Abstract
The aim of this study is to determine the water demand of the varieties RB867515, IAC91-1099, and IAC87-3396 of sugarcane using weighing lysimetry in the environmental conditions of the Cerrado of Goiás, Brazil. The experiment was conducted in Goiânia, GO, Brazil, from March 2016 to March 2018 during the cycles of plant-cane and ratoon cane, lasting 12 months each. For this purpose, 120 pots of 200 L were installed in the experimental area. Eight lines with 15 pots were spaced 1.5 m apart. Three pots were installed on electronic weighing platform scales. The soil water content was monitored by ECH2O EC-5 sensors installed in the sugarcane root zones. Irrigation was performed every two days by replenishing water in the soil up to field capacity. The water demand for the varieties IAC91-1099, RB867515, and IAC87-3396 was on average 9.2, 8.2, and 7.6 L.day−1, respectively, for the first cycle (plant-cane), and 8.4, 7.9, and 6.6 L.day−1, respectively, for the second cycle (ratoon cane). There was a decrease in water consumption of all varieties from the first to the second growing cycle. The variety RB867515 had the smallest decrease (3.7%) between cycles, followed by IAC91-1099 (8.7%), and IAC 87–3396 (13.2%). The differences in average water demand between varieties reached 17.4 and 21.4% in the first and second cycles, respectively, compared to the varieties with a greater (IAC91-1099) and lower (IAC87-3396) water demand. This is due to differences in morphological characteristics (stem diameter, stem height, number of green leaves, and leaf area), which also decreased from the first to the second cycle. There is a need to consider the variety cultivated and the cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is of great importance for the Brazilian economy. Sugar (sucrose) and ethanol are the main products of this crop. In the 2018/2019 harvest, the production was 29.04 million tons of sugar and 33.14 billion liters of ethanol. The Brazilian Midwest region is the second largest producer of sugarcane (136.9 million tons), second only to the Southeast region (400.3 million tons of sugarcane processed in the 2018/2019 harvest) (CONAB 2019).
In recent years, the state of Goiás has significantly increased its planted area with sugarcane (Franco 2014; Wissmann et al. 2014; Shikida, 2013). Among the factors that drive sugar and alcohol production in that state, climate stands out (Manzatto et al. 2009). The climate is predominantly Aw, according to the Köppen-Geiger classification, i.e., tropical with a dry season in winters and humid temperate with dry winters and hot summers (Cardoso et al. 2014). These are ideal climatic conditions for the planting of sugarcane. However, the problem in achieving a high productivity in the Cerrado concerns the occurrence of water deficit during the vegetative period together with the lack of varieties adapted to such conditions.
To face this climatic adversity different strategies of management and positioning of varieties have been used for each environment in addition to irrigation. Therefore, for an adequate estimate of productivity limitation caused by water deficit (Gouvêa et al. 2009) and an adequate water supplementation using irrigation, it is necessary to quantify the atmospheric water demand of sugarcane at different development stages (Andrade Junior et al. 2017a, b). However, the use of crop coefficient values (Kc) traditionally recommended for sugarcane, such as those provided for in the FAO Bulletin 56 (Allen et al. 1998), may differ by up to 40% from the Kc of regions of sugarcane cultivation during the crop development phase (Carvalho et al. 2012; Silva et al. 2013; Lozano et al. 2017). It is thus essential to determine the water demand of different varieties of sugarcane in each production environment.
In the Cerrado, among the varieties with the largest planted area five stand out: RB867515, CTC4, RB966928, RB82579, and IAC91-1099, in that order of importance (Braga Junior et al. 2017a, b). The morphological characteristics of the variety RB867515 are an erect growth and an easy peeling, medium tillering with uniform stems of medium diameter, arched and curved leaves of a medium width, and leaf edges with little aggressive serrates (RIDESA 2010). The variety IAC91-1099 has a good sucrose content, a medium ripening, an erect growth, excellent interlining, and excellent bud sprouting (Landell et al. 2007). The variety IAC87-3396 is characterized by its rusticity, the possibility of use in low-fertility environments, and a moderate resistance to Diatraea saccharalis (Fabricius 1794) (Landell et al. 1997).
The aim of this study is to determine the water demand of the varieties RB867515, IAC 91–1099, and IAC 87–3396 of sugarcane using weighing lysimetry in the environmental conditions of the Cerrado of Goiás, Brazil.
Materials and Methods
This study was carried out in the experimental area of the Federal University of Goiás (UFG) in the city of Goiânia, state of Goiás, Brazil. The altitude is 741 m, the latitude is 16°41′ S and the longitude is 49°16′ W. Two sugarcane cultivation cycles were evaluated, namely plant-cane (first year, 12-month cycle) and ratoon cane (second year, 12-month cycle). According to the Köppen classification, the region's climate is tropical Aw, hot and semi-humid, with a well-defined dry season (April to September).
The experiment was installed in 120 plastic pots of 200 L (0.85 m high and 0.55 m internal diameter) arranged in eight rows of 15 pots each, spaced 0.2 m apart, distanced 1.5 m between lines, with five meters of borders at the ends of the area (Fig. 1c).
Three electronic weighing lysimeters (capacity of 500 kg and accuracy of 0.050 kg) were installed in the experimental area (a). In the same pots, sensors (ECH2O EC-5, Decagon) were also installed (0.05 and 0.50 m depth) to measure the soil volumetric water content (b). The experiment was installed in 120 plastic pots of 200 L (0.85 m high and 0.55 m internal diameter) arranged in eight rows of 15 pots each, spaced 0.2 m apart, distanced 1.5 m between lines
The pots were placed on ceramic blocks (Fig. 1), leveled, and filled with a 0.65-m layer (Fig. 1b) of soil mixture (dystrophic Red Latosol) + peat (black soil with tanned manure) + coarse sand at a ratio of 7:2:1 (Table 1 shows the soil physical analysis); a permeable geotextile blanket and a drainable layer of 0.15 m of gravel no. 2, enriched with 250 g of limestone to raise the base saturation to 50% (Souza and Lobato, 2004), 12 g of P2O5, 23 g of N, and 30 g of K2O, according to the crop recommendations (Rossetto et al. 2008) (sources are simple superphosphate, urea, and potassium chloride). Still in the first year of experiment, 25 g N and 40 g of K2O were applied to pots as cover fertilization. In the second year, 30 g of P2O5, 40 g of N, and 20 g of K2O were applied to pots at the beginning of regrowth.
Sugarcane was planted on March 30, 2016, using three stems and nine vegetative buds per pot in open grooves at the center of pots, 0.15 m deep. Then, the stems were covered with soil and 24 L of water were poured to stimulate sprouting.
After planting, the soil was kept with a moisture close to field capacity for 30 days to stimulate germination and emergence of plants. After germination and emergence, a layer of 0.10 m of soil (soil + peat, 7:2) was added to pots, thus making up a 0.65 m soil layer. After 30 days, the plants were thinned, and four tillers were kept per pot throughout the experiment in both the first and the second year of evaluation.
A drip irrigation system was used with a single line of a 16-mm polyethylene pipe installed over each of the eight rows of pots. It had five 2-L self-compensating drip buttons (Naandanjain), totalizing 10 L h−1 per pot.
Three weighing lysimeters (Fig. 1a) were installed in the experimental area. They consisted of an electronic weighing platform scale (AL 7676–500, Alfa Instruments) with a maximum capacity of 500 kg and an accuracy of 0.050 kg. In the same pots, ECH2O EC-5 (Decagon) sensors were installed to measure the soil volumetric water content.
The weighing platforms (Fig. 1a) were connected to a CR1000 datalogger (Campbell Scientific). The datalogger was programmed to record every second and store averages every five minutes. The data were later entered into an Excel spreadsheet to convert the mass input and output values. The electrical power for the system was supplied by an ATX 400 W power supply with a 12 V constant current. 1,000 mV of excitation was used so that the signal reading range was around 7.5 mV. This setup provides a better performance and a greater sensitivity.
Lysimetric calibration began with the preparation and measurement of standard mass. It was organized in series of 0.25 kg (four units), 1.0 kg (four units), 5.0 kg (two units), 10 kg (five units), and 50 kg (five units), totaling a mass of 315 kg. Bomfim et al. (2004) carried out a procedure similar as this.
At the time of calibration, the datalogger was programmed to perform and store readings every second. Then, each standard mass was added to the weighing platform, starting with 0.25 kg mass increases until reaching a total mass of 60 kg. From then on, 1 kg was added until reaching 300 kg. Each volume of mass was added each 60 s. The data were subsequently tabulated, and the first 20 s were disregarded, thus allowing a balance of the weighing platform. This is a methodology adapted from Bomfim et al. (2004). The lysimetric calibration consisted of converting the load cell readings (mV) into concrete mass values and then correlating these values.
Subsequently, a linear function was fitted to the data resulting from the calibration process. The fitting equation and its respective determination coefficient (R2) were determined as Fig. 2 shows.
Mass variation data on weighing platforms (lysimeters) were computed from May 6, 2016, to March 30, 2018. The variation in soil water storage contained in the pots (ΔARM) was determined by the variation in mass on the weighing platforms at every five minutes. It is expressed by
where mf5 and mi5 are the total mass of the pot at the end and at the beginning of the five-minute periods, respectively.
To determine the water demand of sugarcane (L day−1), a daily accumulation of negative mass values (∑ − ARM24h, kg) was determined and converted into liters (L) of evapotranspirated water, considering a water density equal to 1000 kg m−3.
The irrigation time necessary to replace 100% of the sugarcane water requirement was determined by the average weekly consumption of lysimeters. Irrigation was carried out three times a week. The sugarcane varieties planted in lysimeters 1, 2, and 3 were IAC91-1099, IAC87-3396, and RB867515, respectively.
Two pairs of ECH2O EC-5 sensors (Fig. 1b) were installed in each pot. They were allocated at 0.05 and 0.50 m from the soil surface. The sensors were connected to a EM50 datalogger (Decagon Devices), and the readings were taken and stored every hour. The sensor calibration procedure was performed as Antunes Júnior et al. (2018) described.
The calculation of volumetric moisture using the sensors ECH2O EC-5 was carried out from May 14, 2016, to March 30, 2018. Thus, it was possible to monitor the soil volumetric moisture during the experimental period.
The software MS Excel®, version 2014, was used for tabulation, calculations, and plotting graphs.
Results
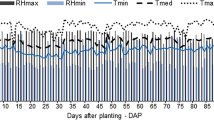
In the first cycle (plant-cane), an irrigation blade of 2,993 L of water was applied; in the second cycle (ratoon cane), 2,725 L of water was applied. Figure 2 shows the climatic variables throughout the experimental period (between 03/30/2016 and 03/30/2018). There was a total rainfall of 2,292 mm. However, due to the pot surface area, only 545 mm was used: 250 and 295 mm in the first and second evaluation cycles, respectively. The average daily air temperature and relative humidity were 23 °C and 70%, respectively. The average incident solar radiation was 15 MJ m−2 during the whole experimental period. There was no significant climatic difference between the two evaluation years (Fig. 3).
In both sugarcane cultivation cycles, the behavior of meteorological variables (Fig. 2) at sprouting (0–30 days) and plant establishment (31–110 days) provided a period of low radiation (12 MJ m−2), temperature (21 °C), and relative humidity (60%). The scarcity of rainfalls also caused this condition. At development (111–320 days), there was an increase in radiation (17 MJ m−2), temperature (26 °C), and relative humidity (80%), in addition to the beginning of the rainy season. Ripening (321–365 days) corresponded to the period during which radiation (16 MJ m−2), temperature (24 °C), and relative humidity (70%) began to decrease and the rainy season ended.
We monitored soil moisture (m3 m−3) using the ECH2O EC-5 sensor in two positions on the soil, as Fig. 4 shows. During the entire cultivation cycle, the soil volumetric moisture was between field capacity and crop critical moisture (70–80% of available water). Therefore, there was no water restriction for plants in lysimeters during both cycles.
Figure 5 shows the water demand (L day−1) for the three varieties of sugarcane evaluated in both cycles. The varieties behaved differently in relation to phenological stages and cultivation cycles. In the plant-cane cycle, the water demand was on average 9.2, 8.2, and 7.6 L day−1 for the varieties IAC 91–1099, RB867515, and IAC 87–3396, respectively. For the ratoon cane cycle, the water demand averaged 8.4, 7.9, and 6.6 L day−1 for the varieties IAC 91–1099, RB867515, and IAC 87–3396, respectively. Thus, from the first to the second year, there was a decrease in water demand of all varieties analyzed.
In the plant-cane cycle, all varieties presented a water demand during sprouting of approximately 2.9 L.day−1. This probably happened because the leaf area was low and the evaporation of the soil corresponded to most of the water demand. At budding (0–30 days), there was a difference of 27.5% and 12.5% in water demand between the variety IAC 911,099 and RB867515 and IAC 87–3396, respectively. At establishment (31–110 days), there was a difference of 37.2% in water demand between the variety IAC 91–1099 and the others. During development (111–320 days), water demand had the greatest variation among different varieties. During this period, the IAC 87–3396 presented the lowest demand (average of 7.71 L day−1), followed by RB867515 and IAC 91–1099, with a demand surpassing that of the former by 10.7 and 31.7%, respectively. Finally, during ripening (321–365 days), the variety RB867515 presented the highest water demand. It was 27.1% higher than the others.
In the second cycle (ratoon cane), at sprouting, the average daily consumption of the three varieties was 2.6 L. During budding (0–30 days), there was a difference of 20% and 30% in water demand between the variety IAC 911,099 and RB867515 and IAC 87–3396, respectively. During establishment, there was an exponential increase in water demand by the variety IAC 91–1099. It was 60.7 and 91.4% higher than that of the varieties RB867515 and IAC 87–3396, respectively. During development, water demand showed the smallest relative difference, varying on average 9.1% between different varieties. Finally, during ripening, the variety RB867515 presented the highest demand, i.e., 6.0 and 30.7% higher than that of the varieties IAC 91–1099 and IAC 87–3396, respectively.
Discussion
Tillering was the main factor responsible for the variation in water demand in the first 110 days of evaluation in both growing cycles. The variety IAC 91–1099 had a number of tillers 53 and 91% higher than that of the varieties RB867515 and IAC 87- 3396, respectively (Antunes Júnior 2020). Libardi et al. (2019) used weighing lysimetry and reported that in a greenhouse, sugarcane seedlings had an initial evapotranspiration of 3.0 L day−1 (zero to seven days after transplanting), increasing to 6.9 L day−1 in just 30 days. The authors attributed these values to seedling density and the specific evaporation area of the trays. These data corroborate the results found here. The variety that had the largest number of tillers had a greater water demand.
The evaluated varieties have different morphological characteristics (Table 2). They may have directly influenced water demand in the climatic conditions of the Cerrado of Goiás during vegetative development. The variety IAC 91–1099 presented a higher consumption of water because it has a high tillering capacity and a larger leaf area (3.4 m2) than those of the varieties RB867515 (3.3 m2) and IAC 87–3396 (3.1 m2), which in turn presented a greater rusticity because of the smaller leaf area, erect growth, and easy leaf removal.
The leaf area is responsible for intercepting solar radiation and transforming it into energy (ATP). A full water availability favors the development of sugarcane, especially during development, promoting the best use of solar radiation and the performance of photosynthesis (Inman-Bamber and McGlinchey 2003). Therefore, the larger the leaf area, the greater the increase in respiration and, consequently, the greater the loss of water by sugarcane.
In both cultivation cycles, during phenological ripening, the varieties RB867515 and IAC 91–1099 showed a tendency to increase water demand. This is associated with the rainy season, as well as with the water demand of the atmosphere during that period, which was concomitant with this vegetative subperiod. Thus, even interrupting irrigation to induce ripening, the rainfalls in March, in both years, conditioned the resumption of development of these varieties. This confirms the reports of Inman-Bamber and Smith (2005), who noted that when sugarcane water needs are met, its leaves grow faster. In just seven days it has as many green leaves as a plant that has not suffered water stress.
For all the varieties analyzed, there was a reduction in water consumption between the two cultivation cycles. There was a reduction in all biometric variables (stem diameter, stem height, number of green leaves, and leaf area) from the first to the second cycle (Fig. 3). This suggests a decrease in the volume of water to be applied over the years. Therefore, water management by irrigation must be carried out differently between crop cycles to make irrigation management more efficient.
Therefore, we suggest caution in the use of crop coefficients (Kc) traditionally recommended for sugarcane. Further research is needed to determine the crop coefficients (Kc) for different varieties of sugarcane at its various vegetative stages and cultivation cycles in the Cerrado's environmental conditions.
Conclusions
There was a decrease in water consumption of all sugarcane varieties from the first to the second growing cycle. The variety RB867515 had the smallest decrease (3.7%) between cycles, followed by IAC 91–1099 (8.7%) and IAC 87–3396 (13.2%). The differences in average water demands between varieties reached 17.4 and 21.4% in the first and second cycles, respectively, compared to the varieties with a greater (IAC 91–1099) and lower (IAC 87–3396) water demand. This is due to differences in the morphological characteristics of these varieties. There is a need to consider the cultivated variety and the cycle.
References
Allen, Richard G., Luis S. Pereira, Raes Dirk, and Smith Martin. 1998. Crop Evapotranspiration: Guidelines for computing crop water requirements. Roma. FAO Irrigation and Drainage Paper 56: 300.
Antunes Júnior, E. J. Necessidade hídrica e irrigação suplementar em cana-de-açúcar no cerrado goiano. 2020. Tese (Doutorado em Agronomia) - Universidade Federal de Goiás. 130.
Bomfim, Guilherme V., Benito M. Azevedo, Thales V. A. Viana, Ronaldo L. M. Borges, and John J. G. Oliveira. 2004. Calibração de um lisímetro de pesagem após dois anos de utilização. Revista Ciência Agronômica 35: 284–290.
Cardoso, Murilo R. D., Francisco F. N. Marcuzzo, and Juliana R. Barros. 2014. Classificação climática de Köppen-Geiger para o estado de Goiás e o Distrito Federal. ACTA Geográfica 8 (16): 40–55. https://doi.org/10.5654/actageo2014.0004.0016.
Carvalho, Daniel F. D., Marcio E. D. Lima, Alexsandra D. D. Oliveira, Hermes S. D. Rocha, and José G. M. Guerra. 2012. Crop coefficient and water consumption of eggplant in no-tillage system and conventional soil preparation. Engenharia Agrícola 32 (4): 784–793. https://doi.org/10.1590/S0100-69162012000400018.
CONAB - Companhia Nacional de Abastecimento. 2019. Acompanhamento da safra brasileira de cana-de-açúcar - safra 2018/19, Fourth survery, Brasília: Conab, 75.
Franco, Iria O. 2014. Expansão da cana-de-açúcar na microrregião sudoeste de Goiás: análise espacial das mudanças do uso e cobertura do solo nos anos de 2001, 2006 e 2011. Boletim Goiano de Geografia 34(3): 481–499. https://www.redalyc.org/pdf/3371/337137823006.pdf
Gouvêa, Julia R. F., Paulo C. Sentelhas, Samuel T. Gazzola, and Marcelo C. Santos. 2009. Climate changes and technological advances: Impacts on sugarcane productivity in tropical Southern Brazil. Scientia Agricola 66 (5): 593–605. https://doi.org/10.1590/S0103-90162009000500003.
Inman-Bamber, Geoff N., and M.G. Mcglinchey. 2003. Crop coefficients and water-use estimates for sugarcane based on long-term Bowen ratio energy balance measurements. Field Crops Research 83: 125–138. https://doi.org/10.1016/S0378-4290(03)00069-8.
Inman-Bamber, Geoff N., and D.M. Smith. 2005. Water relations in sugarcane and response to water deficits. Field Crops Research 92: 185–202. https://doi.org/10.1016/j.fcr.2005.01.023.
Andrade Junior, S. Aderson, Donavan H. Noleto, Edson A. Bastos, Magna S. B. Moura, and João. C.. R. Anjos. 2017a. Demanda hídrica da cana-de-açúcar, por balanço de energia, na microrregião de Teresina Piauí. Agrometeoros 25 (1): 217–226.
Antunes Júnior, Elson J., Alves Junior, José, and Casaroli Derblai. 2018. Calibração do sensor capacitivo EC-5 em um Latossolo em função da densidade do solo. Revista Engenharia na Agricultura 26: 80–88. https://doi.org/10.13083/reveng.v26i1.864
Braga Junior, Rubens L. C., Landell, Marcos G. A, Silva, Daniel N., Bidóia, Marcio A. P., Silva, Thiago N., Thomazinho Junior, J.R., and Silva, Victor H. P. 2017. Censo varietal IAC de cana-de-açúcar na região Centro-Sul do Brasil – Safra 2016/17, Campinas: Instituto Agronômico, 40.
Landell, Marcos G. A., Campana, Mario P., Figueiredo, Pery, Zimback Leo, Silva, Marcelo A., and Prado Hélio. 1997. Novas Variedades de cana-de-açúcar, Campinas: Instituto Agronômico, 28.
Landell, Marcos G. A., Campana, Mario P., Figueiredo Pery, Xavier, Mauro A., Vasconcelos, Antonio C. M., Bidoia, Marcio A., Silva, Daniel N., Anjos, Ivan A., Prado Hélio, Pinto, Luciana R., Souza, Silvana A. C. D., Scarpari, Maximiliano S., Rosa Júnior, Vicente E., Miranda, Leila L. D., Azania, Carlos A. M., Perecin Dilermando, Rossetto Raffaela, Silva, Marcelo A., Martins, Antonio L. M., Gallo Paola, Kanthack, Ricardo A. D., Cavichioli, J.C., Veiga Filho, Alceu A., Mendonça, Jeremias R., Dias, Fabio L. F., and Garcia, Juliana C. 2007. Variedades de cana-de-açúcar para o Centro-Sul do Brasil: 16ª liberação do programa cana IAC (1959–2007), Campinas: IAC, 37.
Libardi, Luis G. P., Rogério T. Faria, Alexandre B. Dalri, Glauco S. Rolim, Luis F. Palaretti, Aderson P. Coelho, and Izabela P. Martins. 2019. Evapotranspiration and crop coefficient (Kc) of pre-sprouted sugarcane plantlets for greenhouse irrigation management. Agricultural Water Management 212: 306–316. https://doi.org/10.1016/j.agwat.2018.09.003.
Lozano, Claudia S., Rezende Roberto, Paulo S. Freitas, Tiago L. Hachmann, Fernando A. Santos, and André F. Andrean. 2017. Estimation of evapotranspiration and crop coefficient of melon cultivated in protected environment. Revista Brasileira De Engenharia Agrícola e Ambiental 21 (11): 758–762. https://doi.org/10.1590/1807-1929/agriambi.v21n11p758-762.
Manzatto, Celso V., Eduardo D. Assad, Jesus F. M. Bacca, Maria J. Zaroni, and Sandro E. M. Pereira. 2009. Zoneamento agroecológico da cana-de-açúcar: expandir a produção, preservar a vida, garantir o futuro, 55. Rio de Janeiro: Embrapa Solos.
Rossetto Raffaela, Dias, Fabio L. F., Vitti, André C., and Prado Junior, José Q. 2008. Fósforo. In Cana-de-açúcar, ed. Miranda, Leila L. D., Vasconcelos Antonio M., and Landell Marcos G.A., Campinas, 271–288.
RIDESA. Rede Interuniversitária para o Desenvolvimento do Setor Sucroalcooleiro. 2010. Catálogo nacional de variedades “RB” de cana-de-açúcar. RIDESA: Curitiba, 136.
Shikida, Pery F. A. 2013. Expansão canavieira no Centro-Oeste: limites e potencialidades. Revista De Política Agrícola 2: 122–137.
Silva, Vicente P. R., Bernardo B. Silva, Walker G. Albuquerque, Cicera J. R. Borges, Inaja F. Sousa, and Dantas Neto José. 2013. Crop coefficient, water requirements, yield and water use efficiency of sugarcane growth in Brazil. Agricultural Water Managment 128: 102–109. https://doi.org/10.1016/j.agwat.2013.06.007.
Souza, Dijalma M. G. and Lobato Edson. 2004. Correção da acidez do solo. In Cerrado: correção do solo e adubação, ed. SOUZA, Dijalma. M. G. and LOBATO Edson, Second edition, Brasília: EMBRAPA, 81–96.
Wissmann, Martin A., Graciela C. Oyamada, Claudia C. Wesendonck, and Pery F. A. Shikida. 2014. Evolução do cultivo da cana-de-açúcar na região Centro-Oeste do Brasil. Revista Brasileira De Desenvolvimento Regional 2 (1): 95–117. https://doi.org/10.7867/2317-5443.2014v2n1p095-117.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Ethical Approval
This work does not study humans or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Antunes Júnior, E.D.J., Alves Júnior, J., Evangelista, A.W.P. et al. Water Demand of Sugarcane Varieties Obtained by Lysimetry. Sugar Tech 23, 1010–1017 (2021). https://doi.org/10.1007/s12355-021-01002-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-021-01002-5