (Shankaranand and Lonsane 1993; Xavier and Lonsane 1994). The organic components in press mud make it a possible source for biogas production by anaerobic digestion. Such a facility was set-up in a sugar factory in western India with the biogas obtained being piped to households in the factory premises (Kumar 1996). The yield was reportedly 165 L biogas/kg press mud with 60% methane content. In another study, press mud treated in a biphasic reactor resulted in a yield of 9m3/ton biogas with 70–75% methane content (Balakrishnan and Batra 2011). Additionally, the resulting sludge had a high N/P/K value and is suited for use as a fertilizer. On the whole, for the production of high-value chemicals from press mud, process development and scale-up, ensuring consistent product quality, etc., still needs to be investigated.
There are continuing studies on the ability of press mud to provide adequate nitrogen and phosphorus for specific crops (Gupta et al. 2008; Jamil et al. 2008; Muhammad and Khattak 2009). In this context, enrichment of press mud by vermicomposting has been studied by mixing with other wastes like cow dung (Prakash and Karmegam 2010), bagasse and sugarcane trash (Kumar et al. 2010). Press mud has also been used in aquaculture for promoting the growth of carp (Keshavanath and Gangadhara 2006). Yet another application is as an adsorbent. Based on its porous structure and presence of polar groups, it is predicted that press mud would be a good biosorbent for metal ions, dyes, etc. (Gupta et al. 2011). Utilizing press mud as a fertilizer either directly or after biocomposting with distillery effluent is a popular practice. Bulk usage is possible, and the approach is perceived to be an environment friendly way of increasing the nitrogen and phosphorous contents in the soil. In contrast, other waste-based soil amenders like municipal waste compost have associated environmental concerns such as accumulation of heavy metal and other pollutants in the soil over time (Déportes et al. 1995). However, recent reports indicate that decomposition of press mud generates acid leachate and also emits significant amounts of greenhouse gases (George et al. 2010). Further, press mud can also lead to immobilization of inorganic nitrogen (Rasul et al. 2006). Because land application is well established, there is relatively less incentive for developing alternative products from this waste.
Bagasse
In this section, the composition and applications of the sugarcane waste, bagasse, are discussed. The composition and major applications were presented step by step in the subsequent subsections for further explanations with more emphasis on the value-added products. Table 7 presents some of the published chemical composition (on average) of the sugarcane bagasse.
Sugarcane bagasse (SCB) is the fibrous lignocellulosic residue of sugarcane after it has been crushed for the extraction of its juice used for sugar and ethanol production (Ajala et al. 2021). It is estimated that about 700 m tons of bagasse are produced annually throughout the World (Monteiro et al. 2016), corresponding to about 25–26% of the total sugarcane production (Frías et al. 2011; Moretti et al. 2018). Bagasse is the most important by-product of sugarcane production and the most abundant agricultural waste in the world (Candido et al. 2017). This waste is mostly used in the generation of electricity for cogeneration boilers in sugar production, and the surplus electricity exported to the grid. The use of SCB as conventional electricity distribution has been reported in even developing countries such as Cuba (Gil et al. 2013) and South Africa (Mashoko et al. 2013). SCB contains several functional groups such as hydroxyl, carbonyl, phenolic, sulphate and amine groups which can be functionalized to new compounds with different qualities (Ding et al. 2014; Gupta et al. 2015). The presence of these functional groups makes SCB a good adsorbent material (Adeniyi et al. 2020c).
Chemical Composition of SCB
The chemical composition of SCB as shown in Table 7 and Fig. 4 contains mainly cellulose, hemicelluloses and lignin, with small amount of ashes and extractives. The complex chemical compositions of the cell walls limit the use of SCB as feed for cattle and ruminants, thus making it more abundant and desirable by-product for commercialization (Chandel et al. 2012). The sugarcane age, method of harvest, soil topography and the extraction method are factors that can affect the composition of the SCB (Bezerra and Ragauskas 2016). SCB is chemically composed of cellulose, hemicellulose, lignin, extractives, and ashes.
Cellulose is a highly linear homopolysaccharide composed of β-1,4-linked anhydro D-glucose units. The primary structure of cellulose is represented by a linear polymer β-glucopyranoside residues, with up to 20,000 residues in polymerization (Candido et al. 2017). It is a homopolymer of glucose, a hexose, and can be converted to the six carbon sugar by hydrolysis (De Moraes Rocha et al. 2015). Cellulose is one of the most abundant, renewable and biodegradable natural polymers existing in many plant-based materials such as SCB and has potential as an excellent industrial material (Corrales et al. 2012; Li et al. 2012). In fact, most of the applications of SCB are as a result of its high cellulose content.
Hemicellulose is the second major fraction of SCB and the second most abundant naturally occurring polysaccharide after cellulose. The hemicellulose in SCB is comprised of β-(1 → 4)-xylopyranose backbone, having about 200 β-xylopyranose residues linked by 1,4-glycosidic bonds (Bezerra and Ragauskas 2016). It is a heterogeneous polymer of pentoses (xylose, arabinose), hexoses (mannose, glucose, galactose) and uronic acids, dominated by xylose, a five carbon sugar (Peng and Wu 2011). Unlike cellulose, hemicellulose is easily hydrolysed by an acid or base because of its non-crystallinity, although the resulting sugar, xylose, is very difficult to ferment. Hemicellulose is the bridge linking cellulose through hydrogen bonds and lignin through covalent bonds, and these bonds can either be ester bond type, ether bond type or glycosidic bond (Hamzeh et al. 2013).
Lignin is a polyphenol that consists of the primary lignols, coumaryl, coniferyl and sinapyl alcohols (José et al. 2015; Lu et al. 2017; Sella Kapu and Trajano 2014). The lignin from SCB is mainly made up of alkyl-aryl ether structures (β-O-4', 83%) followed by minor amounts of phenylcoumarans (β-5', 6%) and other condensed bonds (José et al. 2015). Unlike cellulose and hemicellulose, lignin is not a polymer but an amorphous complex macromolecule consisting of three hydroxycinnamyl monomers that helps in cell wall water transport and provides support to the structure of the cell wall, as well as resistance to pathogenic attacks (De Moraes Rocha et al. 2015; Karp et al. 2013). Two bond types are found in lignin: an ester bond type and an ether bond type that are, respectively, sensitive and insensitive to basic solution (Kumar et al. 2014). The phenyl-propane precursor monomer units present in lignin make it very difficult to biodegrade (Maurya et al. 2015). Indeed, lignin is the recalcitrant in lignocellulosic materials, surrounding cellulose and hemicellulose and preventing them from breaking down without pre-treatment. The chemical structure of lignin is too complex, but the widely accepted basis is that it is formed through anomalous biosynthesis process modelled from its three primary lignols (Lu et al. 2017).
Sugarcane bagasse ash (SCBA) is the by-product of bagasse after being burnt in cogeneration plants. Approximately 7–8% of bagasse consumed are converted to ash which can never be further reduced but landfilled (Bahurudeen et al. 2016; Madurwar et al. 2014). Hence, constituting environmental nuisance SCBA has been reported as a non-biodegradable solid waste produced in high quantity in Brazil (2.5 m tons/year) (Faria et al. 2012), India (44,000 m tons/day) (Bahurudeen et al. 2016) and Thailand (424,700 m tons/year) (Somna et al. 2012). This black solid waste is highly rich in crystalline silica (SiO2) (66.89%), Al2O3 (29.18%), Fe2O3 (29.18%), CaO (1.92%), MgO (0.83%) and SO3 (0.56%) (Kawade et al. 2013). The presence of these compounds in SCBA makes it a very useful additive to cement, concrete and mortars. This application is further discussed in the subsection that follows. The soluble compounds in lignocellulosic materials are collectively called extractives. They are materials in biomass which during pre-treatment dissolves in water or ethanol (Zhou et al. 2017). Extractives, commonly removed from the bagasse, are hydrophobic and can be fatty acids, waxes, proteins, among others (Bezerra and Ragauskas 2016).
Applications of Sugarcane Bagasse
SCB is one of the most highly utilized renewable materials and one of the most widely researched, with De Moraes Rocha et al. (2015), noting that there are over 40 different applications of SCB. Aside from generation of heat in sugar production plants, many value-added products have been produced from SCB, with many of them directly linked to its high content of the crystalline cellulose and fibre. Bulk of the published papers, however, focused on one or more of the following applications.
Second Generation Bioethanol
The global climate change as a result of greenhouse gas emissions, the uncertainty in the prices and sustainability of fossil fuel and its general negative environmental impact have ignited interest in developing alternative sources of energy that are renewable and environmentally friendly (Dias et al. 2012; Velmurugan and Muthukumar 2011). This led to the use of agro products from the industrial production of such products like sugar, starch and oil to produce bioethanol known as first generation (1G) ethanol for vehicle fuel. As of 2012, over 80% of cars manufactured in Brazil are flex-fuel (able to run in gasoline, bioethanol or mixture of both) (Furlan et al. 2013; Hofsetz and Silva 2012). The demand for this bioethanol has, however, resulted in a direct competition with food crops in land usage, and hence, a threat to food security (Bezerra and Ragauskas 2016; Maryana et al. 2014). In a bid to mitigate this problem, inedible feedstock such as lignocellulosic biomass has been used to produce this bioethanol, and they are called second generation (2G) ethanol. In sugar industries, the ethanol produced from the fermentation of sugar is the 1G bioethanol. Half of the bagasse produced are used in the mill plant, while the remnant is dumped in the field. Chandel et al. (2012) remarked that a ton of SCB can yield up to 300 L of ethanol depending on factors such as the quality of the bagasse and the method of ethanol production. The processes involved in the production of bioethanol from lignocellulosic materials are more complex and expensive than the 1G bioethanol production, although the possibility of coproduction with the 1G bioethanol plants can reduce the production cost (Rabelo et al. 2011). The lignin–cellulose–hemicellulose complex, the crystallinity of the cellulose and the moisture content of the SCB are some of the problems inhibiting the fermentation of the lignocellulosic biomass to ethanol (Dantas et al. 2013; Rabelo et al. 2011). These problems can be mollified by following the biological route of production (Fig. 5) where the SCB is first pre-treated and hydrolysed before fermentation takes place.
Pre-treatment is carried out to separate the recalcitrant structure of lignin, increase the surface area of the bagasse and disrupt the cellulose–hemicellulose complex. It is done to make easy the hydrolysis that will follow before the subsequent fermentation. Pre-treatment separates the lignin by breaking the α-aryl ether bonds in its polyphenolic monomers and causes the swelling of cellulose by weakening the hydrogen bond linking the cellulose–hemicellulose complex (Rezende et al. 2011). Several methods have been employed by different researchers for the pre-treatment of SCB, which include alkaline pre-treatment (Canilha et al. 2011), acid pre-treatment (Rezende et al. 2011), steam explosion (Rocha et al. 2012), hot water pre-treatment (Yu et al. 2013), extrusion method (Moro et al. 2017), organosolv pre-treatment (Mesa et al. 2011), liquid ammonia pre-treatment (Hans et al. 2021), among others. Acid pre-treatment is the most widely used method, and according to Canilha et al. (2011), pre-treatment with dilute acid has become the state-of-the-art technology for the pre-treatment of lignocellulosic biomass. On the other hand, Hans et al. (2021) noted that the selective removal of lignin by saponification of the ether bonds and swelling of the cellulose makes alkaline pre-treatment the most efficient method. Organosolv has also been considered the most promising method for potential production of 2G bioethanol (Mesa et al. 2011). However, from the available papers, we can plausibly say that combination of two or more pre-treatment methods will yield better results.
Hydrolysis is carried out after pre-treatment, and it converts cellulose and hemicellulose to glucose and xylose, respectively (Dantas et al. 2013). Hydrolysis of pre-treated SCB can be carried out in two methods: acid hydrolysis and enzymatic hydrolysis. Acid hydrolysis breaks down the heterocyclic ether bonds between sugar monomers in polymeric chain (Velmurugan and Muthukumar 2011), but it has the demerit of generating some products that inhibits the process. Enzymatic hydrolysis, though more commonly used and less toxic, could also be limited in its actions by the bagasse concentrations. However, this could be avoided by performing the hydrolysis in a fed-batch mode (by gradually adding the bagasse to prevent precipitation and maintain low concentration) (De Albuquerque Wanderley et al. 2013). Without pre-treatment, the hydrolysis yield is less than 20% compared to 75–80% for acid hydrolysis and up to 95% for enzymatic hydrolysis using pre-treatment (Dantas et al. 2013).
Fermentation is carried out to finally convert the sugars from cellulose and hemicelluloses to ethanol, and it is usually carried out by yeast or bacteria. Some of the commonly used yeasts include scheffersomyces stipitis, spathaspora passalidarum, S. cerevisiae, kluyveromyces marxianus, among others. One promising means of reducing process time and cost in the production of 2G ethanol is carrying out hydrolysis and fermentation simultaneously, a process known as simultaneous saccharification and fermentation (SSF). SSF is designed to reduce the inhibitory product from hydrolysis and make room for fermentation (de Souza et al. 2012). However, fermentation on its own is always difficult because most of the yeasts that ferment glucose cannot ferment xylose. Xylose, the main hemicellulose sugar, can be fermented by several yeasts, but only 1% can ferment it into ethanol (Nakanishi et al. 2017). This is because the fermentation rate of these yeasts is restricted in xylose, leading to low ethanol tolerance, difficulty in maintaining the rate of oxygen supply and sensitivity to inhibitors generated during pre-treatment and hydrolysis (Ji et al. 2011). Ultimately, temperature, pH, initial xylose volume, accessibility of oxygen, cell concentration and degree of inhibitors are all factors that determine the fermentation of xylose by yeast (Nakanishi et al. 2017). The ethanol obtained after fermentation can then be collected by distillation and purified to meet fuel requirements. Figure 5 shows a schematic representation of the processes involved in the conversion of lignocellulosic biomass to 2G bioethanol.
Nanocellulose
Cellulose is naturally linked in chains by hydrogen bonding to form longer chains called microfibrils, or simply nanocellulose. Nanocellulose is a cellulose in nanometre scale with a size range of 10–350 nm and a surface area higher than cellulose (Plermjai et al. 2018). Based on their size differences, function, method of preparation and source, nanocelluloses, which has both crystalline and amorphous parts, can be divided into cellulose nanocrystals (CNCs), cellulose nanofibrils or nanofibrillated cellulose (CNFs) and bacterial nanocellulose (BNC) (Feng et al. 2018).
CNCs are cellulosic materials that have at least one dimension equal or less than 100 nm with high crystalline nature and high aspect ratio (Kumar et al. 2014) and can take needle-like shape, rod-like shape or spherical shape. Acid hydrolysis has been extensively employed in the extraction of CNCs. When subjected to a strong acid hydrolysis, the unorganized amorphous part of cellulose, being more susceptible to acid attack, is preferentially hydrolysed, releasing the ordered crystalline part in the form of CNC (Kumar et al. 2014; Sofla et al. 2016).
CNFs are nanocelluloses with at least one dimension in the nanometre range, and having a diameter range of 5–60 nm (Feng et al. 2018). CNFs are extracted by mechanical processes such as high-pressure homogenization, ball milling, grinding and high-intensity ultrasonification. BNC is a cellulose with a high degree of crystalline and polymerization, synthesized by microorganisms. The basic microbial producer of BNC is the bacteria, Gluconacetobacter xylinus (Abba et al. 2020; Singh et al. 2021). According to Singh et al. (2021), the synthesis of BNC from G. xylinus follows three steps: (i) polymerization of glucose residue in β-1,4-glucan, (ii) extracellular secretion of linear chain and (iii) arrangement and crystallization of glucan chains through hydrogen bonds and van der Waals forces.
The biodegradability, renewability, high potential of reinforcement in nanocomposites, high specific surface area and rigidity are all qualities of cellulose-based nanomaterials that make them high applicability in different industries. Some potential industrial applications of nanocellulose include high-quality paper products, nanofillers for polymer nanocomposites, thickener in cosmetics, scaffolds for tissue engineering, drug delivery and biomedical applications, supercapacitors, exceptional stabilizing agents in food industries, water-based latex paints as well as for industrial coating and suspensions (Abba et al. 2020; Mandal and Chakrabarty 2011).
Pulp and Paper
In the industrial production of paper, wood and other fibre-containing materials are used as raw materials. Over the years, countries such as China and India without much forest reserves for woody trees have in a bid to reduce their paper import rate, successfully exploited the use of non-wood materials such as SCB to produce pulp and the corresponding paper and paperboards (Rainey and Covey 2016). SCB is one of the most promising non-wood pulp because of its fast growth, high yield and high fibre content. The production of paper from SCB follows four practical processes: (a) removal of the non-fibrous pith cell (parenchymatous tissue) from the bagasse, (b) pulping of the bagasse, (c) bleaching of the pulp and (d) the paper making process. However, the bleaching process can be ignored if non-white paper types are to be produced.
The presence of the pith in SCB is the major constraints in its use for industrial paper production. Pith causes filtration difficulties in the production plants and increases the chemical consumption of the pulping and bleaching processes (Hemmasi et al. 2011). The high hygroscopic nature of the pith means it contains up to 20 times its own weight in water, in comparison with about five times the normal clean bagasse fibre (Lois-Correa 2012), hence, the need for depithing. Depithing can be carried out either by cutting the cane lengthwise and removing the soft central part (pith) (Rainey and Covey 2016), or by the use of depithing equipment such as S.M Caribe, Kimberly KC-4 and Horkel depithers (Lois-Correa 2012). Depithing may not remove the whole pith from the SCB, but it increases its fibre content by up to 80% (Hemmasi et al. 2011).
Pulping is carried out after depithing. Pulping is aimed at removing the lignin and hemicellulose structures of the SCB without depolymerizing the cellulose. The process can be carried out either by chemical method (mostly used) or the mechanical method. Soda, soda anthraquinone and kraft process are the commonly used chemical pulping processes (Rainey and Covey 2016), and about 80–90% of the lignin and 50% of the hemicellulose are removed using these chemical processes (Hamzeh et al. 2013). Remnants of the lignin after pulping may impede the brightness of the pulp and the resulting paper; therefore, additional treatment may be required—bleaching.
Bleaching is commonly carried out with chlorine dioxide (ClO2). The chemical reacts with the pulp to increase its brightness. Afterwards, the pulp is filtered and washed with cold water and little drops of methanol to recover the chemicals and the bleached pulps (Novo et al. 2018). Ecotoxicological issues associated with the use of chlorine chemical for bleaching in the pulp and paper industries led to the development of new bleaching technology known as elemental chlorine-free (ECF) bleaching and subsequently total chlorine-free (TCF) bleaching. These methods are effective in oxidizing lignin remnants and decreasing environmental production loads (Zhang et al. 2018). The bleached pulp is then used, after drying, to produce the paper sheets using an automatic sheet making machine by the standard method. Fibre mat is formed whereby a suspension of fibres is deposited one layer at a time and water drains through it, and then, the fibres overlay one another forming a sheet of paper (Rainey and Covey 2016). The paper sheets are then dried, cut and packaged.
Biosorbent for Heavy Metals Uptake
Increase in population, industrialization and urbanization has all resulted in an increase in heavy metals-containing effluents (Emenike et al. 2021; Iwuozor 2018; Ogunlalu et al. 2021). Different methods have been employed in the removal of these metals from aqueous solutions, and they include precipitation, ion exchange, adsorption, reverse osmosis, filtration membrane, among others (Emenike et al. 2021; Ighalo et al. 2021; Igwegbe et al. 2022). These methods are, however, generally costly to operate and can generate some toxic wastes, hence the need to develop alternative methods that are biodegradable, reusable, less expensive and environmentally friendly (Alomá et al. 2012; Dos Santos et al. 2011; Vera et al. 2019). These properties have been found in biological wastes, generally known as biochar, such as SCB. Biochar is the remnant of sustainable feedstock thermochemical processing such as pyrolysis, gasification and retort carbonization (Iwuozor et al. 2021b). SCB, just like other biochars, have been receiving attention of late for sorption process due to their strong affinity to organic pollutants and heavy metals (Ding et al. 2014), and their porous structures which can provide binding sites for the uptake of heavy metals from aqueous solution, a process known as biosorption (Emenike et al. 2022; Iwuozor et al. 2021c, 2022b).
Biosorption involves the use of inexpensive and non-hazardous technique for the removal of contaminants from aqueous solution using biomass as the biosorbent (Ighalo and Adeniyi 2020b; Khoramzadeh et al. 2013). Mechanisms involved in the biosorption process include inter- and intra-particle diffusions, chelation, precipitation, physical adsorption and complexation (Alomá et al. 2012; Ding et al. 2014). The presence of different functional groups in SCB plays a major role in the sorption process as they can be activated physically through pyrolysis and subsequently treated with steam or CO2 to enhance their sorption of heavy metals (Inyang et al. 2011).
Table 8 presents some published works on the maximum sorption capacity of different heavy metals onto the surface of SCB, reported in decreasing order and to 4 significant figures. These sorption capacities are normally determined experimentally or by use of isotherm parameters. The optimum pH was also reported. This is because in biosorption, pH has great impact on the sorption capacity as it affects the biosorbent surface charge, ionization rate and metal speciation (Alomá et al. 2014). From Table 8, the pH was seen to favour the acidic medium with a range of 2–7. This is probably because SCB has more acidic medium than basic medium as a result of the bagasse functional groups (Vera et al. 2019), and precipitation may occur at higher pH. Another reason for this is because at higher pH, the charges on the surface of the bagasse may likely become negative, resulting in formation of resistant forces between the metallic ions and the biosorbent (Ullah et al. 2013). Rubcumintara (2015) reported the highest maximum sorption capacity of the metals presented in Table 8 as 1498 mg/g uptake of Au3+ from aqueous solution by SCB.
Pozzolanic Material
Ordinary Portland cement (OPC) is often used as a cementing agent to improve the properties of soft clay in civil engineering projects because of its comprehensive strength and compressibility (Jamsawang et al. 2017). OPC, however, poses environmental danger because of the high rate of CO2 it emits (Ighalo and Adeniyi 2020a). Therefore, replacing cement with other lower carbon materials with cementitious properties is one of the four levers for carbon emissions reduction by the World Business Council for sustainable development (Moretti et al. 2018). Agricultural wastes such as silica fume fly ash, rice husk ash and waste paper ash have been used to partially replace cement in concrete and mortar (Meko and Ighalo 2021a, 2021b; Ofuyatan et al. 2021), and recently, SCBA has served this purpose. The bagasse used in sugarcane mill plants for electricity generation if burned under controlled conditions displays pozzolanic properties, allowing their use as cement substitutes in construction (Bahurudeen and Santhanam 2015). Pozzolanic materials are materials rich in silica (SiO2) and alumina (Al2O3) that can react with Ca(OH)2 generated from hydrating cement to form supplementary cementitious materials (Jamsawang et al. 2017). The crystallinity of the silica, the presence of impurities and the particle size of any material are all factors that determine its pozzolanic/cementitious aptness (Ofuyatan et al. 2022).
SCBA, rich in crystalline silica if burned above 700 °C, can be used as an admixture to partially replace 20% wt.% of OPC in concrete, mortar or bricks without any effect on the product in terms of development of early strength, chloride penetration and low water permeability (Alavéz-Ramírez et al. 2012; Amin 2011). This 20% replacement gives concrete and bricks of higher strength and is ascribed to its surface area, amorphous phase and degree of reactivity between the silica and calcium hydroxide in the bagasse ash (Amin 2011). This pozzolanic activity of SCBA could perhaps be due to the likely contamination of bagasse waste with soils before being used in cogeneration plants (Frías et al. 2011). The result of this technique is a decrease in environmental pollution, reduction in construction cost and preservation of natural resources (Faria et al. 2012).
Other Applications
Another important environmentally acceptable application of SCB is in its incorporation into polymer and epoxy composites. This is as a result of its high fibre content, as an increase in fibre content increases the strength of a composite (Adeniyi et al. 2022). SCB has been widely used as a reinforcer to improve the strength of composites, and this has been reported by many researchers (Acharya et al. 2011; Anggono et al. 2019; Monteiro et al. 2016; Singh et al. 2021). This waste has also been applied in thermal insulating board to reduce air conditioning loads (Panyakaew and Fotios 2011), in the synthesis of cellulose acetate for preparation of membrane (Candido et al. 2017), and reported to serve as soil fertilizer (Faria et al. 2012), although their provision of nutrient for this purpose is still in doubt.
In the food industry, nanocelluloses from SCB are used in fillings, crushes, chips, wafers, soups, gravies, puddings, among others. Ditto to its use as a biosorbent, SCB has also been used extensively as an adsorbent for the removal of organic dyes from aqueous solution. Dyes such as Rhodamine B (Zhang et al. 2013), methylene blue (Zhang et al. 2013), Congo red (Zhang et al. 2011), brilliant red (Da Silva et al. 2011), novacron orange (Noreen and Bhatti 2014), eosin yellow (Shaik et al. 2020) and indosol turquoise (Sadaf et al. 2014) have all been removed from aqueous solution using SCB adsorbent. In addition, because of its high silica content, SCBA has been utilized in the production of clay and ceramic materials (Souza et al. 2011), and in the synthesis of different types of zeolites such as Na-A (Moisés et al. 2013) and Na-X (Purnomo et al. 2012), which are also useful in the adsorption process and catalysis.
Despite the diverse applications of SCB, the waste is still being underutilized. A large amount of bagasse is still disposed as waste in the open field, especially domestically, while the locals commonly employed the practice of incineration in a bid to get rid of the waste. These actions result in an increase in the amount of CO2 in the atmosphere, putting the environment at risk. If the incineration is performed in the cogeneration plant, these risk would be avoided since the amount of CO2 released during combustion in ethanol production plant is equivalent to the amount of CO2 consumed during plant photosynthesis as opined by Zanatta et al. (2016).
Finally, from the available studies, the use of SCB for the production of 2G ethanol has not been completely feasible till today. In spite of the amount of resources and capitals infused into the system by governments, it remained highly underutilized and many countries are abandoning the project for the more established 1G ethanol. Nevertheless, not even the 1G ethanol is economically viable. Thus, integration of 1G and 2G bioethanols for better economic and environmental performance is plausibly recommended. This has already been proven by the works of Dias et al. (2012), and Furlan et al. (2013). However, more works need to be performed on this to elucidate more on its potency.
Knowledge Gap, Recommendations and Future Outlook
The novelty of this paper is in evaluating recent research outcomes on the conversion of sugar industry by-products into value-added products. It can lead to guidance for further research in this domain. Some interesting areas were observed by this review which can become the basis for further investigations in the future.
There exists very little knowledge in published literature on the properties of sugarcane leaves. Apart from the study conducted by Yahaya and Shu’aib on the phytochemical characteristics of sugarcane leaves, no other study has evaluated the possible phytochemical as well as antimicrobial characteristics of the leaves of sugarcane. Such study should be encouraged as they could provide more information geared towards the usage of the leaves as food or medicine for humans and also encourage its use as feed for animals.
Bagasse contains large amount of fibre which makes it a good source of dietary fibre. It can be used in the production of biscuits, bread and for other bakery products. One problem that has limited its usage in this regard is the presence of lignin present in the bagasse. The human body lacks the capacity to digest lignin which acts as a covering to the cellulose and hemicellulose present in the bagasse. To solve this problem, research geared towards the delignification of bagasse to make it edible for consumption should be encouraged.
Even after more than a decade of research, further diversification from the sugar–ethanol–electricity scenario to biofuels like biohydrogen and biobutanol, as well as high-end chemicals like sugar replacements, enzymes, organic acids, bioplastics and bioadsorbents, is still in the research phase. Despite substantial study in the domain of process design to develop efficient pre-treatment methods and fermentation processes to achieve better yields of biochemicals, cost-effective processes and energy-efficient downstream processes are still lacking in most cases. One of the technological hurdles for commercialization of biochemicals such as acetic acid, levulinic acid, and adipic acid derived from C5 and C6 sugar feed-stocks is the lack of separation technology to accomplish the requisite purity. Downstream procedures are critical in high-end chemical manufacturing from sugarcane wastes in order to achieve the purity standards required for medicinal and industrial applications. The downstream processes, which account for 50–70% of total production costs, determine the economic sustainability of various bio-based chemical synthesis pathways. This is primarily owing to the low concentration obtained during the conversion stage, such as fermentation, which raises downstream separation costs. As a result, considerable advancements in separation technologies are urgently required. Another barrier in commercializing these bio-based approaches is waste collection supply chain logistics. Because waste is only available during certain times of the year, storage is essential. Improper waste storage can result in decomposition of the wastes, which can be harmful to the environment. Large-scale storage facilities can catch fire in extreme situations because of rising warmth produced by gradual microbial decomposition (Meghana and Shastri 2020).
A collaboration between the sugar industry and the construction industry that aims to use sugar industry waste for sustainable construction practices can provide additional avenues for sustainability in addition to the valorization routes proposed (Gopinath et al. 2018). Holistic study with a thorough understanding of the technological route, accompanying obstacles and exhaustive studies in economic and environmental assessment of a product/process under consideration is critical for the successful implementation of these methods. The system's robustness is provided via many valorization paths. If the sugar mill's sole product is sugar, it is extremely vulnerable to price and demand fluctuations in the market. Diversification of products, on the other hand, allows the flexibility to respond to changing circumstances. This will make the industry more resilient in the long run. Designing such complicated biorefineries, on the other hand, is extremely difficult. Process design, operations and control become more intricate, necessitating the use of advanced methods. Mass and heat integration, process synthesis, improved process control and other process systems engineering technologies must be investigated. Despite the fact that a few life cycle assessment studies have looked at bagasse to bio-based chemicals such as lactic acid, succinic acid and xylitol, studies focusing on techno-economic studies covering various other geographical areas and other wastes such as press mud and spent wash are completely lacking. Systematic research employing techniques like multi-objective optimization and multi-criteria decision making is required. These will aid in measuring trade-offs and offering policy/investment suggestions for specific regions (Meghana and Shastri 2020).
Concluding Remarks
Product, process and value chain design and operation must also include systems engineering methodologies. In this paper, the authors reviewed the variety of applications of sugar industry by-products that has been physically and chemically transformed. It was observed that the degree to which various applications have been researched and adopted varies greatly. It was also observed that the technology for producing power from the by-products has advanced, while the manufacture of value-added chemicals has not. The key technological challenges in this area are downstream separation and purification. Most of the researches that has been carried out on these materials for decades are yet to be commercialized. This appears to be the result of a combination of technological issues and a lack of aggressive industry participation. In contrast, industries' engagement has resulted in scale-up initiatives for applications with high-value markets, such as biofuels. Government cooperation is expected to help commercialize these uses in addition to industry participation. Finally, product, process and value chain design and operation must also include systems engineering methodologies to produce products with a wide range of applications.
References
Abba, M., B.B. Nyakuma, Z. Ibrahim, J.B. Ali, S.I.A. Razak, and R. Salihu. 2020. Physicochemical, morphological, and microstructural characterisation of bacterial nanocellulose from Gluconacetobacter xylinus BCZM. Journal of Natural Fibers. https://doi.org/10.1080/15440478.2020.1857896.
Acharya, S.K., P. Mishra, and S.K. Mehar. 2011. Effect of surface treatment on the mechanical properties of bagasse fiber reinforced polymer composite. BioResources 6 (3): 3155–3165.
Adeniyi, A.G., J.O. Ighalo, and A. Abdulsalam. 2019. Modelling of integrated processes for the recovery of the energetic content of sugarcane bagasse. Biofuels, Bioproducts & Biorefining 13 (4): 1057–1067. https://doi.org/10.1002/bbb.1998.
Adeniyi, A.G., J.O. Ighalo, D.V. Onifade, and S.A. Adeoye. 2020a. Modeling the valorization of poultry litter via thermochemical processing. Biofuels, Bioproducts and Biorefining 14 (2): 242–248.
Adeniyi, A.G., D.V. Onifade, S.A. Abdulkareem, M.K. Amosa, and J.O. Ighalo. 2020b. Valorization of plantain stalk and polystyrene wastes for composite development. Journal of Polymers and the Environment 28 (10): 2644–2651.
Adeniyi, A.G., S.A. Abdulkareem, J.O. Ighalo, D.V. Onifade, and S.K. Sanusi. 2020c. Thermochemical co-conversion of sugarcane bagasse-LDPE hybrid waste into biochar. Arabian Journal for Science and Engineering. https://doi.org/10.1007/s13369-020-05119-9.
Adeniyi, A.G., J.O. Ighalo, and C.A. Adeyanju. 2021. Materials-to-product potentials for sustainable development in Nigeria. International Journal of Sustainable Engineering 14 (4): 664–671. https://doi.org/10.1080/19397038.2021.1896591.
Adeniyi, A.G., S.A. Abdulkareem, M.K. Amosa, M.T. Abdulkareem, and J.O. Ighalo. 2022. Mechanical Crystallographic and microstructural analysis of polymer composites developed from iron filings and polystyrene wastes. Mechanics of Advanced Composite Structures. https://doi.org/10.22075/MACS.2022.20943.1282.
Afiomah, C.S., and K.O. Iwuozor. 2020. Nutritional and phytochemical properties of beta vulgaris Linnaeus (Chenopodiaceae)–A review. Nigerian Journal of Pharmaceutical and Applied Science Research 9 (4): 38–44.
Ajala, E., J. Ighalo, M. Ajala, A. Adeniyi, and A. Ayanshola. 2021. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresources and Bioprocessing 8 (1): 1–25.
Akbar, A., and I. Ali. 2017. Value-added by-products from sugar processing industries. Food Processing By-Products and their Utilization. https://doi.org/10.1002/9781118432921.ch21.
Akbar, N.M. 2006. Study on effluents from selected sugar mills in Pakistan: Potential environmental, health, and economic consequences of an excessive pollution load. Sustainable Development Policy Institute. 1-41.
Akhabue, C., K.S. Otoikhian, D. Bello, A.G. Adeniyi, and J.O. Ighalo. 2019. Effect of dilute acid pre-treatment on the functional complexes and surface morphology of wood sawdust for bioethanol production. In: 18th annual International Materials Congress for the Materials Society of Nigeria (MSN), University of Ilorin, Nigeria, 2019. pp 318–322.
Alavéz-Ramírez, R., P. Montes-Garcia, J. Martínez-Reyes, D.C. Altamirano-Juárez, and Y. Gochi-Ponce. 2012. The use of sugarcane bagasse ash and lime to improve the durability and mechanical properties of compacted soil blocks. Construction and Building Materials 34: 296–305.
Alomá, I., M. Martín-Lara, I. Rodríguez, G. Blázquez, and M. Calero. 2012. Removal of nickel (II) ions from aqueous solutions by biosorption on sugarcane bagasse. Journal of the Taiwan Institute of Chemical Engineers 43 (2): 275–281.
Alomá, I.D.L.C., I. Rodriguez, M. Calero, and G. Blazquez. 2014. Biosorption of Cr6+ from aqueous solution by sugarcane bagasse. Desalination and Water Treatment 52 (31–33): 5912–5922.
Alves de Oliveira, R., C.E. Vaz Rossell, B.H. Lunelli, P.O. Schichi, J. Venus, and R.M. Filho. 2018. Different strategies to improve lactic acid productivity based on microorganism physiology and optimum operating conditions. Industrial & Engineering Chemistry Research 57 (31): 10118–10125.
Amin, N.-U. 2011. Use of bagasse ash in concrete and its impact on the strength and chloride resistivity. Journal of Materials in Civil Engineering 23 (5): 717–720.
Anggono, J., Á.E. Farkas, A. Bartos, J. Móczó, H. Purwaningsih, and B. Pukánszky. 2019. Deformation and failure of sugarcane bagasse reinforced pp. European Polymer Journal 112: 153–160.
Ansari, K.B., and V.G. Gaikar. 2014. Pressmud as an alternate resource for hydrocarbons and chemicals by thermal pyrolysis. Industrial & Engineering Chemistry Research 53 (5): 1878–1889.
Asikin, Y., M. Takahashi, T. Mishima, M. Mizu, K. Takara, and K. Wada. 2013. Antioxidant activity of sugarcane molasses against 2, 2′-azobis (2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chemistry 141 (1): 466–472.
Asikin, Y., M. Takahashi, M. Mizu, K. Takara, H. Oku, and K. Wada. 2016. DNA damage protection against free radicals of two antioxidant neolignan glucosides from sugarcane molasses. Journal of the Science of Food and Agriculture 96 (4): 1209–1215.
Assefa, D., and A. Nurfeta. 2013. Effects of molasses level in a concentrate mixture on performances of crossbred heifer calves fed a basal diet of maize stover. Journal of Cell and Animal Biology 7 (1): 1–8.
Bahurudeen, A., and M. Santhanam. 2015. Influence of different processing methods on the pozzolanic performance of sugarcane bagasse ash. Cement and Concrete Composites 56: 32–45.
Bahurudeen, A., K. Wani, M.A. Basit, and M. Santhanam. 2016. Assesment of pozzolanic performance of sugarcane bagasse ash. Journal of Materials in Civil Engineering 28 (2): 04015095.
Balakrishnan, M., and V. Batra. 2011. Valorization of solid waste in sugar factories with possible applications in India: A review. Journal of Environmental Management 92 (11): 2886–2891.
Barros, S. 2021. Sugar Annual. Sao Paulo ATO. https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Sugar%20Annual_Sao%20Paulo%20ATO_Brazil_04-15-2021.pdf. Accessed 12 Dec 2021.
Baurhoo, B., and A. Mustafa. 2014. Effects of molasses supplementation on performance of lactating cows fed high-alfalfa silage diets. Journal of Dairy Science 97 (2): 1072–1076.
Bento, H.B., A.K. Carvalho, C.E. Reis, and H.F. De Castro. 2020. Single cell oil production and modification for fuel and food applications: Assessing the potential of sugarcane molasses as culture medium for filamentous fungus. Industrial Crops and Products 145: 112141.
Bezerra, T.L., and A.J. Ragauskas. 2016. A review of sugarcane bagasse for second-generation bioethanol and biopower production. Biofuels, Bioproducts and Biorefining 10 (5): 634–647.
Bhatnagar, A., K.K. Kesari, and N. Shurpali. 2016. Multidisciplinary approaches to handling wastes in sugar industries. Water, Air, & Soil Pollution 227 (1): 1–30.
Bhatti, Z.A., M.-U.-H. Rajput, G. Maitlo, Z.A. Solangi, and G.S. Shaikh. 2019. Impact of storage time, rain and quality of molasses in the production of bioethanol. Mehran University Research Journal of Engineering & Technology 38 (4): 1021–1032.
Boviatsi, E., A. Papadaki, M.N. Efthymiou, G.J.E. Nychas, S. Papanikolaou, J.A. da Silva, D.M. Freire, and A. Koutinas. 2020. Valorisation of sugarcane molasses for the production of microbial lipids via fermentation of two Rhodosporidium strains for enzymatic synthesis of polyol esters. Journal of Chemical Technology & Biotechnology 95 (2): 402–407.
Candido, R., G. Godoy, and A.R. Gonçalves. 2017. Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydrate Polymers 167: 280–289.
Canilha, L., V.T. Santos, G.J. Rocha, J.B. Almeida e Silva, M. Giulietti, S.S. Silva, M.G. Felipe, A. Ferraz, A.M. Milagres, and W. Carvalho. 2011. A study on the pretreatment of a sugarcane bagasse sample with dilute sulfuric acid. Journal of Industrial Microbiology and Biotechnology 38 (9): 1467–1475.
Casu, R.E., A. Selivanova, and J.M. Perroux. 2012. High-throughput assessment of transgene copy number in sugarcane using real-time quantitative PCR. Plant Cell Reports 31 (1): 167–177.
Chaisu, K., A.L. Charles, Y.-K. Guu, T.-B. Yen, and C.-H. Chiu. 2014. Optimization lactic acid production from molasses renewable raw material through response surface methodology with Lactobacillus casei M-15. APCBEE Procedia 8: 194–198.
Chan, S., S. Kanchanatawee, and K. Jantama. 2012. Production of succinic acid from sucrose and sugarcane molasses by metabolically engineered Escherichia coli. Bioresource Technology 103 (1): 329–336.
Chandel, A.K., S.S. da Silva, W. Carvalho, and O.V. Singh. 2012. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. Journal of Chemical Technology & Biotechnology 87 (1): 11–20.
Chauhan, J.S., and J.P.N. Rai. 2012. Reuse of distillery wastewater with designed dose and pattern for sugarcane irrigation. CLEAN–Soil, Air, Water 40 (8): 838–843.
Cheng, Y., Y. Yu, C. Wang, Z. Zhu, and M. Huang. 2021. Inhibitory effect of sugarcane (Saccharum officinarum L.) molasses extract on the formation of heterocyclic amines in deep-fried chicken wings. Food Control 119: 107490.
Corrales, R.C.N.R., F.M.T. Mendes, C.C. Perrone, C. Sant’Anna, W. de Souza, Y. Abud, E.P.P. da Silva Bon, and V. Ferreira-Leitão. 2012. Structural evaluation of sugar cane bagasse steam pretreated in the presence of CO2 and SO2. Biotechnology for Biofuels 5 (1): 1–8.
Cueva-Orjuela, J.C., A. Hormaza-Anaguano, and A. Merino-Restrepo. 2017. Sugarcane bagasse and its potential use for the textile effluent treatment. Dyna 84 (203): 291–297.
da Silva, L.G., R. Ruggiero, M. de Patrícia, R.B. Gontijo, B.R. Pinto, E.C. Lima, T.H. Fernandes, and T. Calvete. 2011. Adsorption of Brilliant Red 2BE dye from water solutions by a chemically modified sugarcane bagasse lignin. Chemical Engineering Journal 168 (2): 620–628.
Dantas, G.A., L.F. Legey, and A. Mazzone. 2013. Energy from sugarcane bagasse in Brazil: An assessment of the productivity and cost of different technological routes. Renewable and Sustainable Energy Reviews 21: 356–364.
De Albuquerque Wanderley, M.C., C. Martín, G.J. de Moraes Rocha, and E.R. Gouveia. 2013. Increase in ethanol production from sugarcane bagasse based on combined pretreatments and fed-batch enzymatic hydrolysis. Bioresource Technology 128: 448–453.
De Moraes Rocha, G.J., V.M. Nascimento, A.R. Goncalves, V.F.N. Silva, and C. Martín. 2015. Influence of mixed sugarcane bagasse samples evaluated by elemental and physical–chemical composition. Industrial Crops and Products 64: 52–58.
de Oliveira, F.B., J. Bras, M.T.B. Pimenta, A.A. da Silva Curvelo, and M.N. Belgacem. 2016a. Production of cellulose nanocrystals from sugarcane bagasse fibers and pith. Industrial Crops and Products 93: 48–57.
de Oliveira Lino, F.S., T.O. Basso, and M.O.A. Sommer. 2018. A synthetic medium to simulate sugarcane molasses. Biotechnology for Biofuels 11 (1): 1–12.
de Souza, C.J., D.A. Costa, M.Q. Rodrigues, A.F. dos Santos, M.R. Lopes, A.B. Abrantes, P. dos Santos Costa, W.B. Silveira, F.M. Passos, and L.G. Fietto. 2012. The influence of presaccharification, fermentation temperature and yeast strain on ethanol production from sugarcane bagasse. Bioresource Technology 109: 63–69.
Demoz, Y., A. Assefa, and K. Endale. 2018. Effect of feeding urea-molasses treated TEFF straw on milk yield and composition of cross bred dairy cows. Online Journal of Animal and Feed Research 8 (6): 175–179.
Déportes, I., J.-L. Benoit-Guyod, and D. Zmirou. 1995. Hazard to man and the environment posed by the use of urban waste compost: A review. Science of the Total Environment 172 (2–3): 197–222.
Deseo, M.A., A. Elkins, S. Rochfort, and B. Kitchen. 2020. Antioxidant activity and polyphenol composition of sugarcane molasses extract. Food Chemistry 314: 126180.
Dias, M.O., T.L. Junqueira, O. Cavalett, M.P. Cunha, C.D. Jesus, C.E. Rossell, R. Maciel Filho, and A. Bonomi. 2012. Integrated versus stand-alone second generation ethanol production from sugarcane bagasse and trash. Bioresource Technology 103 (1): 152–161.
Ding, W., X. Dong, I.M. Ime, B. Gao, and L.Q. Ma. 2014. Pyrolytic temperatures impact lead sorption mechanisms by bagasse biochars. Chemosphere 105: 68–74.
Dos Santos, V.C., J.V. De Souza, C.R. Tarley, J. Caetano, and D.C. Dragunski. 2011. Copper ions adsorption from aqueous medium using the biosorbent sugarcane bagasse in natura and chemically modified. Water, Air & Soil Pollution 216 (1): 351–359.
Duga, N.D.F., P.E.A. Imperial, A.N. Soriano, and A.D. Nieva. 2016. Packed bed biosorption of lead and copper ions using sugarcane bagasse. ASEAN Journal of Chemical Engineering 16 (1): 23–37.
El-Gendy, N.S., H.R. Madian, and S.S.A. Amr. 2013. Design and optimization of a process for sugarcane molasses fermentation by Saccharomyces cerevisiae using response surface methodology. International Journal of Microbiology 2013: 1–9.
Ellis, T.P., A.G. Wright, P.M. Clifton, and L.L. Ilag. 2016. Postprandial insulin and glucose levels are reduced in healthy subjects when a standardised breakfast meal is supplemented with a filtered sugarcane molasses concentrate. European Journal of Nutrition 55 (8): 2365–2376.
Emenike, E.C., A.G. Adeniyi, P.E. Omuku, K.C. Okwu, and K.O. Iwuozor. 2022. Recent Advances in Nano-adsorbents for the sequestration of Copper from Water. Journal of Water Process Engineering 47: 102715.
Emenike, E.C., K.O. Iwuozor, and S.U. Anidiobi. 2021. Heavy metal pollution in aquaculture: sources, impacts and mitigation techniques. Biological Trace Element Research. https://doi.org/10.1007/s12011-021-03037-x.
Esparza, I., N. Jiménez-Moreno, F. Bimbela, C. Ancín-Azpilicueta, and L.M. Gandía. 2020. Fruit and vegetable waste management: Conventional and emerging approaches. Journal of Environmental Management 265: 110510.
Fadel, M., A.A. Keera, F.E. Mouafi, and T. Kahil. 2013. High level ethanol from sugar cane molasses by a new thermotolerant Saccharomyces cerevisiae strain in industrial scale. Biotechnology Research International. https://doi.org/10.1155/2013/253286.
Faria, K., R. Gurgel, and J. Holanda. 2012. Recycling of sugarcane bagasse ash waste in the production of clay bricks. Journal of Environmental Management 101: 7–12.
Farooq, U., F.M. Anjum, T. Zahoor, M.A. Sajjad-Ur-Rahman, A.A. Randhawa, and K. Akram. 2012. Optimization of lactic acid production from cheap raw material: Sugarcane molasses. Pakistan Journal of Botany 44 (1): 333–338.
Fatoye, E., and M. Onigbinde. 2020. Dye adsorption with sugarcane bagasse and corn cob. SAU Science-Tech Journal 5 (1): 182–193.
Feng, Y.-H., T.-Y. Cheng, W.-G. Yang, P.-T. Ma, H.-Z. He, X.-C. Yin, and X.-X. Yu. 2018. Characteristics and environmentally friendly extraction of cellulose nanofibrils from sugarcane bagasse. Industrial Crops and Products 111: 285–291.
Ferreira, F., M. Mariano, S. Rabelo, R. Gouveia, and L. Lona. 2018. Isolation and surface modification of cellulose nanocrystals from sugarcane bagasse waste: From a micro-to a nano-scale view. Applied Surface Science 436: 1113–1122.
Franitza, L., M. Granvogl, and P. Schieberle. 2016. Influence of the production process on the key aroma compounds of rum: From molasses to the spirit. Journal of Agricultural and Food Chemistry 64 (47): 9041–9053.
Franitza, L., L. Nicolotti, M. Granvogl, and P. Schieberle. 2018. Differentiation of rums produced from sugar cane juice (rhum agricole) from rums manufactured from sugar cane molasses by a metabolomics approach. Journal of Agricultural and Food Chemistry 66 (11): 3038–3045.
Frías, M., E. Villar, and H. Savastano. 2011. Brazilian sugar cane bagasse ashes from the cogeneration industry as active pozzolans for cement manufacture. Cement and Concrete Composites 33 (4): 490–496.
Furlan, F.F., R. Tonon Filho, F.H. Pinto, C.B. Costa, A.J. Cruz, R.L. Giordano, and R.C. Giordano. 2013. Bioelectricity versus bioethanol from sugarcane bagasse: Is it worth being flexible? Biotechnology for Biofuels 6 (1): 1–12.
Gangavati, P., M. Safi, A. Singh, B. Prasad, and I. Mishra. 2005. Pyrolysis and thermal oxidation kinetics of sugar mill press mud. Thermochimica Acta 428 (1–2): 63–70.
Gasmalla, M., R. Yang, M. Nikoo, and S. Man. 2012. Production of ethanol from Sudanese sugar cane molasses and evaluation of its quality. Journal of Food Processing & Technology 3 (7): 163–165.
George, P.A.O., J.J.C. Eras, A.S. Gutierrez, L. Hens, and C. Vandecasteele. 2010. Residue from sugarcane juice filtration (filter cake): Energy use at the sugar factory. Waste and Biomass Valorization 1 (4): 407–413.
Gharib-Bibalan, S. 2018. High Value-added products recovery from sugar processing by-products and residuals by green technologies: Opportunities, challenges, and prospects. Food Engineering Reviews 10 (2): 95–111.
Ghorbani, F., H. Younesi, A.E. Sari, and G. Najafpour. 2011. Cane molasses fermentation for continuous ethanol production in an immobilized cells reactor by Saccharomyces cerevisiae. Renewable Energy 36 (2): 503–509.
Gil, M.P., A.M.C. Moya, and E.R. Domínguez. 2013. Life cycle assessment of the cogeneration processes in the Cuban sugar industry. Journal of Cleaner Production 41: 222–231.
Gopinath, A., A. Bahurudeen, S. Appari, and P. Nanthagopalan. 2018. A circular framework for the valorisation of sugar industry wastes: Review on the industrial symbiosis between sugar, construction and energy industries. Journal of Cleaner Production 203: 89–108.
Gupta, R., J. Ladha, J. Bains, and J. Singh. 2008. Evaluation of press mud cake as a source of nitrogen and phosphorus for rice–wheat cropping system in the Indo-Gangetic plains of India. Biology and Fertility of Soils 44 (5): 755–762.
Gupta, N., S. Tripathi, and C. Balomajumder. 2011. Characterization of pressmud: A sugar industry waste. Fuel 90 (1): 389–394.
Gupta, A., S. Vidyarthi, and N. Sankararamakrishnan. 2015. Concurrent removal of As (III) and As (V) using green low cost functionalized biosorbent–Saccharum officinarum bagasse. Journal of Environmental Chemical Engineering 3 (1): 113–121.
Gutiérrez-Rivera, B., B. Ortiz-Muñiz, J. Gómez-Rodríguez, A. Cárdenas-Cágal, J.M.D. González, and M.G. Aguilar-Uscanga. 2015. Bioethanol production from hydrolyzed sugarcane bagasse supplemented with molasses “B” in a mixed yeast culture. Renewable Energy 74: 399–405.
Hamouda, H.I., H.N. Nassar, H.R. Madian, S.S.A. Amr, and N.S. El-Gendy. 2015. Response surface optimization of bioethanol production from sugarcane molasses by Pichia veronae strain HSC-22. Biotechnology Research International 2015: 1–10.
Hamzeh, Y., A. Ashori, Z. Khorasani, A. Abdulkhani, and A. Abyaz. 2013. Pre-extraction of hemicelluloses from bagasse fibers: Effects of dry-strength additives on paper properties. Industrial Crops and Products 43: 365–371.
Hans, M., S. Garg, V.O. Pellegrini, J.G. Filgueiras, E.R. de Azevedo, F.E. Guimaraes, A.K. Chandel, I. Polikarpov, B.S. Chadha, and S. Kumar. 2021. Liquid ammonia pretreatment optimization for improved release of fermentable sugars from sugarcane bagasse. Journal of Cleaner Production 281: 123922.
Hemmasi, A.H., A. Samariha, A. Tabei, M. Nemati, and A. Khakifirooz. 2011. Study of morphological and chemical composition of fibers from Iranian sugarcane bagasse. American-Eurasian Journal of Agriculture Environmental Sciences 11 (4): 478–481.
Hinojosa-Nogueira, D., S. Pérez-Burillo, J.Á. Rufián-Henares, and S.P. de la Cueva. 2020. Characterization of rums sold in Spain through their absorption spectra, furans, phenolic compounds and total antioxidant capacity. Food chemistry 323: 126829.
Hofsetz, K., and M.A. Silva. 2012. Brazilian sugarcane bagasse: Energy and non-energy consumption. Biomass and Bioenergy 46: 564–573.
Ighalo, J.O., and A.G. Adeniyi. 2020a. A perspective on environmental sustainability in the cement industry. Waste Disposal and Sustainable Energy 2 (3): 161–164. https://doi.org/10.1007/s42768-020-00043-y.
Ighalo, J.O., and A.G. Adeniyi. 2020b. Statistical modelling and optimisation of the biosorption of Cd(II) and Pb(II) onto dead biomass of pseudomonas aeruginosa. Chemical Product and Process Modelling 16 (1): 20190139. https://doi.org/10.1515/cppm-2019-0139.
Ighalo, J.O., C.A. Igwegbe, C.O. Aniagor, and S.N. Oba. 2021. A review of methods for the removal of penicillins from water. Journal of Water Process Engineering 39: 101886. https://doi.org/10.1016/j.jwpe.2020.101886.
Igwegbe, C.A., I.A. Obiora-Okafo, K.O. Iwuozor, S. Ghosh, S.B. Kurniawan, S. Rangabhashiyam, R. Kanaoujiya, and J.O. Ighalo. 2022. Treatment technologies for bakers’ yeast production wastewater. Environmental Science and Pollution Research 29 (8): 11004–11026.
Inaba, T., D. Watanabe, Y. Yoshiyama, K. Tanaka, J. Ogawa, H. Takagi, H. Shimoi, and J. Shima. 2013. An organic acid-tolerant HAA1-overexpression mutant of an industrial bioethanol strain of Saccharomyces cerevisiae and its application to the production of bioethanol from sugarcane molasses. AMB Express 3 (1): 1–7.
Inyang, M., B. Gao, W. Ding, P. Pullammanappallil, A.R. Zimmerman, and X. Cao. 2011. Enhanced lead sorption by biochar derived from anaerobically digested sugarcane bagasse. Separation Science and Technology 46 (12): 1950–1956.
Iwuozor, K.O. 2018. Removal of heavy metals from their solution using polystyrene adsorbent (foil take-away disposable plates). International Journal of Environmental Chemistry 2 (2): 10. https://doi.org/10.11648/j/ijec.20180202.11.
Iwuozor, K.O. 2019a. Prospects and challenges of using coagulation-flocculation method in the treatment of effluents. Advanced Journal of Chemistry-Section A 2 (2): 105–127.
Iwuozor, K.O. 2019b. Qualitative and quantitative determination of anti-nutritional factors of five wine samples. Advanced Journal of Chemistry-Section A 2 (2): 136–146.
Iwuozor, K.O., and E.E. Gold. 2018. Physico-chemical parameters of industrial effluents from a brewery industry in Imo state, Nigeria. Advanced Journal of Chemistry-Section A 1 (2): 66–78.
Iwuozor, K.O., L.A. Ogunfowora, and I.P. Oyekunle. 2021a. Review on sugarcane-mediated nanoparticle synthesis: A green approach. SugarTech 23: 12. https://doi.org/10.1007/s12355-021-01038-7.
Iwuozor, K.O., J.O. Ighalo, L.A. Ogunfowora, A.G. Adeniyi, and C.A. Igwegbe. 2021. An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. Journal of Environmental Chemical Engineering 9: 105658.
Iwuozor, K.O., I.P. Oyekunle, I.O. Oladunjoye, E.M. Ibitogbe, and T.S. Olorunfemi. 2021c. A review on the mitigation of heavy metals from aqueous solution using sugarcane bagasse. SugarTech. https://doi.org/10.1007/s12355-021-01051-w.
Iwuozor, K.O., J.O. Ighalo, L.A. Ogunfowora, A.G. Adeniyi, and C.A. Igwegbe. 2021d. An empirical literature analysis of adsorbent performance for methylene blue uptake from aqueous media. Journal of Environmental Chemical Engineering 9 (4): 105658. https://doi.org/10.1016/j.jece.2021.105658.
Iwuozor, K.O., E.C. Emenike, C.O. Aniagor, F.U. Iwuchukwu, E.M. Ibitogbe, B.T. Okikiola, P.E. Omuku, and A.G. Adeniyi. 2022. Removal of pollutants from aqueous media using cow dung-based adsorbents. Current Research in Green and Sustainable Chemistry 5: 103300. https://doi.org/10.1016/j.crgsc.2022.100300.
Iwuozor, K.O., V.U. Anyanwu, B.O. Olaniyi, P.S. Mbamalu, and A.G. Adeniyi. 2022a. Adulteration of sugar: A growing global menace. Sugar Tech. https://doi.org/10.1007/s12355-022-01122-6.
Jaimes, J.B., B. Da Silva, J.J. Figueroa, B. Lunelli, R. Maciel Filho, M.W. Maciel, A. Morita, and P. 2014. Coutinho Hybrid route to produce acrylic acid from sugarcane molasses. In: Iconbm: International Conference on Biomass, Pts, 2014.
Jain, R., and P. Venkatasubramanian. 2017. Sugarcane molasses–a potential dietary supplement in the management of iron deficiency anemia. Journal of Dietary Supplements 14 (5): 589–598.
Jamil, M., M. Qasim, and M.S. Zia. 2008. Utilization of pressmud as organic amendment to improve physico-chemical characteristics of calcareous soil under two legume crops. Journal of the Chemical Society of Pakistan 3 (1): 145–150.
Jamir, L., V. Kumar, J. Kaur, S. Kumar, and H. Singh. 2021. Composition, valorization and therapeutical potential of molasses: A critical review. Environmental Technology Reviews 10 (1): 131–142.
Jamsawang, P., H. Poorahong, N. Yoobanpot, S. Songpiriyakij, and P. Jongpradist. 2017. Improvement of soft clay with cement and bagasse ash waste. Construction and Building Materials 154: 61–71.
Jayawickrama, D.R., P.B. Weerasinghe, D.D. Jayasena, and D.C. Mudannayake. 2013. Effects of supplementation of urea-molasses multinutrient block (UMMB) on the performance of dairy cows fed good quality forage based diets with rice straw as a night feeding. Korean Journal of Agricultural Science 40 (2): 123–129.
Ji, J., X. Yang, M. Flavel, Z.P.-I. Shields, and B. Kitchen. 2019. Antioxidant and anti-diabetic functions of a polyphenol-rich sugarcane extract. Journal of the American College of Nutrition 38 (8): 670–680.
Ji, J., M. Flavel, X. Yang, O.C. Chen, L. Downey, C. Stough, and B. Kitchen. 2020. A polyphenol rich sugarcane extract as a modulator for inflammation and neurological disorders. Pharma Nutrition 12: 100187.
Ji, J., X. Yang, M. Flavel, Z.P. Shields, J. Neoh, M.-L. Bowen, and B. Kitchen. 2020b. Age-deterring and skin care function of a polyphenol rich sugarcane concentrate. Cosmetics 7 (2): 30.
Ji, X.-J., H. Huang, Z.-K. Nie, L. Qu, Q. Xu, and G.T. Tsao. 2011. Fuels and chemicals from hemicellulose sugars. Biotechnology in China III: biofuels and bioenergy 128: 199–224. https://doi.org/10.1007/10_2011_124.
José, C., A.G. Lino, J.L. Colodette, C.F. Lima, A. Gutiérrez, Á.T. Martínez, F. Lu, J. Ralph, and J. Rencoret. 2015. Differences in the chemical structure of the lignins from sugarcane bagasse and straw. Biomass and Bioenergy 81: 322–338.
Karp, S.G., A.L. Woiciechowski, V.T. Soccol, and C.R. Soccol. 2013. Pretreatment strategies for delignification of sugarcane bagasse: A review. Brazilian Archives of Biology and Technology 56 (4): 679–689.
Kawade, U., V. Rathi, and V.D. Girge. 2013. Effect of use of bagasse ash on strength of concrete. International Journal of Innovative Research in Science, Engineering and Technology 2 (7): 2997–3000.
Kebede, G., A. Guteta, and F. Dereje. 2018. Characterization of dairy farms, farmers’ training and on-farm evaluation of urea-treated straw and urea-molasses-block (UMB) feeding on performances of lactating crossbred cows in Ada District of East Shoa Zone, Ethiopia. World Journal of Agricultural Sciences 14 (3): 81–88.
Keshavanath, P., and B. Gangadhara. 2006. Evaluation of sugarcane by-product pressmud as a manure in carp culture. Bioresource Technology 97 (4): 628–634.
Khatiwada, D., S. Leduc, S. Silveira, and I. McCallum. 2016. Optimizing ethanol and bioelectricity production in sugarcane biorefineries in Brazil. Renewable Energy 85: 371–386.
Khoja, A.H., E. Ali, K. Zafar, A.A. Ansari, A. Nawar, and M. Qayyum. 2015. Comparative study of bioethanol production from sugarcane molasses by using Zymomonas mobilis and Saccharomyces cerevisiae. African Journal of Biotechnology 14 (31): 2455–2462.
Khoja, A.H., S.M. Yahya, A. Nawar, A.A. Ansari, and M. Qayyum. 2018. Fermentation of sugarcane molasses using Zymomonas mobilis for enhanced bioethanol production. Journal of Advanced Research in Applied Sciences and Engineering Technology 11 (1): 31–38.
Khoramzadeh, E., B. Nasernejad, and R. Halladj. 2013. Mercury biosorption from aqueous solutions by sugarcane bagasse. Journal of the Taiwan Institute of Chemical Engineers 44 (2): 266–269.
Kiani, A., N. Yosefi, and A. Azarfar. 2013. Feeding urea-molasses blocks reduced milk urea nitrogen in Iranian rural dairy cows. Magnesium 1 (1.5): 1.5.
Komesu, A., J.A.R. de Oliveira, L.H. da Silva Martins, M.R.W. Maciel, and R. Maciel Filho. 2017. Lactic acid production to purification: A review. BioResources 12 (2): 4364–4383.
Krishnan, C., L.D.C. Sousa, M. Jin, L. Chang, B.E. Dale, and V. Balan. 2010. Alkali-based AFEX pretreatment for the conversion of sugarcane bagasse and cane leaf residues to ethanol. Biotechnology and Bioengineering 107 (3): 441–450.
Kumar, S. 1996. Biogas from press mud. Bio Energy News 1 (2): 15–17.
Kumar, V., and A. Chopra. 2016. Effects of sugarcane pressmud on agronomical characteristics of hybrid cultivar of eggplant (Solanum melongena L.) under field conditions. International Journal of Recycling of Organic Waste in Agriculture 5 (2): 149–162.
Kumar, R., D. Verma, B.L. Singh, and U. Kumar. 2010. Composting of sugar-cane waste by-products through treatment with microorganisms and subsequent vermicomposting. Bioresource Technology 101 (17): 6707–6711.
Kumar, A., Y.S. Negi, V. Choudhary, and N.K. Bhardwaj. 2014. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. Journal of Materials Physics and Chemistry 2 (1): 1–8.
Lawania, P., and B. Khadda. 2017. Efficacy of urea molasses minerals block on milk production and reproductive performance of Zebu cattle under field condition. Journal of Krishi Vigyan 6 (1): 83–87.
Li, J., X. Wei, Q. Wang, J. Chen, G. Chang, L. Kong, J. Su, and Y. Liu. 2012. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydrate Polymers 90 (4): 1609–1613.
Lois-Correa, J. 2012. Depithers for efficient preparation of sugar cane bagasse fibers in pulp and paper industry. Ingeniería, Investigación y Tecnología 13 (4): 417–424.
López-Gómez, J.P., M. Alexandri, R. Schneider, and J. Venus. 2019. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process Biochemistry 79: 1–10.
Lu, Y., Y.-C. Lu, H.-Q. Hu, F.-J. Xie, X.-Y. Wei, and X. Fan. 2017. Structural characterization of lignin and its degradation products with spectroscopic methods. Journal of Spectroscopy 2017: 8951658. https://doi.org/10.1155/2017/8951658.
Madurwar, M.V., S.A. Mandavgane, and R.V. Ralegaonkar. 2014. Use of sugarcane bagasse ash as brick material. Current Science. https://doi.org/10.18520/CS/V107/I6/1044-1051.
Mahala, A., A. Mokhtar, E. Amasiab, and B. AttaElmnan. 2013. Sugarcane tops as animal feed. International Research Journal of Agricultural Science and Soil Science 3 (4): 147–151.
Maiti, B., A. Rathore, S. Srivastava, M. Shekhawat, and P. Srivastava. 2011. Optimization of process parameters for ethanol production from sugar cane molasses by Zymomonas mobilis using response surface methodology and genetic algorithm. Applied Microbiology and Biotechnology 90 (1): 385–395.
Mandal, A., and D. Chakrabarty. 2011. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydrate Polymers 86 (3): 1291–1299.
Mangwanda, T., J.B. Johnson, J.S. Mani, S. Jackson, S. Chandra, T. McKeown, S. White, and M. Naiker. 2021. Processes, challenges and optimisation of rum production from molasses—A contemporary review. Fermentation 7 (1): 21.
Marlin, L., I. Perada, J. Garciga, E. Barrera, and O. Romero. 2020. Energetic, economic and environmental assessment for the anaerobic digestion of pretreated and codigested press mud. Waste Management 102: 249–259.
Martel, C.A., E. Titgemeyer, L. Mamedova, and B. Bradford. 2011. Dietary molasses increases ruminal pH and enhances ruminal biohydrogenation during milk fat depression. Journal of Dairy Science 94 (8): 3995–4004.
Maryana, R., D. Ma’rifatun, A. Wheni, K. Satriyo, and W.A. Rizal. 2014. Alkaline pretreatment on sugarcane bagasse for bioethanol production. Energy Procedia 47: 250–254.
Mashoko, L., C. Mbohwa, and V. Thomas. 2013. Life cycle inventory of electricity cogeneration from bagasse in the South African sugar industry. Journal of Cleaner Production 39: 42–49.
Maurya, D.P., A. Singla, and S. Negi. 2015. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 5 (5): 597–609.
Mayzuhroh, A., S. Arindhani, and C. Caroenchai. 2016. Studies on bioethanol production of commercial baker’s and alcohol yeast under aerated culture using sugarcane molasses as the media. Agriculture and Agricultural Science Procedia 9: 493–499.
Medeiros, A., M. de Matos, A. de Pinho Monteiro, J. de Carvalho, and C. Soccol (2017) Cachaça and rum. In: Current Developments in Biotechnology and Bioengineering. pp. 451–468. https://doi.org/10.1016/B978-0-444-63666-9.00016-9.
Meghana, M., and Y. Shastri. 2020. Sustainable valorization of sugar industry waste: status, opportunities, and challenges. Bioresource technology 303: 122929.
Meko, B., and J.O. Ighalo. 2021a. Utilization of cordia africana wood sawdust ash as partial cement replacement in C 25 concrete. Cleaner Materials 1: 100012. https://doi.org/10.1016/j.clema.2021.100012.
Meko, B., and J.O. Ighalo. 2021b. Utilization of waste paper ash as supplementary cementitious material in C-25 concrete: Evaluation of fresh and hardened properties. Cogent Engineering 8 (1): 1938366. https://doi.org/10.1080/23311916.2021.1938366.
Mengistu, G., and W. Hassen. 2017. Review on: Supplementary feeding of urea molasses multi-nutrient blocks to ruminant animals for improving productivity. IJVSAH 2: 43–49.
Mesa, L., E. González, C. Cara, M. González, E. Castro, and S.I. Mussatto. 2011. The effect of organosolv pretreatment variables on enzymatic hydrolysis of sugarcane bagasse. Chemical Engineering Journal 168 (3): 1157–1162.
Mohan, N. 2021. An insight to sugar manufacture. Kanpur: Kratika Printers.
Moisés, M.P., C.T.P. da Silva, J.G. Meneguin, E.M. Girotto, and E. Radovanovic. 2013. Synthesis of zeolite NaA from sugarcane bagasse ash. Materials Letters 108: 243–246.
Monteiro, S.N., V.S. Candido, F.O. Braga, L.T. Bolzan, R.P. Weber, and J.W. Drelich. 2016. Sugarcane bagasse waste in composites for multilayered armor. European Polymer Journal 78: 173–185.
Moretti, J.P., S. Nunes, and A. Sales. 2018. Self-compacting concrete incorporating sugarcane bagasse ash. Construction and Building Materials 172: 635–649.
Moro, M.K., R.S.S. Teixeira, A.S.A. da Silva, M.D. Fujimoto, P.A. Melo, A.R. Secchi, and E.P. da Silva Bon. 2017. Continuous pretreatment of sugarcane biomass using a twin-screw extruder. Industrial Crops and Products 97: 509–517.
Muhammad, D., and R. Khattak. 2009. Growth and nutrient concentrations of maize in press mud treated saline-sodic soils. Soil and Environment 28 (2): 145–155.
Mulye, S. (2019) The effect of distillation conditions and molasses concentration on the volatile compounds of unaged rum. Auckland University of Technology.
Nakanishi, S.C., L.B. Soares, L.E. Biazi, V.M. Nascimento, A.C. Costa, G.J.M. Rocha, and J.L. Ienczak. 2017. Fermentation strategy for second generation ethanol production from sugarcane bagasse hydrolyzate by Spathaspora passalidarum and Scheffersomyces stipitis. Biotechnology and Bioengineering 114 (10): 2211–2221.
Nimbalkar, P., M. Khedkar, S. Gaikwad, P. Chavan, and S. Bankar. 2017. Mew insight into sugarcane industry waste utilization (press mud) for cleaner biobutanol production by using C. acetobutylicum NRRL B-527. Applied Biochemistry and Biotechnology 183 (2): 1008–1025.
Noreen, S., and H.N. Bhatti. 2014. Fitting of equilibrium and kinetic data for the removal of Novacron Orange P-2R by sugarcane bagasse. Journal of Industrial and Engineering Chemistry 20 (4): 1684–1692.
Novo, L.P., J. Bras, M.N. Belgacem, and A. da Aprigio Silva Curvelo. 2018. Pulp and paper from sugarcane: Properties of rind and core fractions. Journal of Renewable Materials 6 (2): 160–168.
Ofuyatan, M.O., A.G. Adeniyi, and J.O. Ighalo. 2021. Evaluation of fresh and hardened properties of blended silica fume self-compacting concrete (SCC). Research on Engineering Structures and Materials 7 (2): 211–223.
Ofuyatan, M.O., B.O. Agbawhe, D.O. Omole, C.A. Igwegbe, and J.O. Ighalo. 2022. RSM and ANN modelling of the mechanical properties of self-compacting concrete with silica fume and plastic waste as partial constituent replacement. Cleaner Materials. https://doi.org/10.1016/j.clema.2022.100065.
Ogunlalu, O., I.P. Oyekunle, K.O. Iwuozor, A.D. Aderibigbe, and E.C. Emenike. 2021. Trends in the mitigation of heavy metal ions from aqueous solutions using unmodified and chemically-modified agricultural waste adsorbents. Current Research in Green and Sustainable Chemistry 4: 18. https://doi.org/10.1016/j.crgsc.2021.100188.
Oliveira, R., R. Maciel Filho, and C.V. Rossel. 2016b. High lactic acid production from molasses and hydrolysed sugarcane bagasse. Chemical Engineering Transactions 50: 307–312.
Osman, O.A., N.M. Elkhair, and K.A. Abdoun. 2020. Effects of dietary supplementation with different concentration of molasses on growth performance, blood metabolites and rumen fermentation indices of Nubian goats. BMC Veterinary Research 16 (1): 1–12.
Palmonari, A., D. Cavallini, C. Sniffen, L. Fernandes, P. Holder, L. Fagioli, I. Fusaro, G. Biagi, A. Formigoni, and L. Mammi. 2020. Characterization of molasses chemical composition. Journal of Dairy Science 103 (7): 6244–6249.
Panyakaew, S., and S. Fotios. 2011. New thermal insulation boards made from coconut husk and bagasse. Energy and Buildings 43 (7): 1732–1739.
Partha, N., and V. Sivasubramanian. 2006. Recovery of chemicals from pressmud-a sugar industry waste. Indian Chemical Engineer 48 (3): 160–163.
Peng, Y., and S. Wu. 2011. Fast pyrolysis characteristics of sugarcane bagasse hemicellulose. Cellulose Chemistry and Technology 45 (9): 605.
Phukan, A., and R. Boruah. 1999. Extraction and evaluation of microcrystalline wax from press mud waste of the sugar industry. Separation and Purification Technology 17 (3): 189–194.
Pinilla, L., R. Torres, and C. Ortiz. 2011. Bioethanol production in batch mode by a native strain of Zymomonas mobilis. World Journal of Microbiology and Biotechnology 27 (11): 2521–2528.
Pino, J.A., and E. Roncal. 2016. Validation of a HS-SPME-GC method for determining higher fatty esters and oak lactones in white rums. Food Analytical Methods 9 (7): 1958–1962.
Pino, J.A., S. Tolle, R. Gök, and P. Winterhalter. 2012. Characterisation of odour-active compounds in aged rum. Food Chemistry 132 (3): 1436–1441.
Plermjai, K., K. Boonyarattanakalin, W. Mekprasart, S. Pavasupree, W. Phoohinkong, and W. Pecharapa. 2018. Extraction and characterization of nanocellulose from sugarcane bagasse by ball-milling-assisted acid hydrolysis. In: AIP Conference Proceedings, 2018. vol 1. AIP Publishing LLC, p 020005.
Prakash, M., and N. Karmegam. 2010. Vermistabilization of pressmud using Perionyx ceylanensis Mich. Bioresource Technology 101 (21): 8464–8468.
Prakash, M.D., L. Stojanovska, J. Feehan, K. Nurgali, E.L. Donald, M. Plebanski, M. Flavel, B. Kitchen, and V. Apostolopoulos. 2021. Anti-cancer effects of polyphenol-rich sugarcane extract. PLoS ONE 16 (3): e0247492. https://doi.org/10.1371/journal.pone.0247492.
Purnomo, C.W., C. Salim, and H. Hinode. 2012. Synthesis of pure Na–X and Na–A zeolite from bagasse fly ash. Microporous and Mesoporous Materials 162: 6–13.
Quirk, R., V. Zwieten, S. Kimber, A. Downie, S. Morris, and J. Rust. 2012. Utilization of biochar in sugarcane and sugar-industry management. Sugar Tech 14 (4): 321–326.
Rabelo, S., H. Carrere, R. Maciel Filho, and A. Costa. 2011. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresource Technology 102 (17): 7887–7895.
Radwan, N., K.H. Mo, C.C. Onn, C.G. Ng, and T.-C. Ling. 2021. Waste press mud in enhancing the performance if glass powder unblended cement. Construction and Building Materials 313: 125469.
Raharja, R., U. Murdiyatmo, A. Sutrisno, and A. Wardani. 2019. Bioethanol production from sugarcane molasses by instant dry yeast. In: IOP Conference Series: Earth and Environmental Science, 2019. vol 1. IOP Publishing, p 012076.
Rahiman, F., and E.J. Pool. 2016. The effect of sugar cane molasses on the immune and male reproductive systems using in vitro and in vivo methods. Iranian Journal of Basic Medical Sciences 19 (10): 1125.
Raimondi, I., V. Rodrigues, J. Lima, J. Marques, and L. Vaz. 2020. The potential use of pressmud as reactive material for Cd 2+ removal: Adsorption equilibrium, kinetics, desorption, and bioaccessibility. Water, Air, & Soil Pollution 231 (7): 1–20.
Rainey, T.J., and G. Covey. 2016. Pulp and paper production from sugarcane bagasse. Sugarcane-Based Biofuels and Bioproducts 2017: 259–280.
Ram, D., M. Ram, and R. Singh. 2006. Optimization of water and nitrogen application to menthol mint (Mentha arvensis L.) through sugarcane trash mulch in a sandy loam soil of semi-arid subtropical climate. Bioresource Technology 97 (7): 886–893.
Rasche, L., and R.S. Del Diego. 2020. Pros and cons of sugarcane straw recovery in São Paulo. BioEnergy Research 13 (1): 147–156.
Rasmey, A.-H.M., H.H. Hassan, O.A. Abdulwahid, and A.A. Aboseidah. 2018. Enhancing bioethanol production from sugarcane molasses by Saccharomyces cerevisiae Y17. Egyptian Journal of Botany 58 (3): 547–561.
Rasul, G., A. Appuhn, T. Müller, and R.G. Joergensen. 2006. Salinity-induced changes in the microbial use of sugarcane filter cake added to soil. Applied Soil Ecology 31 (1–2): 1–10.
Rezende, C.A., M.A. De Lima, P. Maziero, E.R. deAzevedo, W. Garcia, and I. Polikarpov. 2011. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnology for Biofuels 4 (1): 1–19.
Rocha, G.D.M., A.R. Gonçalves, B. Oliveira, E. Olivares, and C. Rossell. 2012. Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Industrial Crops and Products 35 (1): 274–279.
Rodrigues, C., L. Vandenberghe, A. Woiciechowski, J. de Oliveira, L. Letti, and C. Soccol. 2017. Production and application of lactic acid. In: Current Developments in Biotechnology and Bioengineering. pp 543–556. https://doi.org/10.1016/B978-0-444-63662-1.00024-5.
Romero, E., J. Scandaliaris, P. Digonzelli, L. Alonso, F. Leggio-Neme, J. Giardina, S. Casen, J. Tonatto, and J. Fernández de Ullivarri. 2007. Sugarcane potential trash estimation: Variety and cane yield effect. Proceedings - International Society of Sugar Cane Technology 2007: 421–425.
Rubcumintara, T. 2015. Adsorptive recovery of Au (III) from aqueous solution using modified bagasse biosorbent. International Journal of Chemical Engineering and Applications 6 (2): 95.
Sadaf, S., H.N. Bhatti, S. Ali, and K.-U. Rehman. 2014. Removal of Indosol Turquoise FBL dye from aqueous solution by bagasse, a low cost agricultural waste: Batch and column study. Desalination and Water Treatment 52 (1–3): 184–198.
Saleh-e-in, M.M., S. Yeasmin, B.K. Paul, M. Ahsan, M.Z. Rahman, and S.K. Roy. 2012. Chemical studies on press mud: A sugar industries waste in Bangladesh. Sugar Tech 14 (2): 109–118.
Salvador-Loreto, I., C.M. Arriaga-Jordán, J.G. Estrada-Flores, F. Vicente-Mainar, A. García-Martínez, and B. Albarrán-Portillo. 2016. Molasses supplementation for dual-purpose cows during the dry season in subtropical Mexico. Tropical Animal Health and Production 48 (3): 643–648.
Sampaio, I.L., T.F. Cardoso, N.R. Souza, M.D. Watanabe, D.J. Carvalho, A. Bonomi, and T.L. Junqueira. 2019. Electricity production from sugarcane straw recovered through bale system: Assessment of retrofit projects. BioEnergy Research 12 (4): 865–877.
Sarker, T.C., S.M.G.G. Azam, and G. Bonanomi. 2017. Recent advances in sugarcane industry solid by-products valorization. Waste and Biomass Valorization 8 (2): 241–266.
Sella Kapu, N., and H.L. Trajano. 2014. Review of hemicellulose hydrolysis in softwoods and bamboo. Biofuels, Bioproducts and Biorefining 8 (6): 857–870.
Shafiqa-Atikah, M., M. Nor-Khaizura, N. Mahyudin, F. Abas, J. Nur-Syifa, and Y. Ummul-Izzatul. 2020. Evaluation of phenolic constituent, antioxidant and antibacterial activities of sugarcane molasses towards foodborne pathogens. Food Research 4 (2): 40–47.
Shaik, O., J. Kavitha, M.H. Varma, and N. Chittibabu. 2020. Biosorption of eosin yellow dye from aqueous solution using sugarcane bagasse: Equillibrium, kinetics and thermodynamics. Materials Today: Proceedings 26: 842–849.
Shankaranand, V., and B. Lonsane. 1993. Sugarcane-pressmud as a novel substrate for production of citric acid by solid-state fermentation. World Journal of Microbiology and Biotechnology 9 (3): 377–380.
Sheikh, G., A. Ganai, F. Sheikh, S.A. Bhat, D. Masood, S. Mir, I. Ahmad, and M.A. Bhat. 2017. Effect of feeding urea molasses treated rice straw along with fibrolytic enzymes on the performance of Corriedale Sheep. Journal of Entomology and Zoology Studies 5 (6): 2626–2630.
Singh, R., A. Dhawan, and P. Saxena. 1999. Potential of press mud - a sugar factory byproduct - in supplemantary diets and its impact on fish growth. Bioresource Technology 67 (1): 61–64.
Singh, P., A. Suman, P. Tiwari, N. Arya, A. Gaur, and A. Shrivastava. 2008. Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World Journal of Microbiology and Biotechnology 24 (5): 667–673.
Singh, S.S., D.R. Salem, and R.K. Sani. 2021. Spectroscopy, microscopy, and other techniques for characterization of bacterial nanocellulose and comparison with plant-derived nanocellulose. In: Microbial and Natural Macromolecules. Pp 419–454. https://doi.org/10.1016/B978-0-12-820084-1.00018-1.
Siverson, A., C. Vargas-Rodriguez, and B. Bradford. 2014. Effects of molasses products on productivity and milk fatty acid profile of cows fed diets high in dried distillers grains with solubles. Journal of Dairy Science 97 (6): 3860–3865.
Sofla, M.R.K., R. Brown, T. Tsuzuki, and T. Rainey. 2016. A comparison of cellulose nanocrystals and cellulose nanofibres extracted from bagasse using acid and ball milling methods. Advances in Natural Sciences: Nanoscience and Nanotechnology 7 (3): 035004.
Somna, R., C. Jaturapitakkul, P. Rattanachu, and W. Chalee. 2012. Effect of ground bagasse ash on mechanical and durability properties of recycled aggregate concrete. Materials & Design 1980–2015 (36): 597–603.
Souza, A., S. Teixeira, G. Santos, F. Costa, and E. Longo. 2011. Reuse of sugarcane bagasse ash (SCBA) to produce ceramic materials. Journal of Environmental Management 92 (10): 2774–2780.
Srivastava, A.K., A.D. Tripathi, A. Jha, A. Poonia, and N. Sharma. 2015. Production, optimization and characterization of lactic acid by Lactobacillus delbrueckii NCIM 2025 from utilizing agro-industrial byproduct (cane molasses). Journal of Food Science and Technology 52 (6): 3571–3578.
St-Pierre, P., G. Pilon, V. Dumais, C. Dion, M.-J. Dubois, P. Dubé, Y. Desjardins, and A. Marette. 2014. Comparative analysis of maple syrup to other natural sweeteners and evaluation of their metabolic responses in healthy rats. Journal of Functional Foods 11: 460–471.
Sun, Y., Z. Xu, Y. Zheng, J. Zhou, and Z. Xiu. 2019. Efficient production of lactic acid from sugarcane molasses by a newly microbial consortium CEE-DL15. Process Biochemistry 81: 132–138.
Tayade, A.S., P. Geetha, S. Anusha, R. Dhanapal, and K. Hari. 2017. Effect of green cane trash blanketing and microbial consortia application on soil compaction and productivity of mechanically harvested sugarcane ratoon crops. Journal of Sugarcane Research 7 (2): 112–120.
Trivedi, S., and S. Shah. 2014. The effect of cane molasses on cow milk productivity. International Journal of Current and Engineering Technology 4: 4157–4161.
Ullah, I., R. Nadeem, M. Iqbal, and Q. Manzoor. 2013. Biosorption of chromium onto native and immobilized sugarcane bagasse waste biomass. Ecological Engineering 60: 99–107.
Umenweke, G., J.O. Ighalo, M. Anusi, B. Itabana, and L. Ekeh. 2021. Selected thermo-chemical biorefining: Evaluation of the current trends and progressions. European Journal of Sustainable Development Research 5 (2): em0154.
Valli, V., A.M. Gómez-Caravaca, M. Di Nunzio, F. Danesi, M.F. Caboni, and A. Bordoni. 2012. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. Journal of Agricultural and Food Chemistry 60 (51): 12508–12515.
Velmurugan, R., and K. Muthukumar. 2011. Utilization of sugarcane bagasse for bioethanol production: Sono-assisted acid hydrolysis approach. Bioresource Technology 102 (14): 7119–7123.
Vera, L.M., D. Bermejo, M.F. Uguña, N. Garcia, M. Flores, and E. González. 2019. Fixed bed column modeling of lead (II) and cadmium (II) ions biosorption on sugarcane bagasse. Environmental Engineering Research 24 (1): 31–37.
Vidra, A., A.J. Tóth, and Á. Németh. 2017. Lactic acid production from cane molasses. Waste Treatment and Recovery 2 (1): 13–16.
Vikash, P.V., P.V. Mandade, and Y. Shastri. 2018. Assessment of bagasse and trash utilization for ethanol production: A case study in india. Environmental Progress & Sustainable Energy 37 (6): 2165–2174.
Wang, B.-S., L.-W. Chang, Z.-C. Kang, H.-L. Chu, H.-M. Tai, and M.-H. Huang. 2011. Inhibitory effects of molasses on mutation and nitric oxide production. Food Chemistry 126 (3): 1102–1107.
Williams, I.O., E.O. Onyenweaku, and I.J. Atangwho. 2016. Nutritional and antimicrobial evaluation of Saccharum officinarum consumed in Calabar, Nigeria. African Journal of Biotechnology 15 (33): 1789–1795.
Windsor, P., S. Nampanya, L. Olmo, S. Khounsy, P. Phengsavanh, and R. Bush. 2020. Provision of urea–molasses blocks to improve smallholder cattle weight gain during the late dry season in tropical developing countries: Studies from Lao PDR. Animal Production Science 61 (5): 503–513.
Wright, A.G., T.P. Ellis, and L.L. Ilag. 2014. Filtered molasses concentrate from sugar cane: Natural functional ingredient effective in lowering the glycaemic index and insulin response of high carbohydrate foods. Plant Foods for Human Nutrition 69 (4): 310–316.
Wu, R., D. Chen, S. Cao, Z. Lu, J. Huang, Q. Lu, Y. Chen, X. Chen, N. Guan, and Y. Wei. 2020. Enhanced ethanol production from sugarcane molasses by industrially engineered Saccharomyces cerevisiae via replacement of the PHO4 gene. RSC Advances 10 (4): 2267–2276.
Xavier, S., and B. Lonsane. 1994. Sugar-cane pressmud as a novel and inexpensive substrate for production of lactic acid in a solid-state fermentation system. Applied Microbiology and Biotechnology 41 (3): 291–295.
Xu, Q., T. Ji, S.-J. Gao, Z. Yang, and N. Wu. 2019. Characteristics and applications of sugar cane bagasse ash waste in cementitious materials. Materials 12 (1): 39.
Yadav, R., S. Prasad, R. Singh, and V. Srivastava. 1994. Recycling sugarcane trash to conserve soil organic carbon for sustaining yields of successive ratoon crops in sugarcane. Bioresource Technology 49 (3): 231–235.
Yahaya, A., Y.S. Shu’aib, D.B. Danladi, and R.A. Idris. 2019. Phytochemical screening of helathy and sugarcane chlorotic streak virus-infected leaves of Saccharum officinarum. FUW Trends in Science & Technology Journal 4 (3): 920–923.
Yu, Q., X. Zhuang, S. Lv, M. He, Y. Zhang, Z. Yuan, W. Qi, Q. Wang, W. Wang, and X. Tan. 2013. Liquid hot water pretreatment of sugarcane bagasse and its comparison with chemical pretreatment methods for the sugar recovery and structural changes. Bioresource Technology 129: 592–598.
Yu, P., X.-B. Xu, and S.-J. Yu. 2017. Inhibitory effect of sugarcane molasses extract on the formation of Nε-(carboxymethyl) lysine and Nε-(carboxyethyl) lysine. Food Chemistry 221: 1145–1150.
Zanatta, E.R., T.O. Reinehr, J.A. Awadallak, S.J. Kleinübing, J.B.O. dos Santos, R.A. Bariccatti, P.A. Arroyo, and E.A. da Silva. 2016. Kinetic studies of thermal decomposition of sugarcane bagasse and cassava bagasse. Journal of Thermal Analysis and Calorimetry 125 (1): 437–445.
Zentou, H., Z.Z. Abidin, M. Zouanti, and D. Greetham. 2017. Effect of operating conditions on molasses fermentation for bioethanol production. International Journal of Applied Engineering Research 12 (15): 5202–5506.
Zhang, Z., L. Moghaddam, I.M. O’Hara, and W.O. Doherty. 2011. Congo Red adsorption by ball-milled sugarcane bagasse. Chemical Engineering Journal 178: 122–128.
Zhang, Z., I.M. O’Hara, G.A. Kent, and W.O. Doherty. 2013. Comparative study on adsorption of two cationic dyes by milled sugarcane bagasse. Industrial Crops and Products 42: 41–49.
Zhang, H., C. Qin, S. Nie, and S. Wang. 2018. Effects of D-hot pretreatment on micro-distribution of residual lignin in sugarcane bagasse pulp and fiber properties. Cellulose 25 (8): 4423–4435.
Zhou, X., W. Li, R. Mabon, and L.J. Broadbelt. 2017. A critical review on hemicellulose pyrolysis. Energy Technology 5 (1): 52–79.
Zhul-quarnain, A., K.O. Iwuozor, I. Modupe, E. Gold, and E.E. Chidubem. 2018. Adsorption of malachite green dye using orange peel. Journal of Biomaterials 2 (2): 10. https://doi.org/10.11648/j.jb.20180202.12.
Funding
There was no external funding for the study.
Author information
Authors and Affiliations
Contributions
KOI contributed to conceptualization; data curation; methodology; investigation; writing—original draft; writing—review and editing; supervision; validation; and project administration. ECE was involved in data curation; methodology; investigation; writing—review and editing; and validation. JOI contributed to data curation; methodology; investigation; writing—review and editing; and validation. SE, PEO and AGA were involved in writing—review and editing, and validation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Human or Animal Rights
This article does not contain any studies involving human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iwuozor, K.O., Emenike, E.C., Ighalo, J.O. et al. Valorization of Sugar Industry’s By-products: A Perspective. Sugar Tech 24, 1052–1078 (2022). https://doi.org/10.1007/s12355-022-01143-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-022-01143-1