Abstract
We carried out a systematic research on antioxidant activities of ethanolic extracts of aerial parts (stems and leaves) of four selected Stevia rebaudiana lines. Total phenolics and flavonoids contents of different S. rebaudiana lines were in the range of 55.64–58.35 mg (gallic acid) g−1 dw and 48.29–60.33 mg (rutin) g−1 dw, respectively. The contents of phenolics and flavonoids, and antioxidant capacities in the leaves of S. rebaudiana were twofold to fivefold significantly higher than that in stems of S. rebaudiana. SR-3 had the highest total phenolics and flavonoids contents and the strongest scavenging abilities to DPPH and ABTS radicals. DPPH and TEAC assays were equal to evaluate the antioxidant ability of ethanolic extract of S. rebaudiana. The obtained results clearly indicated that the leaves of S. rebaudiana had an important value of utilization and development as a natural antioxidant agent and further studies for SR-3 should be put into practice.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many of the free radicals and non-radicals produced in the body as by-products of normal metabolism can induce oxidation stress which has been implicated in the aetiology of diseases including cancer, cardiovascular diseases, neural disorders, diabetes and arthritis (Bruckdorfer 2005). These diseases have gained wide concern for their importance to human health in recent years. With the recognition of the safety of food and drugs deepening, people were eager for searching antioxidants from natural sources such as many herbal and medicinal plants containing antioxidant compounds including phenolics compounds which were known to possess potential antioxidant properties (Aehle et al. 2004; del Baño et al. 2003; Sastre et al. 1998; Van Beek 2002). Phenolics constitute one of the largest categories of phytochemicals, most widely distributed among the plant kingdom, and an integral part of the human diet. Flavonoids composed the largest and most-studied group of plant phenolics are effective scavengers of hydroxyl and peroxyl radicals, and of the superoxide anion (Heim et al. 2002). Plant polyphenolics are multifunctional and can act as hydrogen atom-donating antioxidants, singlet oxygen quenchers and as metal ion chelation (Pengelly 2004). Moreover, the antioxidant activity of medicinal plants depends on the concentration of individual antioxidant entering into the composition (Larson 1988).
Stevia rebaudiana Bertoni is a perennial shrub belonging to the family of Asteraceae (Compositae) native to Brazil and Paraguay, but now grown commercially in a number of countries, particularly in Japan, China, Korea, India, and certain countries of South America. It has been widely used in food, beverage and drugs industry as dietary supplements and key-source sweetener for diabetic and hypertension world. The major constituents in the leaves of S. rebaudiana are the potently sweet diterpenoid glycosides stevioside (ST), rebaudiosides A (RA) and D, and dulcoside A (Prakash Chaturvedula et al. 2011). Recently, phenolics and flavonoids of S. rebaudiana possessing antioxidant, antimicrobial and antifungal abilities have attracted a lot of attention (Abou-Arab and Abu-Salem 2010; Jayaraman et al. 2008).
To the best of our knowledge, little reports are available on the antioxidant activities of constituents in different parts and of different S. rebaudiana lines. In this study, we investigated the contents of phenolics and flavonoids compounds and antioxidant activities of ethanolic extracts of leaves and stems of four selected S. rebaudiana lines.
Materials and Methods
Plant Materials
The S. rebaudiana plants used in this study were supplied by Chengdu Wagott Pharmaceutical Co., Ltd., identified by Prof. Wei Wu of Sichuan Agricultural University as S. rebaudiana Bertoni. After 3 years of planting breeding and selection, four characteristic S. rebaudiana lines were developed: SR-1, SR-2, SR-3 and SR-5 (Yang et al. 2011). Plants of each S. rebaudiana line were planted in the plot on row spacing of 40 cm. 30 plants per line, 3 lines per plot.
Preparation of Ethanolic Extract of S. rebaudiana
The leaves and stems of the four S. rebaudiana lines were separately harvested at the beginning of August in 2010. After being cleared, the harvested parts were dried at 80 °C to constant weight after being processed under 120 °C for 10 min and then powdered. The powdered leaves and stems were weighed to 3.00 g and then were successively extracted with 40, 30 and 30 ml of 75 % ethanol by ultrasound apparatus each for 1 h. After concentrated, the crude extract (CE) was diluted with distilled water to 100 ml and used for the assessment of antioxidant activity. Before the phenolics extract solution of S. rebaudiana was ready for determination, active carbon was used to decolorize the extract solutions.
Determination of the Contents of Phenolics and Flavonoids
The contents of phenolics compounds in ethanolic extracts of stems and leaves of four S. rebaudiana lines were determined with Folin–Ciocalteu reagent according to the method of Prior et al. (2005) with some modifications. Briefly, 0.5 ml CE was mixed with 2.0 ml 20 % of sodium carbonate solution, 1.5 ml Folin–Ciocalteu reagent, fixed volume by distilled water to 5.0 ml, incubated at 55 °C for 1.5 h, and measured the absorbance at 760 nm using a Spectrophotometer (Shimadzu, UV-2450, Japan). Gallic acid was used for constructing the standard curve (1.0–5.0 mg ml−1), and the regression equation was y = 0.0451x + 0.0044 (R2 = 0.9965). The results were expressed as mg gallic acid per gram dry weight (mg gallic acid g−1 dw).
The total flavonoids contents were determined according to a colorimetric method described by China Pharmacopeia (2005) with some modifications. An aliquot of 1 ml CE was put into a 15 ml graduated test tube, and then 0.3 ml 5 % sodium nitrite was added. After 6 min, 0.3 ml 10 % aluminum nitrate was added and after another 6 min, 4 ml 4 % sodium hydroxide was added. After mixing, the volume was filled to 10 ml by distilled water. The solution was kept for 15 min at room temperature, and the absorbance at 510 nm was determined. Rutin was used for constructing the calibration curve. A good linear relationship was obtained over the range of 0–1.0 mg ml−1, and the regression equation was y = 1.0332x + 0.014 (R2 = 0.9990). The results were expressed as mg rutin equivalent (mg rutin g−1 dw).
Evaluation of Antioxidant Activity
DPPH Assay
The DPPH• free radical scavenging activity of CE was determined according to the method of Li et al. (2009) method. Briefly, 0.1 ml of CE (diluted at 1:10) was added to 3.9 ml of a 6 × 10−5 M DPPH solution in methanol. A control sample, containing the same volume of solvent in place of extract solution, was used to measure the maximum DPPH• absorbance. After the reaction in the dark for 30 min, the absorbance at 515 nm was recorded to determine the concentration of remaining DPPH•. Results were expressed as trolox equivalent antioxidant capacity (μM trolox g−1 dw). Trolox standard solutions were prepared at a concentration ranging from 100 to 1,200 μM. The test was carried out in three replicates.
TEAC Assay
The ABTS•+ radical scavenging activity of CE was measured by using the method of Re et al. (1999) with some minor modifications. ABTS•+ was dissolved in ethanol to a concentration of 7 mM. ABTS•+ radical cation was produced by reacting ABTS•+ stock solution with 2.45 mM potassium persulphate and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. The ABTS•+ radical cation solution was diluted with ethanol to an absorbance of 0.70 ± 0.02 at 734 nm and equilibrated at 30 °C. An aliquot of 0.1 ml CE (diluted at 1:10) was mixed with 2.9 ml of diluted ABTS•+ radical cation solution. After reaction at 30 °C for 20 min, the absorbance was measured at 734 nm. Trolox was used as standard antioxidants (200–1,400 μM). The results are expressed as μM trolox g−1 dw. The test was carried out in three replicates.
Results
Phenolics Contents
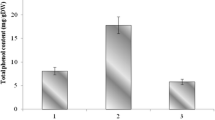
The phenolics contents presented in the ethanolic extract of leaves and stems of four S. rebaudiana lines are shown in Fig. 1. The total content of phenolics of the aerial parts (stems and leaves) of S. rebaudiana was ranged from 55.64 to 58.35 mg gallic acid g−1 dw. Phenolics contents in the leaves of different lines were in the order SR-3 > SR-1 > SR-2 > SR-5 but not significantly different among lines. However, total phenolics contents in the stems of different lines showed significant difference. SR-5 owned the highest stems phenolics content with 18.37 mg gallic acid g−1 dw while SR-3 had the lowest stems phenolics content with 12.95 mg gallic acid g−1 dw. The ethanolic extracts of leaves of S. rebaudiana lines possessed 2 to fourfold higher contents of phenolics than that of stems (p < 0.01).
Phenolics contents in the ethanolic extracts of leaves and stems of different S. rebaudiana lines. Values are expressed as the mean ± SE (n = 3). Bars carrying different letters are significantly different at p < 0.05 among lines. **means significant difference at p < 0.01 between stems and leaves in single line
Flavonoids Contents
The flavonoids contents presented in the ethanolic extract of stems and leaves of S. rebaudiana are shown in Fig. 2. The total contents of flavonoids of the aerial parts (stems and leaves) of S. rebaudiana showed significant difference among lines. SR-3 and SR-5 showed the highest and lowest total flavonoids contents with 60.33 and 48.29 mg rutin g−1 dw in the ethanolic extract, respectively. Leaves flavonoids contents of different lines contained 67–83 % of total flavonoids content, which ranged from 32.36 to 49.90 mg rutin g−1 dw. SR-3 owned the highest leaves flavonoids content. Nevertheless, SR-5 had the highest stems flavonoids content (16.02 mg rutin g−1 dw), followed by SR-2, SR-3, SR-1. The total flavonoids contents in the ethanolic extracts of leaves were twofold to fivefold higher than that of stems (p < 0.01).
Flavonoids contents in the ethanolic extracts of leaves and stems of different S. rebaudiana lines. Values are expressed as the mean ± SE (n = 3). Bars carrying different letters are significantly different at p < 0.05 among lines. **means significant difference at p < 0.01 between stems and leaves in single line
Antioxidant Activity
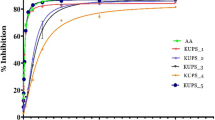
Full form of DPPH and ABTS radicals scavenging abilities of the ethanolic extracts of the aerial parts (stems and leaves) of S. rebaudiana were determined using DPPH• and ABTS•+, and the results were shown in Figs. 3 and 4. The total DPPH radical scavenging abilities in stems and leaves of SR-1, SR-2 and SR-3 were significantly higher than SR-5. However, SR-1, SR-2 and SR-3 shared a similar level of total DPPH radical scavenging ability. The DPPH radical scavenging abilities of the leaves in the four SR lines ranged from 101.88 to 134.58 μM trolox g−1 dw and SR-1 and SR-3 were higher than that in SR-2 and SR-5 (p < 0.05). Furthermore, SR-2, SR-3 and SR-5 possessed relative higher DPPH radical scavenging abilities of stems than SR-1 (Fig. 3). Compared with total DPPH radical scavenging abilities, there was no significant difference in the total ABTS radical scavenging ability among the four SR lines. Nevertheless, The ABTS radical scavenging ability of the leaves in four SR lines ranged from 113.14 to 154.25 μM trolox g−1 dw. SR-1 and SR-3 was significantly higher than SR-2 and SR-5 (p < 0.05) in terms of the ABTS radical scavenging ability of leaves. However, reverse results showing that SR-5 and SR-2 had higher ABTS radical scavenging abilities in stems than SR-1 and SR-3. Specially, the ethanolic extracts of leaves had significantly higher DPPH and ABTS radicals scavenging abilities than those of stems (p < 0.01).
DPPH radical scavenging abilities of the ethanolic extracts of leaves and stems of different S. rebaudiana lines. Values are expressed as the mean ± SE (n = 3). Bars carrying different letters are significantly different at p < 0.05 among lines. **means significant difference at p < 0.01 between stems and leaves in single line
ABTS radical scavenging abilities of the ethanolic extracts of leaves and stems of different S. rebaudiana lines. Values are expressed as the mean ± SE (n = 3). Bars carrying different letters are significantly different at p < 0.05 among lines. ** means significant difference at p < 0.01 between stems and leaves in single line
Discussion
Although flavonoids and phenolics affect the taste of the extract of S. rebaudiana, they have been studied to be effective scavengers of hydroxyl and peroxyl radicals. Results of present study indicated the presence of flavonoids and phenolics in stems and leaves of S. rebaudiana and these compounds could be extracted with ethanol. Different extract methods may affect the proportion of total phenolics in S. rebaudiana leaves. In the present study, the total phenolics content in S. rebaudiana leaves extracted with 75 % ethanol by ultrasound was about 4 %. The content of total phenolics extracted from SR leaves by water was equal with or higher than our results (Kim et al. 2011; Kaushik et al. 2010), while it obtained with a lower percentage of 2.0–3.0 by methanol (Abou-Arab and Abu-Salem 2010). Environments could also impact on the total leaf phenolics content. The leaves of S. rebaudiana plants grown under laboratory conditions contained only 2.5 % total phenolics contents (Tadhani et al. 2007), which was lower than present results with S. rebaudiana plants grown in fields. Furthermore, the contents of total phenolics and flavonoids in S. rebaudiana were higher than that in Plantago major, Equisetum maximum, and Urtica dioica (Pourmorad et al. 2006), which suggested its potential use as the natural source of phenolics and flavonoids.
Different S. rebaudiana lines indicated the inherent differences in antioxidant compounds and antioxidant abilities in present study. Previous study on the four lines showed that SR-2, SR-3 and SR-1 possessed the higher ratio of full form of RA and ST than SR-5 with higher ratio of ST content (Yang et al. 2011). Antioxidant capacity of S. rebaudiana is related to phenolics and flavonoids, as well as diterpene glycosides and inorganic salts containing potassium (Madan et al. 2010; Xi et al. 1998). The results of our study manifested that the contents of antioxidant compounds were closely associated with the antioxidant abilities of the different SR lines. SR-3 had the highest total phenolics and flavonoids contents and the strongest scavenging abilities to DPPH and ABTS radicals, followed by SR-1, SR-2 and SR-5. Therefore, the antioxidant potential development value of SR-3 is worth being further studied.
In addition, the contents and distributions of antioxidant phytochemicals might determine the antioxidant capacity of the plant organ. Recently, it has been reported that RA had significant relationship with the total antioxidant capacity as total phenolics (Tavarini et al. 2010). Diterpenoid glycosides were mainly distributed in the leaves of S. rebaudiana, while almost no diterpenoid glycosides in the stems. In this study, the lines including SR-2, SR-3 and SR-1 which have higher RA, phenolics and flavonoids contents are apt to possess higher antioxidant abilities in leaves while SR-5 is more accessible to possess higher antioxidant ability in stems because of its highest phenolics and flavonoids contents in stems. The connection between total antioxidant capacity and RA content suggest that RA not only possess the good flavor and higher sweetness but also may have the potent antioxidant ability.
Stems of S. rebaudiana plants found to have some flavor enhancers, odourisers and other agents of potential use for improving foodstuffs or alcoholic beverages although they contained little or no sweeteners (Singh and Rao 2005). In present study, the phenolics and flavonoids content in leaves make up 70–80 % of the total phenolics and flavonoids content of the plant. It is suggested that not only sweet diterpene glycosides are abundant in the leaves of S. rebaudiana but also phenolics and flavonoids. Therefore, the leaf of S. rebaudiana can be considered as the main utilizing organ. This result may provide rationale for breeding the S. rebaudiana line with the leaves of high quality and yield.
The obtained findings indicate that no single antioxidant capacity assay will truly reflect the “total antioxidant capacity” of a particular sample and multimethods are proposed in antioxidant ability assays. TEAC and DPPH are classical methods widely used in antioxidant screening of plant extracts due to their simple operation and rapid reaction (Prior et al. 2005). The similar results in TEAC and DPPH assay of S. rebaudiana indicate that the two methods are proper and equal for evaluating the antioxidant capacity of S. rebaudiana extracts.
Conclusions
Stevia rebaudiana have natural antioxidants, which included phenolics, flavonoids and other substances. These bioactive compounds can protect human from some major illnesses. This work indicated that SR-3 might be an ideal line candidate for further research into its uses for food preservation as well as pharmaceutical and natural plant-based products owing to its highest antioxidant activity. Leaf might be the main plant part of bioactive compounds of S. rebaudiana. Moreover, DPPH and ABTS assays can well evaluate the antioxidant capacity of different parts of S. rebaudiana and need to be popularized. The relationships of antioxidant activities and diterpene glycosides in SR-3 need to be studied in the future.
References
Abou-Arab, E.A., and F.M. Abu-Salem. 2010. Evaluation of bioactive compounds of Stevia rebaudiana leaves and callus. African Journal of Food Science 4: 627–634.
Aehle, E., S.R.-L. Grandic, R. Ralainirina, S. Baltora-Rosset, F. Mesnard, C. Prouillet, J.-C. Mazière, and M.-A. Fliniaux. 2004. Development and evaluation of an enriched natural antioxidant preparation obtained from aqueous spinach (Spinacia oleracea) extracts by an adsorption procedure. Food Chemistry 86: 579–585.
Bruckdorfer, K.R. 2005. Oxidative stress. In Cardiovascular disease, diet, nutrition and emerging risk factors (The report of the British nutrition foundation task force), ed. Stanner, S., 78–98. Oxford: Blackwell Publishing.
del Baño, M.J., J. Lorente, J. Castillo, O. Benavente-García, J.A. del Río, A. Ortuño, K.-W. Quirin, and D. Gerard. 2003. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis, antioxidant activity. Journal of Agricultural Food Chemistry 51: 4247–4253.
Heim, K.E., A.R. Tagliaferro, and D.J. Bobilya. 2002. Flavonoids antioxidants, chemistry, metabolism and structure-activity relationships. Journal of Nutrition Biochemistry 13: 572–584.
Jayaraman, S., M.S. Manoharan, and S. lllanchezian. 2008. In-vitro antimicrobial and antitumor activities of Stevia rebaudiana (Asteraceae) leaf extracts. Tropical Journal of Pharmaceutical Research 7: 1143–1149.
Kaushik, R., P. Narayanan, V. Vasudevan, G. Muthukumaran, and A. Usha. 2010. Nutrient composition of cultivated stevia leaves and the influence of polyphenols and plant pigments on sensory and antioxidant properties of leaf extracts. Journal of Food Science and Technology 47: 27–33.
Kim, I.-S., M. Yang, O.-H. Lee, and S.-N. Kang. 2011. The antioxidant activity and the bioactive compound content of Stevia rebaudiana water extracts. LWT-Food Science and Technology 44: 1328–1332.
Larson, R.A. 1988. The antioxidants of higher plants. Phytochemistry 27: 969–978.
Li, H., X.Y. Wang, Y. Li, P.H. Li, and H. Wang. 2009. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chemistry 112: 454–460.
Madan, S., S. Ahmad, G.N. Singh, K. Kohli, Y. Kumar, R. Singh, and M. Garg. 2010. Stevia rebaudiana (Bert.) Bertoni-a review. Indian Journal of Natural Products Research 1: 267–286.
Pengelly, A. 2004. Polyphenolics-Tannins and Flavonoids. In The constituents of medicinal plants. 2nd edition, ed. Pengelly, A., 35. New South Wales: Allen & Unwin.
Pourmorad, F., S.J. Hosseinimehr, and N. Shahabimajd. 2006. Antioxidant activity, phenol and flavonoids contents of some selected Iranian medicinal plants. African Journal of Biotechnology 5: 1142–1145.
Prakash Chaturvedula, V.S., M. Upreti, and I. Prakash. 2011. Diterpene glycosides from Stevia rebaudiana. Molecules 16: 3552–3562.
Prior, R.L., X.L. Wu, and K. Schaich. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural Food Chemistry 53: 4290–4302.
Re, R., N. Pellegrini, A. Proteggente, A. Pannala, M. Yang, and C. Rive-Evans. 1999. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology Medicine 26: 1231–1237.
Sastre, J., A. Millan, J.G. de la Asuncion, R. Pla, G. Juan, F.V. Pallardo, E. O’Connor, J.A. Martin, M.-T. Droy-Lefaix, and J. Viña. 1998. A Ginkgo Biloba extract (EGb 761) prevents mitochondrial aging by protecting against oxidative stress. Free Radical Biology Medicine 24: 298–304.
Singh, S.D., and G.P. Rao. 2005. Stevia, the herbal sugar of 21st century. Sugar Tech 7: 17–24.
Tadhani, M.B., V.H. Patel, and R. Subhash. 2007. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. Journal of Food Composition Analysis 20: 323–329.
Tavarini, S., M. Ribuoli, M. Bimbatti, and L.G. Angelini. 2010. Functional components from Stevia rebaudiana Bert. leaves. Journal of Biotechnology 150: 326.
Van Beek, T.A. 2002. Chemical analysis of Ginkgo biloba leaves and extracts. Journal of Chromatography A 967: 21–55.
Xi, Y., T. Yamaguchi, M. Sato, and M. Takeuchi. 1998. Antioxidant actitvity of Stevia rebaudiana. Nippon Shokuhin Kagaku Kogaku Kaishi 45: 310–316. (in Japanese).
Yang, W.T., W. Wu, Q.R. Cai, Y.W. Xu, and C. Wei. 2011. Comparison on main agronomic traits and glucoside content in different Stevia rebaudiana new lines. Sugar Crops of China 3: 26–29. (in Chinese).
Acknowledgments
The authors thank Miss Q. Liu for providing convenient experiment environment and equipments. We are also grateful to Dr. Y. W. Xu for giving pertinent suggestions in article’s peer view.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zeng, J., Cai, W., Yang, W. et al. Antioxidant Abilities, Phenolics and Flavonoids Contents in the Ethanolic Extracts of the Stems and Leaves of Different Stevia rebaudiana Bert Lines. Sugar Tech 15, 209–213 (2013). https://doi.org/10.1007/s12355-013-0210-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-013-0210-4