Abstract
Stevia rebaudiana extract is globally approved as a low-calorie sweetener. Increasing glycosides content using biotechnological methods is particularly important. In this study, stevia plant was treated with silver nanoparticles at various concentrations (0, 10, 20, and 40 mM). Subsequently, the transcription of key gene levels in the biosynthesis of rebaudioside and stevioside glycosides, including UGT85C2 (UDP-glycosyltransferases), KAH (Kaurenoic acid-13 hydroxylase), UGT74G1, and UGT76G1 was measured using real time PCR assays. The HPLC was used to evaluate the glycosides content. UGT85C2 demonstrated the highest transcriptional level changes in plants treated with silver nanoparticles. The plants treated with silver nanoparticles at concentration of 10 and 20 mM showed a lower gene expression than the control plant, but plant treated with silver nanoparticles at 40 mM concentration showed significantly higher gene expression than the control samples. The results suggested that the treatment with AgNPs at 40 mM leads to similar positive effects on the transcription of all genes. HPLC results also revealed that the plants treated with 40 mM nanoparticles contains a higher glycosides content comparing to the control sample. Thus, the present experiment suggests that silver nanoparticles can act as a strong amplifier of the transcriptional trigger for steviol glycoside biosynthesis pathway genes which have the potential to control the production of steviol glycosides positively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a growing interest in pharmaceutical industries to improve the quality and quantity of active ingredients in medicinal plants (Jasim et al. 2017). One of the techniques to increase the secondary metabolites is based on the biological and non-biological stimuli that can manipulate the metabolism of secondary metabolites and increase production. It should be noted that such stimulants can affect the plant in natural conditions, which leads to the production of a particular metabolite (Mohanpuria et al. 2008).
Naturally, not all elicitors follow a single path, and several factors such as their concentration, properties, size and plant growth stage, physiochemical conditions and callus could have their effects (Angelova et al. 2006).
The first and the most important step to increase metabolic engineering is to identify the pathway, how it is tuned, and the factors that can affect the speed of production of valuable metabolites. One of these methods is the use of such elicitors for enzyme stimulation (Mulabagal and Tsay 2004). Stress improves secondary metabolites production in medicinal plants. It is reported that elicitors have a positive effect on the production of secondary metabolites (Pitta-Alvarez et al. 2000). Recently, it has been found that the exploitation of nanomaterials is effective in the biosynthesis of commercially and economically secondary metabolites in medicinal plants (Gomes et al. 2012).
The interest in nanotechnology has been growing recently due to its tremendous effects in various areas such as pharmaceutical, energy, electronics, and industrial applications. Researchers are interested in using biotechnological techniques to enhance the production of secondary metabolites. These secondary metabolites are used as renewable sources of drugs due to legal restrictions on the use of natural resources for pharmaceutical purposes. The use of plant breeding under the induction of nanoparticles (NPs) can be a useful tool in creating difference between various plants (Gomes et al. 2012; Jain and Spencer 2006). Nanoparticles have been reported as an elicitor in recent years. Due to their small size, nanoparticles penetrate rapidly into plant cells and increase protein levels and stimulate gene expression (Masarovicova and Kralova 2013).
It is reported that the content of artemisinin (Zhang et al. 2013) and diosgenin (Jasim et al. 2017) of the Ag–SiO2 core–shell NP-treated plants has increased. AgNP treatment of C. officinalis L. appears to be a cost-effective and simple method of enhance its medicinal properties (Gupta et al. 2013).
Stevia rebaudiana belongs to Asteraceae family that grows in places with long latitudes and longer days. Stevia is regarded as a dietary sugar and a natural sweetener that can substitute for all types of sweets, candies, and drinks. Stevia extract has antiviral and antioxidant properties (Lemus-Mondaca et al. 2012). Stevia leaves contain a mixture of sweet diterpene glycosides, such as stevioside, isosteviol, dulcoside A, rebaudioside, and steviolbioside (Goyal et al. 2010). Additionally, stevia leaves also contain phytoconstituents such as vitamins, fatty acids, flavonoids, phenolic acids, and proteins (Gupta et al. 2013). These leaves are also known for their high sweet steviol glycosides content (about 4–20% of dry weight) depending on growth and the cultivar conditions (Geuns 2003).
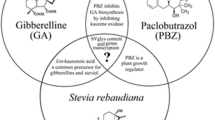
Steviol glycosides (SGs) are low-calorie and natural sweeteners. SG concentrations are 10,000 times higher compared to gibberellic acid, indicating that plants are using major energies to produce SGs. Rebaudioside A and stevioside are the major SGs. Other SGs present in smaller amounts are steviolbioside (SB), dulcoside A (Dul A), and Reb B, C, D, E, F. The main difference between rebaudioside A and stevioside is in their abundance and sweetening power. The stevioside sweetener is 143 times greater than natural sucrose. On the other hand, rebaudioside A is almost 320 times sweeter but less abundant (Richman et al. 1999). SGs are derived from the MEP (2-C-Methyl-d-erythritol-4-phosphate) pathway. This pathway is located within plastids and primarily in leaves (Brandle and Telmer 2007). Some genes involved in steviol glycosylation are unknown, but several important genes have been characterized biochemically (Ceunen and Geuns 2013). Geranylger-anyl-diphosphate (GGDP) is a substrate for the synthesis of SGs which is converted into steviol by the continuous action of four enzymes: copalyldiphosphate synthase (CPPS), kaurene synthase (KS), kaurene oxidase (KO), and kaurenoic acid hydroxylase (KAH) (Brandle and Telmer 2007). Various steviol glycosides are formed by steviol glycosylation using specific glucosyltransferases (GTs). UGT85C2 (UDP-glycosyltransferases), KAH (Kaurenoic acid-13 hydroxylase), UGT74G1, and UGT76G1 are the main enzymes in the biosynthesis pathway of steviol glucosylation (Shibata et al. 1999). The formation of SGs varies significantly with the treatment of growth regulators (Modi et al. 2011; Hajihashemi et al. 2013). Similarly, patterns of biosynthesis genes transcription and the content of diterpene glycoside accumulation depend on the plant’s growth stage which was reported in tomato (Scolnik and Giuliano 1994), Arabidopsis (Che et al. 2006), and cotton (Ghazi et al. 2009).
Considering the significance of the stevia in the pharmaceutical industry, evidently, using the silver nanoparticles as an inducer can alter the expression of the key genes in the production pathway of glycosides and enhance their levels. For this purpose, the effect of various concentrations of AgNP on the changes of gene expression on production of rebaudioside and stevioside glycosides was investigated.
Material and Methodology
Synthesis of AgNP
The silver nanoparticles were synthesized chemically by reducing the salt of silver nitrate with trisodium citrate. All solutions were prepared using distilled water. Silver nitrate (50 ml of 0.001 M) was heated followed by adding 1% trisodium citrate. The solution was stirred continuously until the solution turned dark yellow, signaling the formation of AgNPs. The solution was then left at room temperature. The SEM microscopic images and UV–Vis spectroscopy were used to study the formation of AgNP and their specification.
UV–Vis spectroscopy is one of the most common methods to determine the structural properties of NPs based on AgNP surface plasmon resonance. The presence of AgNPs in the solution is evident from the absorption of light at the 450-nm region.
The SEM was used to analyze the morphology (size and shape) of the particles. Aqueous AgNP sample was loaded on a carbon-coated copper grid, and the solvent evaporated at room temperature for 1 h. The SEM images were recorded using Zeiss—EM10C instrument on grids of carbon-coated copper and 80 kV accelerating voltage with various magnifications.
Treatment of the Plant
The experiment conducted using a completely randomized design with three replications in a research greenhouse belonging to the Sana Institute of Higher Education. S. rebaudiana seedlings were planted and their successful establishment was assured. After 4–6 leaves emerged, the plants were treated with AgNP [in various concentrations of (0, 10, 20, 40 mM)] using spray solution (2 times in 3 weeks). Pods were transported to a greenhouse and were exposed to 16 h of light (8000–10,000 Lux) and 8 h of darkness, with an average temperature of 25 ± 5 °C and a humidity of 60%. The necessary nutrition was provided using Hoagland nutrition solution. All the experiments were done in triplicate (biological replication), and the samples were collected in aluminum foils and stored for molecular assessments at − 80 °C.
Extraction of RNA and Synthesis of cDNA
Fresh 100 mg of the leaf was inserted in the liquid nitrogen and the trizol protocol was used to extract the RNA (Invitrogen, Carlsbad, CA, USA), Then the fresh leaf was exposed to chloroform, trizol, 75% ethanol, and isopropyl alcohol in RNase-free water. Around 2 µg of total RNA treated with DNaseI and was used for the first-strand synthesis of cDNA utilizing 10 mM dNTPs, oligo (dT) primers, and reverse transcriptase according to the instructions by the manufacturer (Thermo Scientific, Germany).
Real-Time Analysis PCR
cDNA aliquots were used as a template for real-time PCR analysis, and primers were extracted using Primer3 online software rechecked with OLIGO5 analyzer software (Table 1). PCR carried out using the one-step SYBR® Green RT-qPCR with Hot Start Taq DNA polymerase (Thermo Scientific) in a Bio-Rad real-time PCR machine (CFX96™ Touch real-time PCR detection system) according to the recommendations of the manufacturer. The following procedure for reactions was applied: 3 min at 95 °C, denaturation at 95 °C for 25 s, annealing at 60 °C for 20 s, and extension at 72 °C for 25 s for 40 cycles. The housekeeping gene was used (Actin) as a reference for normalization, and comparative gene expression method (2−ΔΔcT) for data analysis and determination of the relative amount of gene expression by amplifying the target genes and actin as the reference gene.
Extracting Rebaudioside A and Stevioside and HPLC Analysis
One hundred milligrams of dry leaves was stirred in 10 ml of pure methanol for 25 min. The methanol was evaporated at 48 °C followed by adding n-hexane (25 ml) for neutralization. After the solvent evaporated, 5 ml of the water/acetonitrile solution was added and then filtered for HPLC analysis using an Aqua C-18–125A (150 × 4.0 mm, 5 microns) from Phenomenex (Torrance, CA, USA). Ten microliters of the extract was injected into a chromatography column with 15-cm-long Cosmosil NH2-MS specimen with 4.6 mm diameter and a diameter of 5 µm attached to the Unicam Crystal 200 model HPLC device. The 8:2 ratio of acetonitrile/water mobile phase was passed through the column at a rate of 1 ml/min. A diode array detector at a wavelength of 210 nm was used. The pump pressure was at 800 psi and compared the amount of each substance to standard courier based on the surface area under their curve and output courier’s inhibition time (Rahi et al. 2010).
Statistical Analysis
This experiment was performed in a completely randomized design (CRD) with three biological replicates for each treatment. The variance between various means and its impact was analyzed using one-way ANOVA and least significant difference (LSD) methods in SPSS software (version 23, SPSS, Chicago, IL). A comparison of means was executed using SAS software (SAS Institute, Cary, NC) and was also used to draw MS Office graphs.
Results
SEM analysis and UV–Vis absorbance at 400 nm demonstrated that the produced AgNPs were spherical with an average of 25 nm (Fig. 1).
Gene Expression
In this study, the analysis of the key genes expression in the biosynthesis pathway of the stevioside and rebaudioside (UGT85C2, KAH, UGT74G1, and UGT76G1) revealed that the glycosides were significantly different from control plant (water-treated leaves) in stevia leaves. Thus, their expression changed significantly after the treatment with AgNPs of various concentrations (0, 20, 20, and 40 mM). The key genes (UGT85C2, KAH, UGT74G1, and UGT76G1) responded differently to various concentrations of AgNPs.
The difference in KAH transcription levels was obvious among the various concentrations. It was observed that plants treated with 40 mM AgNP concentration showed the highest accumulation of KAH transcripts (2.8-fold) compared with the control plant. Plants treated with lower concentrations (10 and 20 mM) showed a low level of KAH expression compared with the control plant, and the lowest gene expression was recorded in plants treated with 10 mM AgNPs (Fig. 2).
UGT85C2 transcript analysis showed an increase in mRNA levels in response to AgNP treatment plant (Fig. 3). AgNP-treated plants at 40 mM showed a peak of transcription for UGT85C2 compared to the control plant (9.9-fold). Lower concentration of AgNPs (10 and 20 mM) showed lower expression levels, and the 20 mM concentration of AgNP indicated the slightest changes in UGT85C2 gene expression.
Among the concentrations of AgNPs, the leaves treated with 40 mM concentration AgNPs showed the highest UGT76G1 gene expression level compared with the control plant (0.9 fold). The lowest gene expression was recorded in plants treated with AgNPs at 10 mM concentration (Fig. 4).
Between various AgNP concentrations in treated plants, 40 mM concentration of AgNPs indicated the highest UGT74G1 gene expression level compared with the control plant (1.6-fold). Lower concentration of AgNP treatment (10 and 20 mM) exhibited a lesser expression level, and thus, no major changes were recorded between 10 and 20 mM AgNP concentrations in treated plants (Fig. 5).
HPLC Analysis of Rebaudioside A and Stevioside Content
Based on valid standards, HPLC analysis of S. rebaudiana extract revealed the presence of two major peaks: rebaudioside A and stevioside. Other minor peaks were assigned as: Dulbecauside A and rebaudioside B, C, and F.
Rebaudioside A and stevioside retention times were 5.05 ± 0.8 min and 6 ± 0.2 min, respectively.
Overall, the present study revealed that the treatment of plants with AgNP at various concentrations can lead to alteration of glycosides content in stevia plants (rebaudioside A and stevioside content) at 40 mM AgNP concentration compared with the control plant. As demonstrated in the results, the differences between treatments and the control sample are significant (P ≤ 0.05). As shown in Fig. 6, the result was recorded with 0.6–47 mg/g dry w under various concentrations of biosynthesized AgNP treatments.
Effect of AgNP applications in (in different concentrations AgNP0, AgNP10, AgNP20, and AgNP40 mM) on main glycosides (mg/g dry.w) level in leaves tissues of S. rebaudiana plants. The mean values were obtained from three independent experiments. *Significantly (P ≤ 0.05) different according to LSD least significant difference. In each column, means that have at least one letter in common did not show any significance difference
Discussion
Elicitors have a positive effect on the production of secondary metabolites (Pitta-Alvarez et al. 2000; Ramezani et al. 2017a, b; Majlesi et al. 2018; Ramezani et al. 2018a, b). In this study, S. rebaudiana seedling was grown and treated with AgNP as elicitors. The statistical analysis revealed a significant increase in glycosides, especially rebaudiosid A and stevioside. According to the analysis of gene expression, the transcription level of key genes (UGT85C2, KAH, UGT74G1, and UGT76G1) in the rebaudioside A and sativoside biosynthesis pathways increases in plants treated with AgNPs. Therefore, the highest expression was observed in stevia treated with 40 mM AgNP.
The maximum of glycosides content was recorded in S. rebaudiana plant treated with AgNP at 40 mM concentration.
Overexpression of several glycosyltransferase genes caused a significant increase in their respective glycosides (Jackson et al. 2001). Until now, many types of researche have demonestrated the effect of NPs on plant’s secondary metabolism. For instance, after TiO2NPs treatment, the concentration of phenolic compounds in the extracellular medium increased by 27%, and 22% respectively, in Haematococcus pluvialis and Arthrospira platensis (Comotto et al. 2014). This increase is contributed to the lipid peroxidation, oxidative stress (H2O2 production), and CAT activity. Diosgenin concentration increased when Trigonella foenum-graecum L. was exposed to AgNPs (Vecerova et al. 2016). Ferulic acid and isovitexin were barley enhanced after treatment with AgNPs (Jasim et al. 2017). AgNPs can upregulate key genes of flavonoid and anthocyanin biosynthesis pathway in A. thaliana (Garcia-Sanchez et al. 2015).
The above-mentioned reports affirm the NP-mediated modulation of secondary metabolism of the plant. Other studies confirm that the nanoparticles can modulate natural products through interfering with various signaling pathways, however, the exact mechanism of this regulation is not fully understood. According to some studies, it is suggested that the plant’s early responses to NP treatment are increased in the upregulation of mitogen-activated protein kinase (MAPK) cascades, ROS levels, and cytoplasmic Ca2+ efflux due to the following finding. AgNPs were recognized using plasma membrane-bound receptors, and afterward, a ROS induction and Ca2+ burst in A. thaliana were stimulated (Sosan et al. 2016; Kohan-Baghkheirati and Geisler-Lee 2015). Ca2+ levels and some of the proteins linked with signaling pathways were upregulated in AgNPs treated with O. sativa roots (Mirzajani et al. 2014). It is believed that AgNPs, or released ions, blocked the metabolism of the cell by binding to Ca2+/Na+ ATPases activity, Ca2+ channels, and Ca2+ receptors. NPs imitate Ca2+ In the cytosol in calcium-binding proteins of NP-specific proteins (Kha et al. 2017). MAPK phosphorylation, and subsequently downregulating transcription factors activation, changed the secondary metabolism transcription in plants (Schluttenhofer and Yuan 2015; Phukan et al. 2016; Eom and Choi 2010; Lim et al. 2012). ROS production and solubility of nanoparticles affect their activity (Navarro et al. 2008).
Based on the results of this work, the treated stevia with AgNPs causes the membrane lipid peroxidation that results in an increase in the ROS formation. The increase in rebaudioside A and stevioside also improves the anticancer and cytotoxic activity of this extract, rendering it a potential therapeutic material for treating cancer and other diseases.
Based on the results, AgNP-treated plants have a concentration-dependent behavior. It was observed that in a higher concentration of AgNP (40 mM), more key genes expression involved in the biosynthesis pathways of main glycosides and the main content of glycoside.
Thus, nanoparticles can easily enter plant cells which results in the secondary metabolites production (Kim et al. 2007). El-Temsah and Joner (2012) reported that various concentrations and sizes of AgNPs can have different effects on seed germination.
Moteriya et al. research (2014) revealed an increase in reduction power by increasing AgNP concentrations. Also, Kanipandian et al. (2014) observed similar results in green synthesized AgNP of Cleistanthus collinus extract.
Nanoparticles can enter plant cells through the apoplast and cross the plasma membrane through endocytosis; they can move further from one part to another via symplastic flow (Rico et al. 2011). Also, the transport of nanoparticles into subcellular organelles (e.g., the vacuoles, nucleus, and plastids) is proven. After entering the nucleus, nanoparticles enhance gene transcription levels (Chichiriccó and Poma 2015; Da Costa and Sharma 2016). The biosynthesis pathway of steviol glycosides is currently an interesting field of study. This can be explained at the genetic level, the scientific knowledge about steviol glycosides biosynthesis pathway is limited (Richman et al. 1999). Furthermore, this pathway has four common intermediate steps with Gibberellic acid (GA) biosynthesis pathway (Brandle and Telmer 2007) which is a critical role in the development and growth of the plant.
Based on the results, treating stevia with AgNPs can be a good strategy for enhancing its medicinal properties.
AgNP can act as elicitors and effectively increase the transcription of key genes involved in stevia’s main pathway of glycosides biosynthesis and subsequently increase rebaudioside A and stevioside.
The transcription patterns of stevioside biosynthetic genes varied with different treatments. Result of a study shows that the transcription of KS1-1, KAH, and UGT76G1 have no significant changes under PEG, GA, and PBZ treatments (Hajihashemi et al. 2013). Kumar et al. (2012) reported that there is no upregulation of KAH gene in S. rebaudiana after treatment of plant with GA. There is a positive correlation between KAH transcription and stevioside accumulation (Kumar et al. 2012), which is reinforced by the results of this experiment and other previous researches on stevioside content (Hajihashemi et al. 2012). It can be proposed that change in KAH transcription affects stevioside content by alterations in their precursors.
It was observed that both the treatments and stress can affect the genes expression involved in the same biosynthesis pathway and that leads to the more complicated mechanism (Hajihashemi et al. 2012; Kumar et al. 2012).
It was established that transcription patterns of different members of the same gene family act differently in different treated plants, and the same was reported by Song et al. (2011) in maize treatment.
Our study reveals that expression of genes, in particular, KAH gene, increased at 40 mM AgNP treatment, which resulted in the increase in stevioside and rebaudioside content, which was supported by HPLC measurement.
Hajihashemi et al. (2013) have reported that stevioside and Rebaudiosid A content decreased in PBZ and PEG treatments, but UGT74G1 transcription was not significantly changed after treatments.
It can be concluded that change in content of stevioside and rebaudiosid A is due to its precursor and UGT76G1 transcription after treatment with PBZ and PEG. Glycosyltransferase genes overexpression led to a significant increase in their glycoside content (Jackson et al. 2001).
In our study, after treatment of AgNP, all studied gene expressions were increased especially KAH, and these results showed that every treatment has different actions in plants.
We have observed that an increase in stevioside and rebaudioside by AgNP treatments resulted in the same positive effect on genes transcription and glycoside accumulation. This investigation aimed to reveal a possible correlation between the transcription of some genes involved in glycosides biosynthesis and glycosides contents in S. rebaudiana treated with AgNP.
There are major challenges for the production of secondary metabolites, and using AgNP as an elicitor proves to be a promising method for producing rebaudioside A and stevioside by triggering transcription level of main genes in the biological synthesis pathway of its natural form.
References
Angelova, Z., S. Georgiev, and W. Roos. 2006. Elicitation of plant. Biotechnology & Biotechnological Equipment 20: 72–83.
Brandle, J.E., A. Richman, A.K. Swanson, and B.P. Chapman. 2002. Leaf ESTs from Stevia rebaudiana: A resource for gene discovery in diterpene synthesis. Plant Molecular Biology 50: 613–622.
Brandle, J.E., and P.G. Telmer. 2007. Steviol glycoside biosynthesis. Phytochemistry 68: 1855–1863.
Ceunen, S., and J.M.C. Geuns. 2013. Steviol glycosides: Chemical diversity, metabolism, and function. Journal of Natural Product 76: 1201–1228.
Che, P., S. Lall, D. Nettleton, and S.H. Howell. 2006. Gene transcript accumulation programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiology 141: 620–637.
Chichiriccó, G., and A. Poma. 2015. Penetration and toxicity of nanomaterials in higher plants. Nanomaterials 5: 851–873.
Comotto, M., A.A. Casazza, B. Aliakbarian, V. Caratto, M. Ferretti, and P. Perego. 2014. Influence of TiO2 nanoparticles on growth and phenolic compounds production in photosynthetic microorganisms. Scientific World Journal 9: 324–333.
Da Costa, M.V.J., and P.K. Sharma. 2016. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54: 110–119.
El-Temsah, Y.S., and E.J. Joner. 2012. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environmental Toxicology 27: 42–49.
Eom, H.J., and J. Choi. 2010. P38 MAPK activation, DNA damage, cell cycle arrest and apoptosis as mechanisms of toxicity of silver nanoparticles in Jurkat T cells. Environmental Science & Technology 44: 8337–8342.
Garcia-Sanchez, S., I. Bernales, and S. Cristobal. 2015. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics 16: 341–349.
Geuns, J.M.C. 2003. Molecules of interest – stevioside. Phytochemistry 64: 913–921.
Ghanati, F., and S. Bakhtiarian. 2014. Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L. (Asteraceae). Tropical Journal of Pharmaceutical Research 13(11): 1783–1789.
Ghazi, Y.A., S. Bouro, T. Arioli, E.S. Dennis, and J. Danny. 2009. Transcript profiling during fiber development identifies pathways in secondary metabolism and cell wall structure that may contribute to cotton fiber quality. Plant and Cell Physiology 50: 1364–1381.
Gomes, S.I.L., S.C. Novais, and C. Gravato. 2012. Effect of Cu-nanoparticles versus one Cu-salt: Analysis of stress biomarkers response in Enchytraeus albidus (Oligochaeta). Nanotoxicology 6: 134–143.
Goyal, S.K., G.R.K. Samsher, and R.K. Goyal. 2010. Stevia (Stevia rebaudiana) a bio-sweetener: A review. International Journal of Food Science and Nutrition 61: 1–10.
Gupta, E., S. Purwar, S. Sandaram, and G.K. Gai. 2013. Nutritional and therapeutic values of Stevia rebaudiana: A review. Journal of Medicinal Plants Research 7: 3343–3353.
Hajihashemi, S., J.M.C. Geuns, and A. Ehsanpour. 2012. Physiological analysis of Stevia rebaudiana after treatment with polyethylene glycol, paclobutrazol and gibberellic acid. In Proceedings of the 6th Eustas Stevia Symposium, Stevia: Six months beyond authorisation (ed JMC Geuns), 157–180, Belgium: KULeuven. ISBN: 9789074253208.
Hajihashemi, S., J.M.C. Geuns, and A.A. Ehsanpour. 2013. Gene transcription of steviol glycoside biosynthesis in Stevia rebaudiana Bertoni under polyethylene glycol, paclobutrazol and gibberellic acid treatments in vitro. Acta Physiologiae Plantarum 35: 2009–2014.
Jackson, R.G., E.K. Lim, Y. Li, M. Kowalczyk, G. Sandberg, J. Hoggett, D.A. Ashford, and D.J. Bowles. 2001. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. Journal of Biological Chemistry 276: 4350–4356.
Jain, S.M., and M.M. Spencer. 2006. In Floriculture and ornamental biotechnology: Advances and tropical issues, ed. J.A. Teixeira da Silva, Vol. 1, 589–600. Global Science Books, ISSN 1749–0294 (Paper), 1749–0308 (Online), 1749–0316 (CD-ROM).
Jasim, B., R. Thomas, J. Mathew, and E.K. Radhakrishnan. 2017. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharmaceutical Journal 25: 443–447.
Kanipandian, N., S. Kannan, R. Ramesh, P. Subramanian, and R. Thirumurugan. 2014. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Materials Research Bulletin 49: 494–502.
Kha, M.N., M. Mobin, Z.K. Abbas, K.A. Almutairi, and Z.H. Siddiqui. 2017. Role of nanomaterials in plants under challenging environments. Plant Physiology and Biochemistry 110: 194–209.
Kim, J.S., E. Kuk, and K.N. Yu. 2007. Antimicrobial effects of silver nanoparticles. Nanomedicine 3: 95–101.
Kohan-Baghkheirati, E., and J. Geisler-Lee. 2015. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterial 5: 436–467.
Kumar, H., K. Kaul, S. Bajpai-Gupta, V. Kumar Kau, and S. Kumar. 2012. A comprehensive analysis of fifteen genes of steviol glycosides biosynthesis pathway in Stevia rebaudiana (Bertoni). Gene 492: 276–284.
Lemus-Mondaca, R., A. Vega-Gálvez, L. Zura-Bravo, and K. Ah-Hen. 2012. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chemistry 132: 1121–1132.
Lim, D., J.Y. Roh, H.J. Eom, J.Y. Choi, J. Hyun, and J. Choi. 2012. Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditis elegans. Environmental Toxicology and Chemistry 31: 585–592.
Majlesi, Z., M. Ramezani, and M. Gerami. 2018. Investigation on some main glycosides content of Stevia rebaudian B under different concentration of commercial and synthesized silver nanoparticles. Pharmacheutical and Biomedical Research 4(1): 1–10.
Masarovicova, E., and K. Kralova. 2013. Metal nanoparticles and plants. Ecological Chemistry and Engineering S 20: 9–22.
Mirzajani, F., H. Askari, S. Hamzelou, Y. Schober, A. Rompp, and A. Ghassempour. 2014. Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicology and Environmental Safety 108: 335–339.
Modi, A.R., Y.M. Shukla, N.S. Litoriya, N.J. Patel, and S. Narayanan. 2011. Effect of gibberellic acid foliar spray on growth parameters and stevioside content of ex vitro grown plants of Stevia rebaudiana B. Journal of Medicinal Plants 3: 157–160.
Mohanpuria, P., N.K. Rana, and S.K.J. Yadav. 2008. Bio-synthesis of nanoparticles: Technological concepts and future applications. Nanoparticle Research 10: 507–517.
Moteriya, P., H. Padalia, R. Jadeja, and S. Chanda. 2014. Screening of silver nanoparticle synthetic e cacy of some medicinal plants of Saurashtra region: A review, Natural Products: Research Reviews, Gupta VK Ed., 3, Indian Institute of Integrative Medicine, JammuTavi, India (2014) (In press).
Mulabagal, V., and H.S. Tsay. 2004. Plant cell cultures—An alternative and efficient source for the production of biologically important secondary metabolites. International Journal of Applied Sciences and Engineering 2: 29–48.
Navarro, E., A. Baun, and R. Behra. 2008. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17: 372–386.
Phukan, U.J., G.S. Jeena, and R.K. Shukla. 2016. WRKY transcription factors: Molecular regulation and stress responses in plants. Frontier in Plant Science 7: 760–767.
Pitta-Alvarez, S.I., T.C. Spollansky, and A.M. Giulietti. 2000. The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzyme and Microbial Technology 26: 491–504.
Rahi, P., V. Pathania, A. Gulati, B. Singh, R.K. Bhanwra, and R. Tewari. 2010. Stimulatory effect of phosphate-solubilizing bacteria on plant growth, stevioside and rebaudioside-A contents of Stevia rebaudiana Bertoni. Applied Soil Ecology 46: 222–229.
Ramezani, M., M. Gerami, and Z. Majlesi. 2018a. Comparison between various concentrations of commercial and synthesized silver nanoparticles on biochemical parameters and growth of Stevia rebaudian B. Plant Physiology Reports 24: 1–12.
Ramezani, M., F. Ramezani, F. Rahmani, and A. Dehestani. 2018b. Exogenous potassium phosphite application improved PR-protein expression and associated physio-biochemical events in cucumber challenged by Pseudoperonospora cubensis. Scientia Horticulturae 234: 335–343.
Ramezani, M., F. Rahmani, and A. Dehestani. 2017a. The effect of potassium phosphite on PR genes expression and the phenylpropanoid pathway in cucumber (Cucumis sativus) plants inoculated with Pseudoperonospora cubensis. Scientia Horticulture 234: 335–343.
Ramezani, M., F. Rahmani, and A. Dehestani. 2017b. Study of physio-biochemical responses elicited by potassium phosphite in downy mildew-infected cucumber plants. Archive of Phytopathology and Plant Protectection 50(11–12): 540–554.
Richman, S.A., M. Gijzen, A.N. Starratt, Z. Yang, and J.E. Brandle. 1999. Diterpene synthesis in Stevia rebaudiana: Recruitment and upregulation of key enzymes from the gibberellin biosynthetic pathway. The Plant Journal 19: 411–421.
Rico, C.M., S. Majumdar, M. Duarte-Gardea, J.R. Peralta-Videa, and J.L. Gardea-Torresdey. 2011. Interaction of nanoparticles with edible plants and their possible implications in the food chain. Journal of Agricultural and Food Chemistry 59: 3485–3498.
Schluttenhofer, C., and L. Yuan. 2015. Regulation of specialized metabolism by WRKY transcription factors. Plant Physiology 167: 295–306.
Scolnik, P.A., and G. Giuliano. 1994. Regulation of carotenoid biosynthesis genes during plant development. Pure and Applied Chemistry 66: 1063–1068.
Shibata, H., Y. Sawa, T. Oka, S. Sonoke, K.K. Kim, and M. Yoshioka. 1999. Steviol and steviol-glycoside-glucosyltransferase activities in Stevia rebaudiana Bertoni purification and partial character-ization. Archive of Biochemistry and Biophysics 321: 390–396.
Song, J., B. Guo, F. Song, H. Peng, Y. Yao, Y. Zhang, Q. Sun, and Z. Ni. 2011. Genome-wide identification of gibberellins metabolic enzyme genes and expression profiling analysis during seed germination in maize. Gene 482: 34–42.
Sosan, A., D. Svistunenko, D. Straltsova, K. Tsiurkina, I. Smolich, and T. Lawson. 2016. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant Journal 85: 245–257.
Vecerova, K., Z. Vecera, B. Docekal, M. Oravec, A. Pompeiano, and J. Tríska. 2016. Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environmental Pollution 218: 207–218.
Zhang, B., L.P. Zheng, W. Li, and J. Wang. 2013. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag–SiO2 core–shell nanoparticles. Current Nanoscience 9: 363–370.
Acknowledgements
The authors would like to thank the SANA Institute, Sari, IRAN for staff collaboration.
Funding
The authors acknowledged the following support: the IRAN University of Medical Sciences (Grant number 96-04-130-32710, Tehran, IRAN).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The experiments performed under control of ethics committee of IRAN University of Medical Sciences with ethics number IR.IUMS.REC 1396.32710.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramezani, M., Asghari, S., Gerami, M. et al. Effect of Silver Nanoparticle Treatment on the Expression of Key Genes Involved in Glycosides Biosynthetic Pathway in Stevia rebaudiana B. Plant. Sugar Tech 22, 518–527 (2020). https://doi.org/10.1007/s12355-019-00786-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00786-x