Abstract
Stevia rebaudiana, a natural sweetener and a non-caloric sugar substitute, is known to exhibit several medicinal properties including anti-diabetic effects. However, its mechanism of action, as an antidiabetic agent, is not completely understood. In this study, aqueous, methanol, and ethanol extracts of leaves were used. Among all the extracts, the methanolic extract exhibited the highest TPC (41.00 ± 0.69 mg GAE/g DW), TFC (22.66 ± 0.036 mg QE/g DW), FRAP activity (2.50 ± 0.09 mmol of Fe2+/g DW) along with lowest IC50 values for ABTS (3.8 ± 0.01 µg/mL), and nitric oxide radical scavenging activity (150 ± 0.05 µg/mL), whereas ethanolic extract showed the lowest IC50 value of 41 ± 0.20 µg/mL against DPPH than the aqueous (80 ± 0.09 µg/mL) and methanolic (70 ± 0.10 µg/mL). However, aqueous extract showed a strong correlation between phenolic content and antioxidant capacities with R2 values of 0.961 (DPPH), 0.990 (ABTS), and 0.916 (nitric oxide) assays, respectively. Additionally, the aqueous extract inhibited 17.41 ± 0.11% activity of α-amylase and 8.30 ± 0.95% of α-glucosidase. Moreover, the antiglycation properties of the aqueous extract were confirmed with observations like reduction in browning (34.95 ± 11.09%), fructosamine formation (80 ± 5.12%), carbonyl content (11.68 ± 2.12%), and protein aggregation, in an in vitro glycation system. Furthermore, the extract showed high efficiency in protection against H2O2-induced DNA damage. It can also be used as a source of antiglycation and antioxidant agent for the management of glycation-induced disorders like diabetes. This report also shows a positive correlation between phytochemicals present in the Stevia and their associated antioxidant power. Moreover, these antioxidant compounds can prevent the consequences of AGEs formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is one of the most common health hazards affecting 425 million people worldwide with almost 72 million diabetic individuals from India (International Diabetes Federation 2017). Diabetes mellitus is a chronic metabolic disorder that is associated with hyperglycemia. Insulin resistance is an eminent characteristic of both insulin-dependent and non-insulin-dependent diabetes (Lilloja et al. 1993). The skeletal muscle influences the decrease in insulin-induced glucose utilization among diabetic patients. Glucose transport is the rate-limiting step for glucose utilization in muscle, a process that is defective in diabetic individuals (Klip et al. 1992). An increase in activity of pancreatic enzymes α-amylase and α-glucosidase (Jaradat et al. 2020) is also observed in diabetes which accounts for the increase in glucose level. Several researchers report obesity to be concomitant with diabetes as approximately 90% of patients with insulin-resistant diabetes to have a body mass index, BMI ≥ 25.0 kg/m2, are overweight (Boden 2002). Obesity has been designated as a chronic disease by the American Medical Association in 2013. Similarly, it has also been observed that prolonged diabetic conditions lead to oxidative stress (Ullah et al. 2016) which accounts for several secondary complications like retinopathy, nephropathy, neuropathy, cardiomyopathy, aging, rheumatoid arthritis, and osteoporosis.
During a long-standing hyperglycemic state in diabetes mellitus, glucose forms covalent adducts with the plasma proteins through a non-enzymatic process known as glycation (Singh et al. 2014). Glycation is a non-enzymatic reaction between the free amino (–NH2) group in proteins and the carbonyl group of reducing sugars, leading to the generation of Amadori products. These products eventually undergo dehydration, rearrangement, and cyclization process to finally form advanced glycation end products (AGEs) (Wu and Yen 2005). The latest reports have shown that few plants possess both antioxidant and anti-AGEs generating properties (Unuofin et al. 2020). The widely used natural antioxidants like L-ascorbic acid and α-tocopherol are known to exhibit lower activities as compared to synthetic antioxidants such as butylated hydroxytoluene and butyl hydroxyanisole. However, synthetic antioxidants can promote carcinogenesis (Andre et al. 2010). Hence, to replace or at least minimize the use of synthetic antioxidants, there is an increasing interest in plant-based antioxidants that display minimal side effects (Zengin et al. 2015). In the case of hyperglycemia, the use of sweeteners with a low glycemic index is recommended. This has led to extensive use of artificial sweeteners which is accompanied by extreme dire effects (Sorensen et al. 2014). In turn, this scenario has resulted in the popularity of natural sweeteners that show negligible side effects (Surana et al. 2006). However, the role of anti-AGEs generation property of many natural sweeteners is still not reported.
Stevia rebaudiana has been in demand by the food industry due to its no caloric value and sweetness, which is 250–300 times more than sugar (Abbas et al. 2017). The dry leaf of Stevia also contains alkaloids, flavonoids, etc. (Rokosa et al. 2020). The safety of the Stevia plant for humans is not widely studied, but the traditional use of Paraguayans over 1500 years proves the safety of this plant (Singh and Rao 2005), whereas stevioside is used in large amounts in Japan with no side effects for more than 20 years (Saraiva et al. 2020). Pure Stevia extract has been approved and used in many countries (Samuel et al. 2018). The use of Stevia in food and drugs has been reported for many health benefits against oxidative stress, diabetes, hypertension, cancer, dental caries, etc. (Sharangi and Bhutia 2016). Considering the useful characteristics of this plant, the objectives of this study were to investigate the phytochemicals, antioxidant, and antiglycation properties of S. rebaudiana, Bertoni variety Morita II, and their correlation with each other.
Materials and Methods
Plant Materials and Chemicals
The plants of S. rebaudiana, Bertoni variety Morita II, were obtained from Organic Innovation, Guwahati, Assam, India. The plant was identified as Stevia rebaudiana (Ref No. RC-14/2020-21) by taxonomist Dr. Keshava H Korse, Bhandimane Life Science Research Foundation, Karnataka. The sample consisting of Stevia leaves was taken from the same batch, and the sample collection was done at the age of three months. The maintenance of plants was done in a greenhouse, and the leaves were obtained. Plant leaves were washed, air-dried for 7–8 days at 28 ± 2 °C, ground into powder, and stored until further use.
All analytical-grade chemicals and reagents were used in the experiments. Naphthylethylenediamine dihydrochloride (NED), sodium nitroprusside (SNP), sulphanilamide 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid (GA), quercetin (Q), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), quercetin, 2,4,6-tripyridyl-S-triazine (TPTZ), p-nitrophenyl glucopyranoside (pNPG), 2,4-dinitrophenylhydrazine (DNPH), α-amylase, and α-glucosidase were purchased from SRL Pvt. Ltd. (Mumbai). The bovine serum albumin (BSA) and methylglyoxal (MG) were procured from Sigma-Aldrich (USA), and pBR322 plasmid DNA was obtained from Thermo Fisher Scientific.
Extract Preparation
The aqueous extract was prepared using the protocol described by Woelwer et al. (2010) with some modifications. The powdered leaves (5 g) of uniform particle size were added into the distilled water (50 mL), vortexed for 1 h in a water bath at 100 °C, and centrifuged for 15 min at 4500 rpm. The methanolic and ethanolic extracts of dried leaves were prepared according to Al-Manhel et al. (2015). The leaf powder (5 g) of uniform particle size was mixed in 50 mL of methanol or ethanol and kept in a shaking incubator at 200 rpm for 24 h. The solutions obtained were filtered with Whatman no.1 filter paper and stored at 4–8 °C until further use.
Determination of Total Phenolic (TPC) and Flavonoid Content (TFC)
The phenolic and flavonoid contents were obtained for all extracts by using the Folin–Ciocalteu method (Singleton and Rossi 1965) and the aluminum chloride method (Dewanto et al. 2002), respectively. A 250 μL volume of the extract (10 mg/mL) was used for all assays. For TPC measurement, the standard curve of gallic acid (0–250 μg/mL) was used and the results were demonstrated in mg of gallic acid equivalents (GAE)/g DW of a sample (y = 0.008x + 0.026; R2 = 0.991). Similarly, for TFC measurement, a standard curve of quercetin (0–100 μg/mL) was plotted and the results were mentioned in mg of quercetin equivalents (QE)/g DW of sample (y = 0.026x + 0.038; R2 = 0.998).
Reactive Oxygen and Nitrogen Assays
The ROS assays (DPPH, ABTS, FRAP) and RNS assay (nitric oxide scavenging) were performed with all extracts to determine their anti-oxidant and nitrogen scavenging potential.
DPPH Assay
The DPPH activity was determined using the method of Mitra and Uddin (2014), with some modifications. The reaction mixture consisted of 1.8 mL of 0.2 mM DPPH in methanol and 0.2 mL of different concentrations (0–250 μg/mL) of extract. The tubes were incubated for 30 min at 28 ± 2 °C in the dark, and the absorbance was taken at 517 nm. The % inhibition of DPPH was measured using the formula:
\({\text{\% ~Inhibition}} = \frac{{{\text{Absorbance~}}\left( {{\text{Blank}} - {\text{Test}}} \right)}}{{{\text{Absorbance~}}\left( {{\text{blank}}} \right)}}{\text{x}}100\).
ABTS Assay
ABTS assays were carried out as suggested by Ayyashet al. (2018), with some modifications. Various concentrations (0–10 μg/mL) of extracts were mixed with ABTS solution and incubated in dark for 30 min at 28 ± 2 °C. The absorbance was measured at 734 nm. The % inhibition of ABTS was measured using the formula:
FRAP Assay
This assay was performed using a method given by Benzie and Strain (1996). The FRAP reagent was made by the addition of 300 mM acetate buffer (pH 3.6) with 10 mM TPTZ (in 40 mM HCl) and 20 mM FeCl3.6H2O in the ratio of 10:1:1. Sample (20 μL) and distilled water (180 μL) were added to 3 mL of working FRAP reagent and kept at 37 °C for 4 min. The absorbance was measured at 593 nm, and the FRAP value was calculated as mmol of Fe2+ equivalent/g DW of a sample using the standard graph of FeSO4 with a linearity range of 0 to 1 mM (y = 72.62x + 0.032; R2 = 0.998).
Nitric oxide Assay
The nitric oxide scavenging activity was measured by the Griess–Ilosvay reaction (Mandal et al. 2011). Various concentrations of extract (0–500 μg/mL) were incubated at 25 °C for 2.5 h. To these extracts (0.5 mL), 1 mL sulfanilamide (0.33%) was added and the mixture was kept for 5 min. This was followed by the addition of 1 mL NED (0.1% w/v). The resulting mixture was incubated at 28 ± 2 °C for 0.5 h. The absorbance was measured at 540 nm, and the % inhibition was measured using the formula:
Antidiabetic Assays
In vitro α-amylase Inhibitory Assay
The effect of aqueous, methanolic, and ethanolic extracts of Stevia (1 mg/mL) on α-amylase activity was carried out as per the modified method of Ali et al. (2006). The 100 µL extract and 250 µL α-amylase solution (100 U/mL) were pre-incubated for 10 min at 37 °C, followed by the addition of 250 µL of 10% starch. The mixture was incubated at 37 °C for 10 min; 500 µL of dinitrosalicylic acid (DNS) was added and boiled for 5 min. The absorbance was measured at 595 nm, and α-amylase inhibitory activity was calculated as follows:
α-glucosidase Inhibitory Assay
The effect of aqueous, methanolic, and ethanolic extracts of Stevia (1 mg/mL) on α-glucosidase activity was performed as per Kim et al. (2000). A 100 µL sample of α-glucosidase (100 U/mL) was preincubated with 50 µL of the extract for 10 min. To start the reaction, 50 µL of 5 mM pNPG was added. The reaction mixture was incubated at 37 °C for 30 min and stopped by adding 500 µL of 5 mM sodium carbonate (Na2CO3). The α-glucosidase activity was calculated by measuring the p-nitrophenol released from pNPG at 405 nm by using the formula given below:
Antiglycation Assays
Albumin Glycation
The antiglycation activity of Stevia was determined using the BSA assay with slight modifications (Kumar et al. 2020). BSA (10 mg/mL) and fructose (100 mg/mL) were incubated in 0.1 M phosphate buffer (pH 7.4) containing 3 mM sodium azide (NaN3) with or without aqueous extract at 37 °C for four weeks. The solutions were dialyzed against phosphate buffers. All samples, viz. positive control, i.e., BSA + Fructose (B + F), negative controls, i.e., BSA (B) and BSA + Stevia (B + S), and test, i.e., BSA + Fructose + Stevia (B + F + S), were kept at 20 °C until analysis. Results of the test samples were calculated as % inhibition concerning the positive control, taken as 100%.
Measurement of Browning
The intensity of the browning was measured at 420 nm (Kumar and Ali 2019).
Determination of Fructosamine
To evaluate the fructosamine content, nitro blue tetrazolium (NBT) assay was carried out as per Banan and Ali (2016) with slight modifications. Each sample (10 μL) and NBT reagent (0.5 mM, 100 μL) prepared in a sodium carbonate buffer (0.1 M, pH 10.4) were incubated at 25 ± 2 °C for 15 min, and the absorbance was measured at 530 nm.
Estimation of Protein Carbonyl Content
Protein content was determined using the DNPH methodology proposed by Meeprom et al. (2013) with minor modifications. Each sample (100 µL) was mixed with 400 μL DNPH (10 mM in 2.5 M HCl) and incubated in the dark for 1 h at 28 ± 2 °C. This step was followed by the addition of 0.5 mL of 20% TCA (w/v), incubation for 5 min on ice, and centrifugation at 10,000 rpm at 4 °C for 10 min. The supernatant was discarded, and the 500 μL of ethanol-ethyl acetate mixture (1:1 v/v) was added to the pellet thrice for washing and diffused in 100 μL of 6 M guanidine hydrochloride. The absorbance was recorded at 370 nm.
Estimation of Amyloid β-structure
Congo red binding to the amyloid aggregation was measured according to Bouma et al. (2003). For this purpose, 50 µL of each sample was incubated with 50 µL of 100 µM Congo red, prepared in 10% ethanol/PBS (v/v), for 20 min at 25 ± 2 ºC, and absorbance was taken at 530 nm.
Estimation of Total AGEs
In vitro antiglycation activity was estimated by BSA-fluorescent assay using fluorometry as described by Ali et al. (2017). The changes in fluorescence intensity of each sample were measured from 370 to 440 nm using a spectrofluorometer (Varian, Cary Eclipse model). The % inhibition of glycation was reported as follows:
In vitro Glycation of Plasmid DNA
The glycation-mediated DNA damage was studied as per the method of Ali and Sharma (2015). pBR322 plasmid DNA (0.25 μg), lysine (20 mM), MG (20 mM), and FeCl3 (100 μM) were incubated with and without ethanolic extract in phosphate buffer (100 mM; pH-7.4). All samples including pBR322, glycated system, i.e., lysine + MG + ferric chloride (Lys + MG + FeCl3), pBR322 + Stevia, and glycated system + Stevia, were incubated at 37 ± 1 °C for 3 h. After 3 h of incubation, samples were analyzed using 1% agarose gel electrophoresis and visualized in Gel-Doc.
Statistical Analysis
Analysis was performed using Graphpad Prism (version 8). The results were obtained from three independent experiments performed in triplicates and represented as mean ± SD. One-way analysis of variance (ANOVA) was used to analyze the data for significance. Tukey's multiple comparisons tests were used to determine differences between treatments, and results were expressed as p < 0.05*, p < 0.01**, p < 0.001***.
Results and Discussion
Determination of TPC and TFC
The TPC and TFC of all extracts, with a significance level of p < 0.001 (Fig. 1), are summarized in Table 1. The methanol extract showed the highest yield of phenolic and flavonoid content. Aqueous extracts proved to be second best for the extraction of phenolic, and ethanol showed only 54% efficiency as compared to methanol. In the case of flavonoids, the efficiency of methanol was followed by ethanol. Here, the aqueous extract showed a 75% yield as compared to methanol. The observed variation in the efficiency of solvents may be due to the differences in polarities of compounds (Ngo et al. 2017). Also, the high molecular weight and complex structure of flavonoids make them less soluble in water as compared to organic solvents (Zaidan et al. 2019). Our findings were supported by previous studies on Stevia which reported that the solvent used for extraction significantly affected the estimated TPC (Ruiz et al. 2015; Zeng et al. 2013; Ruiz-Ruiz et al. 2015) and TFC content (Zaidan et al. 2019; Ruiz-Ruiz et al. 2015; Tadhani et al. 2007).
Reactive oxygen and Nitrogen Assays
As the polyphenol content increases, the antioxidant activity is also increased. In many plant species, a linear relationship has been observed between total phenolic and antioxidant activities (Oktay et al. 2003).
DPPH and ABTS Assays
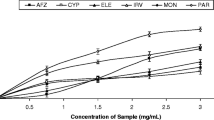
The hydrogen donating ability of the extract to DPPH is because of their antioxidant potential (Baumann 1979). DPPH assay is a widely accepted method because it takes less time to analyze the antioxidant activity, and it was reported that these free radicals can also inhibit lipid peroxidation (Khan et al. 2013). There is a direct correlation between the concentration of extracts and their DPPH and ABTS scavenging activities (Fig. 2). The lowest IC50 values were observed among all three extracts for ethanolic and methanolic extracts against DPPH and ABTS, respectively (Table 2). Few examples of reported IC50 values for ethanolic extracts against DPPH are 93.46 µg/mL (Shukla et al. 2009) and 23.7 ± 0.711 µg/mL (Jahan et al. 2010). These differences may be due to different extraction methods used. In our study, the IC50 value of aqueous extract for DPPH (335.9 µg/mL) is four times less than that reported by Ruiz-Ruiz et al. (2015). This difference may be attributed to the different growth environments of plants resulting in diverse primary and secondary metabolite production and accumulation (Marrassini et al. 2018). The reported IC50 values for aqueous and methanolic extracts against ABTS are 1.67 ± 1.61 and 2.85 ± 0.92 µg/mL, respectively, in a study reported by Phansawan and Poungbangpho (2007), which are comparable to our results, whereas according to Tadhani et al. (2007) and Ghanta et al. (2007) the IC50 values are 10–15 times higher than those indicated in the current study. Again, these differences may be due to plants’ different climatic, ecological, and geographical conditions (Celiktas et al. 2007). All extracts showed comparable antioxidant activity as gallic acid at the same concentration of 10 µg/mL, with a significance level of p < 0.001 (Fig. 3). Hence, this extract can be used as a primary antioxidant that readily donates a proton which can be used as a free radical inhibitor.
FRAP Assay
Direct donation of electrons by extracts leads to the reduction of ferric ions. The ferrous reducing capacity of aqueous extract was increased with an increase in the phenolic content (Connor et al. 2002). In the present study, the methanolic extract showed the highest antioxidant potential and their correlation was significant at p < 0.01 (Table 2). The calculated values for different solvent extract varied between 2.50 (methanolic) and 1.28 Fe2+mmol/g DW (ethanolic). These values are higher than the reported values of 1.05, 1.00, and 0.813 mmol Fe2+/g in hydroalcoholic, aqueous, and methanolic extracts, respectively (Silva et al. (2018), Alvarez-Robles et al. (2016), and Tavarini and Angelini (2013)), whereas Ortiz-Viedma et al. (2017) found FRAP activity ranging from 0.12 to 0.18 mmol Fe2+/g in different extracts of Stevia. These differences could be related to Stevia variety, time of harvest, and the extracting capacity of the solvent used in their study (Silva et al. 2018).
Nitric Oxide Assay
Nitric oxide is an important regulatory compound that is essential for many physiological processes like immune response, signal transmission, control of blood pressure, and vasodilatation. It also plays a key role in different types of inflammatory processes (Jagetia et al. 2000; Shukla et al. 2012). Nitric oxide scavenging activity showed its direct correlation, in a concentration-dependent manner, with different concentrations of extracts (Fig. 4). Among all three extracts, the aqueous extract showed three times higher IC50 value (98.73 µg/mL) with positive correlation, at a significance level of p < 0.01 (Table 2), than that reported by Shukla et al. (2012). The ethanolic extract also showed a higher IC50 value (132.05 µg/mL) than the one reported by Shukla et al. (2009). These differences in the IC50 values may be due to differences in the variety of Stevia used in the study or biochemical relation between plants and their environment. This is moderated, especially in the production of secondary metabolites, which exert their biological functions and responsibilities for the environmental changes, inhibition of protein synthesis, loss of enzyme activity, and it also contributes to cell death (Ozcan and Ogun 2015; Marrassini et al. 2018). If the level of RNS increases, it causes oxidative damage to protein, lipid, and DNA, which are responsible for altered membrane properties that cause DNA damage.
Correlation Between Phytochemicals and Antioxidant
Many studies, including the current study, have consistently reported a close and strong association between aqueous extract and polyphenols (Fig. 5). The strong linear relationship of TPC with antioxidant capacity also has been reported in many medicinal plants like Fumaria officinalis, Rosa Damascene, Foeniculum Vulgare, Stachys lavandulifolia, Stevia rebaudiana, and Salvia hydrangea (Oktay et al. 2003, Shukla et al. 2009 and Safari et al. 2018). In this study, R2 obtained for correlation between DPPH and ABTS was 0.984, 0.963, and 0.609 in aqueous, methanolic, and ethanolic extracts, respectively (Table 3). In a similar approach, a significant correlation was reported for medicinal plants between ABTS assay and FRAP values by Rajurkar et al. (2011). The high content of phenolics and flavonoids can be attributed to elevated levels of antioxidant activity in leaf extracts (Khiraoui et al. 2017).
Antidiabetic Assays
In vitro α-amylase and α-glucosidase Inhibitory Assays
Nowadays, there is increasing attention toward natural inhibitors over synthetic inhibitors due to the side effects of the latter compounds, like gastrointestinal distress (Randhir et al. 2008). Synthetic inhibitors also show low efficacy and other unfavorable effects like discoloration of skin, pruritus, etc. (Wondafrash et al. 2020). Plant phenolics can partially inhibit α-amylase activity; therefore, they can be used as therapeutic agents in the management of secondary complications of diabetes (Chethan et al. 2008). The present study showed that the aqueous extract inhibited α-amylase and α-glucosidase to the maximum extent (Table 4) with a significance level of p < 0.001. These observed values are approximately half of those reported by Ruiz-Ruiz et al. (2015). In a recent study, Zaidan et al. (2019) reported an IC50 value of 13.73 μg/mL (for α-amylase), in Stevia leaf extracts. In the present study, aqueous extracts showed the maximum activity compared to other extracts which might be due to the steviol glycosides content (Rasouli et al. 2017) and hence can be used for the control of diabetes (Ruiz-Ruiz et al. 2015).
Antiglycation Assays
Measurement of Browning
Browning has been used as a primary indicator of glycation (Banan and Ali 2016). Figure 6a shows that the test sample caused a 34.95% ± 11.09 decrease in the browning as compared to the positive control. These observations were significant at p < 0.05. Similar reports have suggested the preventive effect of other plant extracts on glycation. In a recent study by Pandey et al. (2018), it was observed that Nigella sativa caused a 47.96% decrease in the browning of protein by fructose.
Estimation of Fructosamine
An increase in the concentration of oxo-aldehyde and fructosamine is due to the rise in the production of AGEs (Rahbar and Figarola 2003). The effect of the test sample on fructosamine levels is represented in Fig. 6b. The fructosamine production, in the positive control, substantially increased after 4 weeks of incubation. On the contrary, the production of fructosamine was reduced by 47.06% ± 4.67 (p < 0.05) in the test sample. In similar studies by Perera et al. (2016) and Nunthanawanich et al. (2016), 5 mg/mL and 1 mg/mL leaf extract of Costus Speciosus and Moringa oleifera led to 10.05% and 49.56% inhibition of AGEs, respectively, as compared to 45–48% inhibition by 1 mg/mL of aminoguanidine (AG), used as a positive control. Thus, it can be interpreted that Stevia is comparable to Moringa oleifera, which is reported to be a potent antiglycating agent. Also, it is highly possible that the reduction of fructosamine content (an indicator of early glycation products) by Stevia may be associated with the formation of AGEs to a lesser extent due to its inhibitory action on the Amadori products, which act as raw materials for the formation of AGEs (Bartosz et al. 2015).
Estimation of Protein Carbonyl Content
The radical scavenging activity of Stevia extract was estimated by the bleaching rate of DNPH which acts as a stable free radical. Upon reduction by any antioxidant or radical species, absorption of DNPH decreases from 515 to 370 nm which is an indicator of high free radical scavenging activity (Ramkissoon et al. 2013). The color change from deep-violet to light-yellow was plotted on the graph. As shown in Fig. 6c, the radical scavenging activity of the test sample significantly diminished with an increase in carbonyl content (p < 0.05). After 4 weeks of incubation, it was observed that there was a reduction in carbonyl content to 46.18% ± 0.47 in the test sample when compared with the control. Jariyapamornkoon et al. (2013) reported that red grape skin extract reduced the carbonyl content by 37.7% to 41.7% at 0.062–0.500 mg/mL concentration, while it reduced the carbonyl content of AG by 45.1% at 0.5 mg/mL. In this respect, Stevia might show significant inhibitory effects on albumin glycation even at concentrations less than 1 mg/mL. In a previous study, it was suggested that the antiglycation agents may act by intercepting the oxidation of Amadori products as well as metal-catalyzed sugar resulting in the delay of AGEs formation (Sajithlal et al. 1998). Therefore, it can be concluded that Stevia extract might prevent the oxidation of albumin incubated with sugar by decreasing the generation of the carbonyl group. This further suggests its potential as an antiglycating agent.
Determination of Amyloid β-structure
The late stage of non-enzymatic glycation comprises protein aggregates, and hence, it is used to estimate the degree of modification occurring within the secondary structure of the protein (Brownlee 1995). In this study, it was observed that the test sample reduced the protein aggregation index (PAI) to 45.74 ± 12.14%, at a significance level of p < 0.05 (Fig. 6d). In a similar report, Adisakwattana et al. (2014) reported that at a 0.25–1.00 mg/mL Mesona Chinensis extract showed a reduction in protein aggregation in a dose-dependent manner (8.1%, 9.7%, and 10.3%, respectively) in Congo red assay. Several mechanisms have been proposed indicating the fact that the polyphenols break the cross-linking structure found in AGEs and block the carbonyl group found in reducing sugars (Wu et al. 2011). This supports our finding that Stevia extract, with high polyphenolic content, may be a prime contributor to the prevention of AGEs formation. Mass spectrometry analysis of Stevia can be performed to confirm the role of polyphenolic compounds in the attenuation of glycation activity as reported by Deve et al. (2014).
Measurement of Total Fluorescent AGEs
Total AGEs formation and inhibition were evaluated by a fluorometric method. When fluorescence of a sample was inhibited, as compared to the positive control, complete inhibition of fluorescent AGEs was presumed. These results suggest that the presence of Stevia extract reduced the formation of fluorescent AGEs by 81.79% (Fig. 7). Similar effects were observed by Moe et al. (2018), where the same concentration (1 mg/mL) of ring extract of Garcinia Mangostana and rutin reduced the formation of total AGEs by 82.37 ± 1.78% and 87.68 ± 1.75%, respectively. Thus, Stevia can be considered as potent as rutin (positive control). Our results were consistent with Deetae et al. (2012) who showed the anti-AGE formation capacity of aqueous extract of Stevia leaves (> 80%). In an earlier study, Jariyapamornkoon et al. (2013) reported that 0.5 mg/mL of red grape skin extract and AG caused a 63.52% and 73.3% decrease, respectively, in total AGEs. Recently, Escutia-Lopez et al. (2020) also supported this fact as aqueous extract of Stevia exhibited 96.5% inhibition at 100 mg/ml in their study. In this study, the intensity of fluorescence for glycated BSA was quenched with an obvious redshift in the presence of Stevia extract, suggesting its ability to prevent AGE formation (Vivian and Callis 2001).
Correlation Between Different Antiglycation Assays
A correlation analysis was performed to obtain the relationship between various antiglycation assays of Stevia (Fig. 8). A strong relation was noticed between total AGEs and various stages of glycation (browning and fructosamine, carbonyl, and protein aggregation) with a significance level of p < 0.01. Thus, these results suggest that Stevia extract can be a potential candidate in the protection from the initial, intermediate stages as well as late stages of glycation, thereby impacting the total AGEs formation.
Correlation between total AGEs and various antiglycation assays of Stevia (1: total AGEs, 2: browning, 3: fructosamine content, 4: carbonyl content, 5: protein aggregation). Results are expressed as mean ± SD of triplicate tests, with different significance levels. All columns share the same p-value (< 0.01)
In vitro Glycation of pBR322 Plasmid DNA
The effect of Maillard reaction products, on the structure of genomic DNA, was analyzed. The results presented predict that DNA strand breakage may have been prompted by simultaneous incubation of lysine and MG. The increased damage of plasmid pBR322 was caused due to ferric ions (Fe3+). Fenton (1894) first reported that trace metals such as iron may react with H2O2, generate hydroxyl radical, and ultimately cause breakage in the DNA strand. The concept of hydroxyl radical generation with copper and iron was later reported by Kang et al. (2003). In this study, the incubation of DNA (pBR322) with a glycated system caused strand breaks and resulted in an increase in the open circular form of DNA (Fig. 9; Lane 2) as compared to non-glycated DNA (Fig. 9; Lane 1). When glycated DNA was incubated with Stevia extract, it showed protection against H2O2-induced plasmid DNA damage due to glycation (Fig. 9; Lanes 3 and 4). In a similar study, Suji and Sivakami (2006) reported that AG and Fe led to the generation of H2O2, causing breakage of the DNA strand via MG radical anion and cross-linked MG radical cation formation because of MG-induced albumin modification. They further stated that these intermediaries are responsible for generating sites for radical cation and cross-linking of proteins. In the current investigation, confirmation of ROS-induced oxidative modifications, leading to the marked rise in DNA damage and protein carbonyl in BSA, was observed. In recent times, appreciable interest has been dedicated to phytochemicals as they act as free radical scavengers which lead to the prevention of DNA damage as well as lysine/MG-induced protein glycation (Wu and Yen 2005).
Conclusion
An investigation of antioxidation and anti-glycation properties of Stevia leaf extract was carried out. The aqueous leaf extract of S. rebaudiana showed significant antioxidant capacity because of the presence of high phenolic compounds. Statistically, strong correlations were observed between the antioxidant capacity of Stevia and high phenolic and flavonoid contents. Literature reveals that antioxidant compounds can suppress protein oxidation as well as the formation of AGEs. Considerable antioxidant activity of aqueous leaf extract of S. rebaudiana provides a preliminary approach for its recommendation as a potent source of natural antioxidants with beneficial health benefits. Since there is a high demand for healthy food products with natural sweeteners, there is the scope of development of functional foods with these agents. Further investigation on the bioactive compounds present in Stevia responsible for the observed activities is needed.
References
Abbas Momtazi-Borojeni, A., S.A. Esmaeili, E. Abdollahi, and A. Sahebkar. 2017. A review on the pharmacology and toxicology of steviol glycosides extracted from Stevia rebaudiana. Current Pharmaceutical Design 23(11):1616–1622. https://doi.org/10.2174/1381612822666161021142835.
Adisakwattana, S., T. Thilavech, and C. Chusak. 2014. Mesona chinensis Benth extract prevents AGE formation and protein oxidation against fructose-induced protein glycation in vitro. BMC Complementary and Alternative Medicine 14(1):130–139. https://doi.org/10.1186/1472-6882-14-130.
Al-Manhel, A.J., and A.K. Niamah. 2015. Effect of aqueous and alcoholic plant extracts on inhibition of some types of microbes and causing spoilage of food. Pakistan Journal of Food Sciences 25(3):104–109. https://doi.org/10.4172/2155-9600.S5-006.
Ali, A., T.A. More, and A.K. Hoonjan. 2017. Antiglycating potential of acesulfame potassium: An artificial sweetener. Applied Physiology, Nutrition, and Metabolism 42(10):1054–1063. https://doi.org/10.1139/apnm-2017-0119.
Ali, A., and R. Sharma. 2015. A comparative study on the role of lysine and BSA in glycation-induced damage to DNA. Bioscience and Bioengineering Communications 1:38–43. https://doi.org/10.18801/bbc.010119.05.
Ali, H., P.J. Houghton, and A. Soumyanath. 2006. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthusamarus. Journal of Ethnopharmacology 107(3):449–455. https://doi.org/10.1016/j.jep.2006.04.004.
Alvarez-Robles, M.J., A. Lopez-Orenes, M.A. Ferrer, and A.A. Calderon. 2016. Methanol elicits the accumulation of bioactive steviol glycosides and phenolics in Stevia rebaudiana shoot cultures. Industrial Crops and Products 87:273–279. https://doi.org/10.1016/j.indcrop.2016.04.054.
Andre, C., I. Castanheira, J.M. Cruz, P. Paseiro, and A. Sanches-Silva. 2010. Analytical strategies to evaluate antioxidants in food: A review. Trends in Food Science and Technology 21(5):229–246. https://doi.org/10.1016/j.tifs.2009.12.003.
Ayyash, M., A.K. Al-Nuaimi, S. Al-Mahadin, and S. Liu. 2018. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chemistry 239:588–597. https://doi.org/10.1016/j.foodchem.2017.06.149.
Banan, P., and A. Ali. 2016. Preventive effect of phenolic acids on in vitro glycation. Annals of Phytomedicine 5(2):97–102. https://doi.org/10.21276/ap.2016.5.2.12.
Baumann, J. 1979. Prostaglandin synthetase inhibiting Oxygen radical scavenging properties of some flavonoids and related phenolic compounds. Naunyn-Schmiedeberg’s Archives of Pharmacology 308:27–32.
Benzie, I.F., and J.J. Strain. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry 239(1):70–76. https://doi.org/10.1006/abio.1996.0292.
Boden, G. 2002. Pathogenesis of type 2 diabetes Insulin resistance. Endocrinology and Metabolism Clinics of North America 30(4):801–815. https://doi.org/10.1016/S0889-8529(05)70216-4.
Bouma, B., L.M. Kroon-Batenburg, Y.P. Wu, B. Brunjes, G. Posthuma, P. Grete, E.E. Voest, and M. Gebbink. 2003. Glycation induces formation of amyloid cross-β structure in albumin. Journal of Biological Chemistry 278(43):41810–41819. https://doi.org/10.1074/jbc.M303925200.
Brownlee, M. 1995. The pathological implications of protein glycation. Clinical and Investigative Medicine 18(4):275–281. https://europepmc.org/article/med/8549013.
Celiktas, O.Y., E. Bedir, and F.V. Sukan. 2007. In vitro antioxidant activities of Rosmarinus officinalis extracts treated with supercritical carbon dioxide. Food Chemistry 101(4):1457–1464. https://doi.org/10.1016/j.foodchem.2006.03.055.
Chethan, S., Y.N. Sreerama, and N.G. Malleshi. 2008. Mode of inhibition of finger millet malt amylases by the millet phenolics. Food Chemistry 111(1):187–191. https://doi.org/10.1016/j.foodchem.2008.03.063.
Connor, A.M., J.J. Luby, J.F. Hancock, S. Berkheimer, and E.J. Hanson. 2002. Changes in fruit antioxidant activity among blueberry cultivars during coldtemperature storage. Journal of Agricultural and Food Chemistry 50(4):893–898. https://doi.org/10.1021/jf011212y.
Deetae, P., P. Parichanon, P. Trakunleewatthana, C. Chanseetis, and S. Lertsiri. 2012. Antioxidant and antiglycation properties of Thai herbal teas in comparison with conventional teas. Food Chemistry 133(3):953–959. https://doi.org/10.1016/j.foodchem.2012.02.012.
Deve, A.S., K. Kumaresan, and V.S. Rapheal. 2014. Extraction process optimization of polyphenols from Indian Citrus sinensis–as novel antiglycative agents in the management of diabetes mellitus. Journal of Diabetes and Metabolic Disorders 13(1):11–21. https://doi.org/10.1186/2251-6581-13-11.
Dewanto, V., X. Wu, K.K. Adom, and R.H. Liu. 2002. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry 50(10):3010–3014. https://doi.org/10.1021/jf0115589.
Escutia-López, K.N., M. González-Montoya, R. Ortiz, E. Cano-Sampedro, Y. Rodríguez- Rivero, M.E. Sánchez-Pardo, and R. Mora-Escobedo. 2020. Effect of aqueous Stevia rebaudiana Bertoni extract in antioxidant and antiglycation capacity in vitro. Revista Bio Ciencias 7 e875:1–16. https://doi.org/10.15741/revbio.07.e875.
Fenton, H.J. 1894. Oxidation of tartaric acid in presence of iron. Journal of the Chemical Society 65:889–910. https://doi.org/10.1039/CT8946500899.
Ghanta, S., A. Banerjee, A. Poddar, and S. Chattopadhyay. 2007. Oxidative DNA damage preventive activity and antioxidant potential of Stevia rebaudiana (Bertoni) Bertoni, a natural sweetener. Journal of Agricultural and Food Chemistry 55(26):10962–10967. https://doi.org/10.1021/jf071892q.
International Diabetes Federation (IDF). 2017. IDF Diabetes Atlas, 8th Edn. Brussels:International Diabetes Federation. http://www.diabetesatlas.org/resources/2017-atlas.html. Accessed 3 Apr 2021.
Jagetia, G.C., M.S. Baliga, K.J. Malagi, and M.S. Kamath. 2000. The evaluation of the radioprotective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to γ-radiation. Phytomedicine 9(2):99–108. https://doi.org/10.1078/0944-7113-00095.
Jahan, I.A., M. Mostafa, H. Hossain, I. Nimmi, A. Sattar, A. Alim, and S.M.I. Moeiz. 2010. Antioxidant activity of Stevia rebaudiana Bert. leaves from Bangladesh. Bangladesh Pharmaceutical Journal 13(2):67–75.
Jaradat, N.A., N. Al-Maharik, S. Abdallah, R. Shawahna, A. Mosa, and A. Qtishat. 2020. Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Industrial Crops and Products 158:1–11. https://doi.org/10.1016/j.indcrop.2020.112946.
Jariyapamornkoon, N., S. Yi Chok-anun, and S. Adisakwattana. 2013. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complementary and Alternative Medicine 13(1):171–180. https://doi.org/10.1186/1472-6882-13-171.
Kang, J.H. 2003. Oxidative damage of DNA induced by methylglyoxal in vitro. Toxicology Letters 145(2):181–187. https://doi.org/10.1016/S0378-4274(03)00305-9.
Khan, M.A., A.A. Rahman, S. Islam, P. Khandokar, S. Parvin, M.B. Islam, M. Hossain, M. Rashid, G. Sadik, S. Nasrin, M.N.H. Mollah, and A.H.M. Khurshid Alam. 2013. A comparative study on the antioxidant activity of methanolic extracts from different parts of Morus alba L. (Moraceae). BMC Research Note 6(1):24–33. https://doi.org/10.1186/1756-0500-6-24.
Khiraoui, A., A. Hasib, C. Al Faiz, F. Amchra, Z.M. Bakha, B. Abdelali. 2017. Stevia rebaudiana Bertoni (Honey Leaf): A magnificent natural bio-sweetener, biochemical composition, nutritional and therapeutic values. Journal of Natural Sciences Research 7(14):75–85. https://core.ac.uk/reader/234657460.
Kim, J.S., C.S. Kwon, and K.H. Son. 2000. Inhibition of alpha-glucosidase and amylase by luteolin, a flavonoid. Bioscience, Biotechnology, and Biochemistry 64(11):2458–2461. https://doi.org/10.1271/bbb.64.2458.
Klip, A., A. Marette, D. Dimitrakoudis, T. Ramlal, A. Giacia, Z.Q. Shi, and M. Vranic. 1992. Effect of diabetes on glucoregulation: From glucose transporters to glucose metabolism in vivo. Diabetes Care 15(11):1747–1766. https://doi.org/10.2337/diacare.15.11.1747.
Kumar, D., and A. Ali. 2019. Antiglycation and antiaggregation potential of thymoquinone. Natural Volatiles and Essential Oils 6(1):25–33. https://dergipark.org.tr/en/pub/nveo/issue/46268/528760.
Kumar, D., S.G. Bhatkalkar, S. Sachar, and A. Ali. 2020. Studies on the antiglycating potential of zinc oxide nanoparticles and its interaction with BSA. Journal of Biomolecular Structure and Dynamics. https://doi.org/10.1080/07391102.2020.1803137.
Lilloja, S., D.M. Mott, R. Ferraro, J.E. Foley, E. Ravussin, W.C. Knowler, P.H. Bennett, and C. Bogardus. 1993. Insulin resistance and insulin secretory dysfunction as precursors of non- insulin-dependent diabetes mellits: Prospective studies of Pima Indians. New England Journal of Medicine 329 (27):1988–1992. https://doi.org/10.1056/NEJM199312303292703.
Mandal, S., B. Hazra, R. Sarkar, S. Biswas, and N. Mandal. 2011. Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evidence-Based Complementary and Alternative Medicine 2011:1–11. https://doi.org/10.1093/ecam/nep072.
Marrassini, C., I. Peralta, and C. Anesini. 2018. Comparative study of the polyphenol content-related anti-inflammatory and antioxidant activities of two Urera aurantiaca specimens from different geographical areas. Chinese Medicine 13(1):1–12. https://doi.org/10.1186/s13020-018-0181-1.
Meeprom, A., W. Sompong, C.B. Chan, and S. Adisakwattana. 2013. Isoferulic acid, a new anti-glycation agent, inhibits fructose-and glucose-mediated protein glycation in vitro. Molecules 18 (6): 6439–6454. https://doi.org/10.3390/molecules18066439.
Mitra, K., and N. Uddin. 2014. Total phenolics, flavonoids, proanthrocyanidins, ascorbic acid contents and in-vitro antioxidant activities of newly developed isolated soya protein. Journal of Agriculture and Food Sciences 2(5):160–168. http://www.resjournals.org/JAFS/PDF/2.
Moe, T.S., H.H. Win, T.T. Hlaing, W.W. Lwin, Z.W. Htet, and K.M. Mya. 2018. Evaluation of in vitro antioxidant, antiglycation and antimicrobial potential of indigenous Myanmar medicinal plants. Journal of Integrative Medicine 16(5):358–366. https://doi.org/10.1016/j.joim.2018.08.001.
Ngo, T.V., C.J. Scarlett, M.C. Bowyer, P.D. Ngo, and Q.V. Vuong. 2017. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia Chinensis L. Journal of Food Quality 2017:1–8. https://doi.org/10.1155/2017/9305047.
Nunthanawanich, P., W. Sompong, and S. Sirikwanpong. 2016. Moringa oleifera aqueous leaf extract inhibits reducing monosaccharide-induced protein glycation and oxidation of bovine serum albumin. Springerplus 5(1):1098–2004. https://doi.org/10.1186/s40064-016-2759-3.
Oktay, M., I. Gulcin, and O.I. Kufrevioglu. 2003. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Science and Technology 36(2):263–271. https://doi.org/10.1016/S0023-6438(02)00226-8.
Ortiz-Viedma, J., N. Romero, L. Puente, K. Burgos, M. Toro, L. Ramirez, A. Rodriguez, J. Barros-Velazquez, and S.P. Aubourg. 2017. Antioxidant and antimicrobial effects of Stevia (Stevia rebaudiana Bert.) extracts during preservation of refrigerated salmon paste. European Journal of Lipid Science and Technology 119(10):1600467–1600475. https://doi.org/10.1002/ejlt.201600467.
Ozcan, A., and M. Ogun. 2015. Biochemistry of reactive oxygen and nitrogen species. Basic Principles and Clinical Significance of Oxidative Stress 3:37–58. https://doi.org/10.5772/61193.
Pandey, R., D. Kumar, and A. Ali. 2018. Nigella sativa seed extracts prevent the glycation of protein and DNA. Current Perspectives on Medicinal and Aromatic Plants 1(1):1–7. https://dergipark.org.tr/en/pub/cupmap/issue/38636/425212.
Perera, H.K., W.K. Premadasa, and J. Poongunran. 2016. α-glucosidase and glycation inhibitory effects of costus speciosus leaves. BMC Complementary and Alternative Medicine 16(1):1–9. https://doi.org/10.1186/s12906-015-0982-z.
Phansawan, B., and S. Poungbangpho. 2007. Antioxidant capacities of Pueraria Mirifica, Stevia rebaudiana Bertoni, Curcuma longa Linn., Andrographis Paniculata (Burm. f.) Nees and Cassia alata Linn.for the development of dietary supplement. Kasetsart Journal (natural Science) 3:407–413.
Rahbar, S., and J.L. Figarola. 2003. Novel inhibitors of advanced glycation end products. Archives of Biochemistry and Biophysics 419(1):63–79. https://doi.org/10.1016/j.abb.2003.08.009.
Rajurkar, N.S., and S.M. Hande. 2011. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian Journal of Pharmaceutical Sciences 73 (2):146–151. https://doi.org/10.4103/0250-474x.91574.
Ramkissoon, J.S., M.F. Mahomoodally, N. Ahmed, and A.H. Subratty. 2013. Antioxidant and anti–glycation activities correlate with phenolic composition of tropical medicinal herbs. Asian Pacific Journal of Tropical Medicine 6(7):561–569. https://doi.org/10.1016/S1995-7645(13)60097-8.
Randhir, R., Y.I. Kwon, and K. Shetty. 2008. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innovative Food Science and Emerging Technologies 9(3):355–364. https://doi.org/10.1016/j.ifset.2007.10.004.
Rasouli, H., S.M.B. Hosseini-Ghazvini, H. Adibi, and R. hodarahmi. 2017. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food and Function 8(5):1942–1954. https://doi.org/10.1039/c7fo00220c.
Rokosa, M.T., and D. Kulpa. 2020. Micropropagation of Stevia rebaudiana plants. Ciencia Rural 50(1):1–9. https://doi.org/10.1590/0103-8478cr20181029.
Ruiz, J.C., Y.B. Ordoñez, A.M. Basto, and M.R. Campos. 2015. Antioxidant capacity of leaf extracts from two Stevia rebaudiana Bertoni varieties adapted to cultivation in Mexico. Nutrición Hospitalaria 31(3):1163–1170. https://doi.org/10.3305/nh.2015.31.3.8043.
Ruiz-Ruiz, J.C., Y.B. Moguel-Ordoñez, A.J. Matus-Basto, and M.R. Segura-Campos. 2015. Antidiabetic and antioxidant activity of Stevia rebaudiana extracts (Var. Morita) and their incorporation into a potential functional bread. Journal of Food Science and Technology 52(12):7894–7903. https://doi.org/10.1007/s13197-015-1883-3.
Safari, M.R., O. Azizi, S.S. Heidary, N. Kheiripour, and A.P. Ravan. 2018. Antiglycation and antioxidant activity of four Iranian medical plant extracts. Journal of Pharmacopuncture 21(2):82–89. https://doi.org/10.3831/KPI.2018.21.010.
Sajithlal, G.B., P. Chithra, and G. Chandrakasan. 1998. The role of metal-catalyzed oxidation in the formation of advanced glycation end products: An in vitro study on collagen. Free Radical Biology and Medicine 25(3):265–269. https://doi.org/10.1016/S0891-5849(98)00035-5.
Samuel, P., K.T. Ayoob, B.A. Magnuson, U. Wölwer-Rieck, P.B. Jeppesen, P.J. Rogers, I. Rowland, and R. Mathews. 2018. Stevia leaf to Stevia sweetener: Exploring its science, benefits, and future potential. The Journal of Nutrition 148(7):1186S-1205S. https://doi.org/10.1093/jn/nxy102.
Sharangi, A.B. and P.H. Bhutia. 2016. Stevia: Medicinal Miracles and therapeutic magic. International Journal of Crop Science and Technology, 2(2):45–59. https://dergipark.org.tr/en/pub/ijcst/issue/29923/322310.
Shukla, S., A. Mehta, V.K. Bajpai, and S. Shukla. 2009. In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food and Chemical Toxicology 47(9):2338–2343. https://doi.org/10.1016/j.fct.2009.06.024.
Shukla, S., A. Mehta, P. Mehta, and V.K. Bajpai. 2012. Antioxidant ability and total phenolic content of aqueous leaf extract of Stevia rebaudiana Bert. Experimental and Toxicologic Pathology 64(7–8):807–811. https://doi.org/10.1016/j.etp.2011.02.002.
Singh, V.P., A. Bali, N. Singh, and A.S. Jaggi. 2014. Advanced glycation end products and diabetic complications. The Korean Journal of Physiology and Pharmacology: Official Journal of the Korean Physiological Society and the Korean Society of Pharmacology 18(1):1–14. https://doi.org/10.4196/kjpp.2014.18.1.1.
Silva, C.S., A. Oliveira, S.V. Pinto, M.C. Manso, and A. Ferreira da Vinha. 2018. Natural resources with sweetener power: phytochemistry and antioxidant characterisation of Stevia rebaudiana (Bert.), sensorial and centesimal analyses of lemon cake recipes with S. rebaudiana incorporation. Egitania Sciencia 23:141–159. http://hdl.handle.net/10284/8044.
Singh, S.D., and G.P. Rao. 2005. Stevia: The herbal sugar of 21st Century. Sugar Tech 7(1):17–24. https://doi.org/10.1007/BF02942413.
Singleton, V.L. and J.A. Rossi. 1965. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 16(3):144–158. https://www.ajevonline.org/content/16/3/144.
Saraiva, A., C. Carrascosa, D. Raheem, F. Ramos, and A. Raposo. 2020. Natural sweeteners: The relevance of food naturalness for consumers, food security aspects sustainability and health impacts. International Journal of Environmental Research and Public Health. 17(17):6285–6307. https://doi.org/10.3390/ijerph17176285.
Suji, G., and S. Sivakami. 2006. DNA damage by free radical production by aminoguanidine. Annals of the New York Academy of Sciences 1067(1):191–199. https://doi.org/10.1196/annals.1354.023.
Sorensen, L.B., T.H. Vasilaras, A. Astrup, and A. Raben. 2014. Sucrose compared with artificial sweeteners: A clinical intervention study of effects on energy intake, appetite, and energy expenditure after 10 wk of supplementation in overweight subjects. The American Journal of Clinical Nutrition 100(1):36–45. https://doi.org/10.3945/ajcn.113.081554.
Surana, S.J., S.B. Gokhale, R.A. Rajmane, and R.B. Jadhav. 2006. Non-saccharide natural intense sweeteners–An overview of current status. Natural Product Radiance 5(4):270–278. http://hdl.handle.net/123456789/7965.
Tavarini, S. and L.G. Angelini. 2013. Stevia rebaudiana Bertoni as a source of bioactive compounds: The effect of harvest time, experimental site and crop age on steviol glycoside content and antioxidant properties. Journal of the Science of Food and Agriculture 93(9):2121–2129. https://doi.org/10.1002/jsfa.6016.
Tadhani, M.B., V.H. Patel, and R. Subhash. 2007. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. Journal of Food Composition and Analysis 20(3–4):323–329. https://doi.org/10.1016/j.jfca.2006.08.004.
Ullah, A., A. Khan, and I. Khan. 2016. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharmaceutical Journal 3(5):547–553. https://doi.org/10.1016/j.jsps.2015.03.013.
Unuofin, J.O., and S.L. Lebelo. 2020. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxidative Medicine and Cellular Longevity 2020:1–36. https://doi.org/10.1155/2020/1356893.
Vivian, J.T., and P.R. Callis. 2001. Mechanisms of tryptophan fluorescence shifts in proteins. Biophysical Journal 80(5):2093–2109. https://doi.org/10.1016/S0006-3495(01)76183-8.
Woelwer-Rieck, U., C. Lankes, and A. Wawrzun. 2010. Improved HPLC method for the evaluation of the major steviol glycosides in leaves of Stevia rebaudiana. European Food Research and Technology 231(4):581–588. https://doi.org/10.1007/s00217-010-1309-4.
Wondafrash, D.Z., T.Z. Desalegn, E.M. Yimer, A.G. Tsige, B.A. Adamu, and K.A. Zewdie. 2020. Potential effect of hydroxychloroquine in diabetes mellitus: A systematic review on preclinical and clinical trial studies. Journal of Diabetes Research 2020:1–10. https://doi.org/10.1155/2020/5214751.
Wu, C.H., S.M. Huang, J.A. Lin, and G.C. Yen. 2011. Inhibition of advanced glycation end product formation by food stuffs. Food and Function 2(5):224–234. https://doi.org/10.1039/C1FO10026B.
Wu, C.H., and G.C. Yen. 2005. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation end products. Journal of Agricultural and Food Chemistry 53(8):3167–3173. https://doi.org/10.1021/jf048550u.
Zaidan, U.H., N.I. Zen, N.A. Amran, S. Shamsi, and S.S. Abd Gani. 2019. Biochemical evaluation of phenolic compounds and steviol glycoside from Stevia rebaudiana extracts associated with in vitro antidiabetic potential. Biocatalysis and Agricultural Biotechnology 18:101049–101057. https://doi.org/10.1016/j.bcab.2019.101049.
Zeng, J., W. Cai, W. Yang, and W. Wu. 2013. Antioxidant abilities, phenolics and flavonoids contents in the ethanolic extracts of the stems and leaves of different Stevia rebaudiana Bert lines. Sugar Tech 15(2):209–213. https://doi.org/10.1007/s12355-013-0210-4.
Zengin, G., G.O. Guler, A. Aktumsek, R. Ceylan, C.M. Picot, and M.F. Mahomoodally. 2015. Enzyme inhibitory properties, antioxidant activities, and phytochemical profile of three medicinal plants from Turkey. Advances in Pharmacological Sciences 2015:1–8. https://doi.org/10.1155/2015/410675.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ali, A., Shahu, R., Balyan, P. et al. Antioxidation and Antiglycation Properties of a Natural Sweetener: Stevia rebaudiana. Sugar Tech 24, 563–575 (2022). https://doi.org/10.1007/s12355-021-01023-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-021-01023-0






 : aqueous extract;
: aqueous extract;  : methanolic extract;
: methanolic extract;  : ethanolic extract). Results are expressed as mean ± SD of triplicate tests
: ethanolic extract). Results are expressed as mean ± SD of triplicate tests

 : aqueous extract;
: aqueous extract;  : methanolic extract;
: methanolic extract;  : ethanolic extract). Results are expressed as mean ± SD of triplicate tests
: ethanolic extract). Results are expressed as mean ± SD of triplicate tests



