Abstract
Stevia rebaudiana contains steviol glycosides (SVglys) responsible for its sweet taste. The knowledge of some shared steps between gibberellin (GA) and SVglys biosynthesis made it imperative to study the effect of paclobutrazol (PBZ; a GA biosynthesis inhibitor) and GA itself on SVglys accumulation and transcription of their correlated genes. Plants were treated with GA and PBZ (10 mg L−1) in controlled greenhouse conditions. The results showed that GA and PBZ treatments increased or decreased, respectively, stevioside, rebaudioside A, B, C, F, rubusoside, steviolbioside, dulcoside A, and total SVglys contents. The rebaudioside A/stevioside ratio is considered as a parameter to measure the quality of Stevia extract. PBZ treatment increased the rebaudioside A/stevioside ratio which means less aftertaste bitterness of Stevia extracts. GA treatment had no significant effect on this ratio. The transcription of ent-KO, ent-KS1, ent–KAH, UGT76G1, UGT85C2 and UGT74G1 were maximally increased by GA and minimally by PBZ treatments, respectively. This might explain the higher or lower accumulation of SVglys by treatments with GA or PBZ, respectively. The results revealed the correlation between gene transcription and SVglys accumulation which is worth to study in depth to produce Stevia with higher SVglys and sweeter taste.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
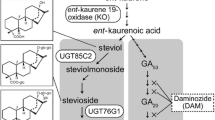
The sweet Stevia rebaudiana (Bertoni) plant accumulates the diterpenic steviol glycosides (SVglys). There are more than 30 SVglys in S. rebaudiana with different concentrations (Ceunen and Geuns 2013; Wölwer-Rieck 2012). The most abundant SVglys in Stevia are stevioside (ST) and rebaudioside A (Reb A). Reb A, with a sweeter taste than ST, is used in drinks and foods (Soejarto 2002; Tanaka 1997). The Reb A/ST ratio is considered as a parameter to determine the quality of the Stevia extract taste (Ceunen and Geuns 2013; Yadav et al. 2011). Other SVglys consist of Reb C–F, dulcoside A (Dul A), steviolbioside (SB) in a total amount of 1–2% (Geuns 2003; Makapugay et al. 1984; Starratt et al. 2002). In Stevia, the enzymes of kaurene synthase (KS), kaurene oxidase (KO, a P450 mono-oxygenase) and kaurenoic acid 13-hydroxylase (KAH) convert copalyl pyrophosphate into steviol (SV). Then, specific glucosyltransferases form different SVglys by SV glucosylation (GTs; Figs. 1, 4) (Brandle and Telmer 2007; Shibata et al. 1995).
S. rebaudiana is currently considered as an alternative substitute for sucrose. Tribes from Brazil and Paraguay used S. rebaudiana as medicinal tea for years (Singh and Rao 2005). SVglys are introduced as a food additive in many countries. Highly purified SVglys and Reb A received the generally recognized as safe status in the United State of America (Wölwer-Rieck 2012). Antioxidant activities (Ahmad et al. 2010; Hajihashemi and Geuns 2013), anti-carcinogenic, anti-hyperglycaemic (Dey et al. 2013), anti-pathogenic (Fazal et al. 2011) and anti-hypersensitive properties (Liu et al. 2003) have been reported for SVglys.
Both SV and GA have a tetra-cyclic diterpene skeleton (Fig. 1). Ent-KO and ent-KS function in SVglys biosynthesis as well as GA biosynthesis and ent-kaurenoic acid is a common precursor for them (Figs. 1, 4). Ent-KO is up-regulated in mature Stevia leaves (Humphrey et al. 2006) whereas in Cucurbita maxima, Arabidopsis and Pisum sativum, ent-KO transcription is low in mature leaves (Davidson et al. 2005; Helliwell et al. 1999).
Triazole fungicides (e.g. paclobutrazol, hexaconazoleand propiconazole), with plant growth regulating side-effects, inhibit GA biosynthesis (Fletcher et al. 2010; Hedden and Graebe 1985). Paclobutrazol (PBZ) affects the isoprenoid pathway and blocks GA biosynthesis at the ent-KO step by inhibiting oxidation of kaurene to kaurenoic acid (Fletcher et al. 2010; Sankar et al. 2007).
S. rebaudiana is a new introduced crop and its physiological properties have not been studied extensively. Some steps are common between the SVglys and GAs biosynthesis pathway. PBZ blocks the GA biosynthesis (Figs. 1, 4). According to this knowledge, the present study has been designed to unravel the mechanism by which the vital step of SVglys biosynthesis in S. rebaudiana is regulated by GA and PBZ treatments and to study the possible correlation to genes transcription as well. The aim of this study was to (1) measure SVglys contents, (2) measure the transcription of some genes involved in GAs and SV biosynthesis, (3) and characterize the correlation between SVglys contents and the transcription of the genes analyzed.
Materials and methods
In vitro S. rebaudiana plants were provided by the Laboratory of Functional Biology, KULeuven, Leuven, Belgium. Plants were propagated by tissue culture technique. After 2 months, plants with a similar size were cultured into pots (1.5 L) filled with a mixture of vermiculite and perlite. The plants were grown under controlled greenhouse conditions with a photoperiod of 16 h light and 8 h dark. The air temperature ranged from 22 ± 4 °C during the day to 15 ± 3 °C during the night. The humidity level was between 40 and 60%. The irrigation was done every other day and the plants were fertilized by a nutrient solution (pH 6.0) every week. The plants (6 months old) were irrigated with 0 (distilled water), and 10 mg L−1 of PBZ and GA solutions. PBZ and GA treatments were carried out every 4 days, for 1 month. Two days later, the newly emerged leaves of the treatments were harvested for further analysis. Leaves were immediately frozen in liquid nitrogen and stored in a freezer at −80 °C. Some of the harvested leaves were freeze-dried for SVglys analysis. Plant culture and treatments were repeated thrice at different times. In each experiment, there were six replicates per treatment.
Water extraction of SVglys
The freeze-dried leaves (20 mg) were extracted three times by boiling for 15 min in 300 µL HPLC quality water. After centrifugation, the pellet was re-extracted twice with 300 µL HPLC quality water. The supernatants were mixed and centrifuged to remove particles (Ceunen et al. 2012). The samples were not purified to avoid possible losses. The samples were stored at −80 °C for further analysis.
Analysis of SVglys
The amount of SVglys was determined according to Ceunen et al. (2012). Twenty µL of extract was injected in the HPLC (Shimadzu, Deurne, Belgium) with two Grace Alltima C18 columns in series. The solvent flow was 1 mL min−1 and the gradient of acetonitrile (AcCN): 1.0 mM phosphoric acid was as described by Ceunen et al. (2012). Different SVglys (Reb A, ST, Reb B, Reb C, Reb F, Dul A, SB, Rub) were measured. The standard of Reb A with purity >99% was used to quantify the results (Geuns 2010). The SVglys amounts were reported as percent of the leaf dry mass (LDM).
Analysis of gene transcription using RT-q PCR
RNA extraction of frozen leaves was done by the RNA Mini Kit. For cDNA synthesis, a Master Mix containing 2.3 µg of total RNA was prepared in a total volume of 25 µL (Hajihashemi et al. 2013). The house-keeping genes of β-Actin and 18S rRNA were used for quantification. The OligoCalc software was used to design primers (RT-qPCR; Table 1) (Hajihashemi et al. 2013).
Real-time PCR is based on the fluorescence signal emitted by SYBR Green I bound to the PCR product. House-keeping gene transcripts were taken as a reference. The qPCR kit Master Mix was used to prepare SYBR Green reaction mix. The SYBR green one-step RT-qPCR assay was done in a 96-well plate. The reaction mixture of PCR was composed of 2 µL of cDNA (5 ng µL−1) in a 10 µL volume. The reactions were done in an ABI prism 7000 SDS as described by Hajihashemi et al. (2013). Obtained data were analysed according to Step One software (Version 2.1).
Statistical analysis
A Randomized Complete Block Design was used for all experiments with three biological and technical replications. The Duncan test (SPSS, version 16) was used to assess significant differences (at the 5% level) between means. The results of statistical analysis are shown by superscript letters to reveal significant differences.
Results
Figure 2 shows that PBZ induced shorter plants with smaller internodes while GA treatment resulted in longer plants. To compare the effect of GA and PBZ on Stevia, first we measured the SVglys contents. The accumulation of SVglys in leaves of Stevia decreased with about 53% by PBZ treatment (Fig. 3). ST, Reb A, Reb B, C, F, Dul A, Rub, and SB amounts were less than those of control plants. S. rebaudiana usually accumulates mainly ST, about three times more than Reb A (ST/Reb A ratio about 3.33). PBZ treatment decreased ST and Reb A content by about 60 and 37%, respectively. PBZ treatment led to a significant increase of the Reb A/ST ratio. GA treatment significantly increased the amount of SVglys by about 20% leading to increases of the different SVglys: ST, Reb A, Reb B, C, F, Dul A, Rub, and SB (Fig. 3). However, GA treatment had no significant effect on the Reb A/ST ratio.
In the next step of the study, the RT-q PCR analysis on treated plants was performed. The RT-q PCR results were normalized to the level of 18S rRNA and β-Actin. The results relative to β-Actin were reported because the expression patterns of samples relative to 18S rRNA and β-Actin were almost the same. The same melting curve was obtained for all samples. Samples were good purifies because no signal was observed in the negative control samples.
The ent-KS1, ent-KO, ent–KAH genes are involved in the biosynthesis of both SV and GA. The transcription of ent-KS1, ent-KO and ent–KAH were significantly decreased by PBZ treatment by about 22, 82 and 19%, respectively (Fig. 4). On the contrary, GA treatment significantly increased the transcription of ent-KS1, ent-KO and ent–KAH by about 19, 133 and 20%, respectively (Fig. 4). The transcription of UGT74G1, UGT76G1 and UGT85C was significantly decreased by PBZ treatment by about 20, 40 and 40%, respectively (Fig. 4). GA application increased the transcription of UGT74G1, UGT76G1 and UGT85C by about 16, 26 and 27%, respectively (Fig. 4). The most reduction or increase in gene transcription of six analyzed genes was observed with ent-KO by PBZ or GA treatments, respectively.
The biosynthetic pathway of steviol glycosides. The transcription of ent-KS1, ent-KO, ent-KAH, UGT85C2, UGT74G1 and UGT76G1 of S. rebaudiana involved in the SVglys biosynthesis, relative to that of β-Actin in plants subjected to gibberellin (GA) and paclobutrazol (PBZ) treatments. kaurene synthase (KS), kaurene oxidase (KO), kaurenoic acid hydroxylase (KAH), UDP-dependent glycosyltransfrases (UGT). Treatments with the same lower-case letters were not significantly different based on mean comparison by Duncan’s test at p < 0.05
Discussion
S. rebaudina has a huge economic potential because of its SVglys accumulation. Since many years, SVglys products have been used as a sweetener in different countries (Ceunen and Geuns 2013; Geuns 2003; Woelwer-Rieck et al. 2010). A critical point in S. rebaudiana is to study the elements which may affect SVglys accumulation. Ent-copapalyl pyrophosphate serves as an intermediate for biosynthesis of steviol and different SVglys are formed by glucosylation of steviol (Brandle and Telmer 2007; Ceunen and Geuns 2013; Geuns 2003), hence analysis and regulation of genes in the SVglys biosynthesis assumes a central position in the manipulation of SVglys contents in S. rebaudiana. Previous studies showed the important role of ent-KS, ent-KO and GTs genes in regulating SVglys contents (Richman et al. 2005; Humphrey et al. 2006; Kumar et al. 2012). The knowledge of some shared steps in biosynthesis pathway of GA and SVglys made it imperative to study the effect of a GA biosynthesis inhibitor (PBZ) and GA itself on SVglys accumulation in the present study. Interestingly, the obtained results showed that GA and PBZ treatments resulted in positive and negative effects, respectively, on ST, Reb A, Reb B, C, F, Dul A, Rub and SB contents. Also, GA and PBZ treatments significantly increased and decreased, respectively, the total SVglys content. ST has three units of d-glucose attached to a steviol ring, whereas, Reb A has four of these units (Kennelly 2002; Starrat et al. 2002; Ceunen and Geuns 2013). This difference makes Reb A sweeter than ST. Commercially, it is worth to study the production of Stevia plants with higher Reb A than ST content or to make a selection of such plants with less aftertaste bitterness. It is valuable to report that PBZ increased the Reb A/ST ratio which means a sweeter taste of Stevia leaves extract of PBZ-treated plants while GA treatment had no effect on this ratio. Development of new varieties of S. rebaudiana with a higher content of Reb-A and a reduced content of ST is the aim of plant breeders (Ceunen and Geuns 2013; Dacome et al. 2005; Yadav et al. 2011).
It was interesting to reveal the correlation between SVglys content with the transcription of some key genes involved in the SVglys biosynthesis pathway. PBZ is a critical limiting treatment, adversely affecting GA biosynthesis (Fletcher et al. 2010; Sankar et al. 2007). Evidently, the negative effect of PBZ on SVglys was observed at this study. The finding that PBZ reduced SVglys contents while GA increased them is reasonable, as PBZ negatively down-regulate but GA up-regulated the transcription of six involved genes in SVglys biosynthesis of ent-KS1, ent-KO, ent–KAH, UGT74G1, UGT76G1 and UGT85C2. The transcript levels of ent-KAH and UGTs showed a linear correlation during vegetative growth (Ceunen and Geuns 2013). The inhibition or stimulation of ent-KS1 and ent-KO transcriptions with PBZ and GA treatments, respectively, suggest a correlation between SVglys contents and their ent-kaurenoic acid precursor. GA is derived from the same ent-kaurenoid precursor as SVglys, and ent-KS and ent-KO are involved in their biosynthesis (Brandle and Telmer 2007). Kumar et al. (2012) reported a positive correlation between SVglys accumulation and ent-KO transcription, which is supported by the results of the present experiment. According to Fig. 2, the plant height decreased in PBZ-treated plants and increased in GA-treated plant which reflects the down-regulated and up-regulated transcription of ent-KS and ent-KO genes involved in GA biosynthesis pathway. The negative effect of PBZ on plant growth is correlated to GA biosynthesis inhibition, more specifically at the ent-KO step (Fletcher et al. 2010; Sankar et al. 2007). Based on a previous study (Hajihashemi et al. 2013), ent-KO transcription significantly decreased in the PBZ-treated plants while it increased in GA-treated plants which also supports the results of the present study. Hajihashemi et al. (2013) similarly reported that the transcription of ent-KS1 decreased in PBZ-treated plants under in vitro conditions. On the contrary, the result of the previous study on ent-KAH transcription does not support the result of this study on PBZ treatment. That study showed that GA treatment had no significant effect on ent-KS and ent-KAH transcriptions under in vitro culture conditions (Hajihashemi et al. 2013) while in the present study, their transcriptions significantly increased by GA treatment. The observed differences about the transcription levels of ent-KS1 and ent-KAH under in vitro culture conditions (Hajihashemi et al. 2013) and greenhouse conditions might be correlated to different parameters such as plant age, growth conditions, concentration of treatment solutions and others which need more investigation. Both ent-KS and ent-KO are highly expressed in mature leaves in S. rebaudiana while they are only highly expressed in the very young, actively growing leaves in other plants (Humphrey et al. 2006; Richman et al. 1999). A linear correlation was observed between low SVglys content with the very low transcription of ent-KS, ent-KO, and UGTs in S. rebaudiana roots (Humphrey et al. 2006; Richman et al. 1999) which is in according to the results of PBZ treatment in this study. It is suggested that the expression of the ent-KS and ent-KO genes is mainly limited to GA biosynthesis occurring in the root tip (Richman et al. 1999).
According to the CAZy classification, the glucosyltransferases of UGT74G1, UGT76G1 and UGT85C2 belong to Family 1 (GT1). Terpenoids, alkaloids, cyanogenic glucosides, glucosinolates, flavonoids, isoflavonoids and other phenylpropanoids belong to the plant CAZy Family 1 (Mohamed et al. 2011). Our results showed that the transcription of UGT85C2 and UGT76G1 significantly decreased by PBZ treatment which corroborates results of Hajihashemi et al. (2013). The UGT85C2 enzyme adds a C-13-glucose to SV to form steviolmonoside. The correlation between transcription of UGT85C2 and total SVgly contents suggests UGT85C2 as a rate-limiting step in SVgly biosynthesis (Mohamed et al. 2011). UGT76G1 is suggested to regulate the synthesis of ST and Reb A (Richman et al. 2005; Richman et al. 1999). In this study, the transcription of UGT74G1 significantly decreased by PBZ treatment, which is different from in vitro studies (Hajihashemi et al. 2013). In this study, GA treatment increased the transcription of UGT74G1, UGT76G1 and UGT85C2 contrary to the results from in vitro conditions.
It is known that plant responses to treatments are associated with the plant species, concentration and time of treatments and environmental conditions (Chaves et al. 2002). Hajihashemi et al. (2013) treated Stevia plant by adding 2 mg L−1 GA and PBZ to MS culture medium containing explants with just two auxiliary buds without roots and leaves while in the present study 6 month old plants were treated with 10 mg L−1 of GA and PBZ. Changes in SVglys contents and the transcription of genes were shown to be dependent of growth stage (Ceunen et al. 2012; Ceunen and Geuns 2013; Yang et al. 2015). The harvesting time of Stevia also affected the quality and yield of SVglys in leaves (Pal et al. 2014). In conclusion, it can be strongly suggested that the Stevia response to GA and PBZ was influenced not only by the treatment itself but also by the concentration of the substances administered, the growth conditions, and the plant growing stage which had a major influence on SVglys accumulation and some genes involved. PBZ inhibit GA production by blocking ent-KO step. The results showed the highest reduction and increse in ent-KO transcription by PBZ and GA treatments, respectively. The linear correlation observed between ent-KO expression with five others studied genes suggests one possible mode of regulation involves metabolon formation (Ceunen and Geuns 2013). Another important point might be the positive effect of PBZ treatment on the Reb A/ST ratio which resulted in a better taste of the Stevia extract. It is an important issue to do more research on the production of S. rebaudiana with a sweeter taste and less bitter taste via bioengineering.
The response of S. rebaudiana (Bertoni) Bertoni to different treatments outlined in this study confirms that SVglys accumulation is affected by PBZ and GA treatments closely related to the transcription of the genes involved. Six genes of ent-KS1, ent-KO, ent–KAH, UGT74G1, UGT76G1 and UGT85C2 were transcribed maximally by GA and minimally by PBZ treatments which resulted in a greater accumulation of SVglys by GA treatment and a smaller one by PBZ treatment, respectively. The transcription of genes involved in the same biosynthesis pathway can differ individually depending upon the growth (in vitro vs. greenhouse). This complicates the explanation of the involved mechanisms. Ent-KO transcription changed by PBZ and GA treatments in both this study and the previous study (Hajihashemi et al. 2013). It might be suggested to classify the genes involved in the SVglys biosynthesis pathway into two groups of early and late induced genes. The gene of ent-KO can be classified as the early induced genes in response to PBZ and GA treatments. With increasing concentrations of PBZ and GA, the other genes of the pathway become engaged (e.g. ent-KS1, ent–KAH, UGT74G1, UGT76G1 and UGT85C2) which might be classified as late induced genes. Overall, these findings provide new insights into the complicated response mechanisms in Stevia plants. Moreover, it should be considered that these studies open new perspectives for the study of other genes involved in GAs and SVglys biosynthesisin S. rebaudiana to reveal the mechanism of gene expression regulation by GA and PBZ treatments. Another valuable result obtained in the present study is the increased Reb A/ST ratio in PBZ-treated plants resulting in a sweeter taste of the Stevia extracts.
References
Ahmad N, Fazal H, Abbasi B, Farooq S (2010) Efficient free radical scavenging activity of Ginkgo biloba, Stevia rebaudiana and Parthenium hysterophorus leaves through DPPH (2, 2-diphenyl-1-picrylhydrazyl). Int J Phytomed 2:231–239
Brandle JE, Richman A (2008) Compositions and methods for producting steviol and steviol glycosides. US Patent 2008/0064063, 13 March 2008
Brandle J, Telmer P (2007) Steviol glycoside biosynthesis. Phytochemistry 68:1855–1863
Ceunen S, Geuns JM (2013) Steviol glycosides: chemical diversity, metabolism, and function. J Nat Prod 76:1201–1228
Ceunen S, Werbrouck S, Geuns JM (2012) Stimulation of steviol glycoside accumulation in Stevia rebaudiana by red LED light. J Plant Physiol 169:749–752
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot 89:907–916
Dacome AS, Da Silva CC, Da Costa CE, Fontana JD, Adelmann J, Da Costa SC (2005) Sweet diterpenic glycosides balance of a new cultivar of Stevia rebaudiana (Bert.) Bertoni: isolation and quantitative distribution by chromatographic, spectroscopic, and electrophoretic methods. Process Biochem 40:3587–3594
Davidson SE, Smith JJ, Helliwell CA, Poole AT, Reid JB (2004) The pea gene LH encodes ent-kaurene oxidase. Plant Physiol 134(3):1123–1134
Dey A, Kundu S, Bandyopadhyay A, Bhattacharjee A (2013) Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. Compte Rendus Biol 336:17–28
Fazal H, Ahmad N, Ullah I, Inayat H, Khan L, Abbasi BH (2011) Antibacterial potential in Parthenium hysterophorus, Stevia rebaudiana and Ginkgo biloba. Pak J Bot 43:1307–1313
Fletcher RA, Gilley A, Sankhla N, Davis TD (2010) Triazoles as plant growth regulators and stress protectants. Hortic Rev 24:55–138
Geuns JM (2003) Stevioside. Phytochemistry 64:913–921
Geuns JM (2010) Stevia and steviol glycosides. Euprint, Heverlee
Hajihashemi S, Geuns JM (2013) Free radical scavenging activity of steviol glycosides, steviol glucuronide, hydroxytyrosol, metformin, aspirin and leaf extract of Stevia rebaudiana. Free Radic Antioxid 3:S34–S41
Hajihashemi S, Geuns JM, Ehsanpour A (2013) Gene transcription of steviol glycoside biosynthesis in Stevia rebaudiana Bertoni under polyethylene glycol, paclobutrazol and gibberellic acid treatments in vitro. Acta Physiol Plantarum 35:2009–2014
Hedden P, Graebe JE (1985) Inhibition of gibberellin biosynthesis by paclobutrazol in cell-free homogenates of Cucurbita maxima endosperm and Malus pumila embryos. J Plant Growth Regul 4:111–122
Helliwell CA, Poole A, Peacock WJ, Dennis ES (1999) Arabidopsis ent-kaurene oxidase catalyzes three steps of gibberellin biosynthesis. Plant Physiol 119:507–510
Humphrey TV, Richman AS, Menassa R, Brandle JE (2006) Spatial organisation of four enzymes from Stevia rebaudiana that are involved in steviol glycoside synthesis. Plant Mol Biol 61:47–62
Kennelly E (2002) Sweet and non-sweet constituents of Stevia rebaudiana. In: Kinghorn AD (ed) Stevia, the genus Stevia. Medicinal and aromatic plants—industrial profiles, vol. 19. Taylor & Francis, London, pp 68–85
Kumar H, Kaul K, Bajpai-Gupta S, Kaul VK, Kumar S (2012) A comprehensive analysis of fifteen genes of steviol glycosides biosynthesis pathway in Stevia rebaudiana (Bertoni). Gene 492:276–284
Liu JC, Kao PK, Chan P, Hsu YH, Hou CC, Lien GS, Hsieh MH, Chen Y-J, Cheng JT (2003) Mechanism of the antihypertensive effect of stevioside in anesthetized dogs. Pharmacology 67:14–20
Makapugay H, Nanayakkara N, Kinghorn A (1984) Improved high-performance liquid chromatographic separation of the Stevia rebaudiana sweet diterpene glycosides using linear gradient elution. J Chromatogr A 283:390–395
Mohamed AA, Ceunen S, Geuns JM, Van den Ende W, De Ley M (2011) UDP-dependent glycosyltransferases involved in the biosynthesis of steviol glycosides. J Plant Physiol 168:1136–1141
Pal PK, Mahajan M, Prasad R, Pathania V, Singh B, Ahuja PS (2014) Harvesting regimes to optimize yield and quality in annual and perennial Stevia rebaudiana under sub-temperate conditions. Ind Crop Prod 65:556–564
Richman AS, Gijzen M, Starratt AN, Yang Z, Brandle JE (1999) Diterpene synthesis in Stevia rebaudiana: recruitment and up-regulation of key enzymes from the gibberellin biosynthetic pathway. Plant J 19:411–421
Richman A, Swanson A, Humphrey T, Chapman R, McGarvey B, Pocs R, Brandle J (2005) Functional genomics uncovers three glucosyltransferases involved in the synthesis of the major sweet glucosides of Stevia rebaudiana. Plant J 41:56–67
Sankar B, Jaleel CA, Manivannan P, Kishorekumar A, Somasundaram R, Panneerselvam R (2007) Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachis hypogaea L. Colloids Surf B: Biointerfaces 60:229–235
Shibata H, Sawa Y, Oka T, Sonoke S, Kim KK, Yoshioka M (1995) Steviol and Steviol-Glycoside: glucosyltransferase activities in Stevia rebaudiana Bertoni-purification and partial characterization. Arch Biochem Biophys 321:390–396
Singh S, Rao G (2005) Stevia: the herbal sugar of 21st century. Sugar Tech 7:17–24
Soejarto DD (2002). Botany of stevia and Stevia rebaudiana. A. Douglas Kinghorn, 18
Starratt AN, Kirby CW, Pocs R, Brandle JE (2002) Rebaudioside F, a diterpene glycoside from Stevia rebaudiana. Phytochemistry 59:367–370
Tanaka O (1997) Improvement of taste of natural sweeteners. Pure Appl Chem 69:675–684
Woelwer-Rieck U, Tomberg W, Wawrzun A (2010) Investigations on the stability of stevioside and rebaudioside A in soft drinks. J Agric Food Chem 58(23):12216–12220
Wölwer-Rieck U (2012) The leaves of Stevia rebaudiana (Bertoni), their constituents and the analyses thereof: a review. J Agric Food Chem 60:886–895
Yadav AK, Singh S, Dhyani D, Ahuja PS (2011) A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can J Plant Sci 91:1–27
Yang Y, Huang S, Han Y, Yuan H, Gu C, Wang Z (2015) Environmental cues induce changes of steviol glycosides contents and transcription of corresponding biosynthetic genes in Stevia rebaudiana. Plant Physiol Biochem 86:174–180
Acknowledgements
The authors acknowledge the assistance and helpful discussions of Bart Panis, Filip Rolland, Hilde Verlinden, Tom Struyf, Edwige Andre, Stijn Ceunen, Amal Mohamed, Uria Bartholomees and Sofie Deroover.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Jan Geuns from KULeuven University.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hajihashemi, S., Geuns, J.M.C. Steviol glycosides correlation to genes transcription revealed in gibberellin and paclobutrazol-treated Stevia rebaudiana . J. Plant Biochem. Biotechnol. 26, 387–394 (2017). https://doi.org/10.1007/s13562-017-0399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-017-0399-5