Abstract
It is necessary to practice efficient irrigation in any agricultural development strategy under the context of climate change and competition from other economic sectors. Irrigation based on the relationship between photosynthetic activity and change of soil moisture could be an effective practice. Herein, we investigated the response of sugarcane photosynthetic parameters on drought stress at yield formation stage. The experiment was carried out by withholding water for 3 weeks from 190 days after transplanting and then re-watering until the end of experimental period. Data were collected for photosynthetic parameters including potential photosynthetic rate, stomatal conductance, transpiration rate, internal CO2 concentration, SPAD, and soil physical parameters. The results showed that sugarcane photosynthetic parameters decreased with decreasing soil moisture content to certain extent and the stayed static before dropping off again. Internal CO2 concentration increased when soil moisture reached the most severe, and it caused a standstill in photosynthesis. However, photosynthesis also recovered with soil moisture recovery. Photosynthetic parameters could be used as indicators to evaluate drought stress’s effect as well as to determine the time to re-irrigate to maintain normal growth of the plants. Soil volume moisture content of 15% (recorded by the 5TE sensor) or at pF of 2.8 (recorded by the MPS-6 sensor) seems to be the initial time to start irrigation to keep standard growth. Soil moistures at 10% and 3.8 pF, respectively, could be the critical points beyond which irrigation was found to result in photosynthetic disorders.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture system is the largest consumer of water resources. Over 70% of the globally available freshwater withdrawals are used for agricultural irrigation. By estimation, the global water demand for agriculture will increase by further 20% in the next 50 years due to irrigational needs (Global Agriculture http://www.globalagriculture.org). However, there is a greater shortage of freshwater source to be used for agriculture because of the impacts of climate change and the competition from other economic sectors. Therefore, water saving and efficient water use should be the important parts of any agricultural development strategy. Recently, the use of soil moisture sensor in smart agriculture is one of the handy and simple ways to manage agricultural irrigation. It is easy to set up an automatic irrigation system by using the output data from soil moisture loggers to connect to a smart computer. However, it is imperative to detect the time to start irrigation.
Sugarcane is one of the most important cash crops that accounts for 80% of global sugar production. It is also used as an important source to produce alternative fuel. Approximately 40–50% of the world bio-ethanol production is based on sugarcane (Zuurbier and Vooren 2008). At the different growth stages, sugarcane’s green tissues contain 60–80% water. Thus, in its life cycle, sugarcane requires a huge amount of water, with 1500–2000 mm of annual rainfall to create an optimum production (Food and Agriculture Organization of the United Nations http://www.fao.org). Hence, the water deficit that brings about reductions in photosynthesis, growth and biomass accumulation may be the reason for revenue failure. Basnayake et al. (2012) reported that water deficits by 50% irrigation and rainfall dependence reduce total dry matter and cane yield of sugarcane genotypes by 20–56% and 17–52%, respectively. Water supplement to compensate for water shortage from rainfall is therefore a requisite. However, when increasing irrigation to an optimum level or soil moisture attaining an optimum content, more water applied or higher soil moisture content will not provide any more advantages. Wiedenfeld and Enciso (2008) reported that there were no significant differences in cane yield and sugar yield when increasing water application from 80 to 120% of crop water requirement. Similarly, the cane and sugar yields increased significantly when water application was increased from 0.6 to 1.0 IW/CPE (irrigation water/cumulative pan evaporation), but not significantly when increasing irrigation levels up to 1.2 IW/CPE (Singh and Mohan 1994; Bahrani et al. 2009). The decision of time of irrigation to maintain high and economic yield becomes important. Based on the changes of soil moisture, Ibrahim (1978, 2006) reported that starting irrigation at 40–50% depletion of available soil moisture (DASM) is the best to keep the highest yields, but irrigation at 60–70% DASM gives economic yields. Delaying irrigation to 70% DASM was detrimental to the growth of sugarcane and resulted in economic losses of sugar yield. The changes in physiological traits (such as stomatal conductance, transpiration rate, internal CO2 concentration, and photosynthetic rate) along with morphological traits (such as stalk and leaf growth) are the most common initial adaptation when sugarcane plant is subjected to mild-to-moderate dehydration (Inman-Bamber and Smith 2005; Ferreira et al. 2017). These physiological and morphological changes could be used as indicators to decide an irrigation schedule. In fact, leaf and stalk extension and green leaves number were suggested as indicators to decide the irrigation time to avoid reductions in biomass accumulation, with an irrigation trigger point at which stalk elongation is reduced by 50% because of water deficit (Inman-Bamber 2004). Because photosynthesis is highly sensitive to water deficit (Ghannoum 2009), it not only easily reduces when soil moisture content decreases, but also recovers when re-watering (Dinh et al. 2017b). In this study, we have tried to apply the photosynthetic parameters as indicators to decide irrigation schedule. The daily changes of soil moisture and photosynthesis of sugarcane when plant is subjected to water deficit and after re-watering was investigated to point out the critical soil moisture value that will be the helpful information for a smart irrigation system in sugarcane.
Materials and Methods
Experimental Design
A root-box experiment was conducted from April 20, 2016, to December 16, 2016, under glasshouse conditions at the University of the Ryukyus, Okinawa, Japan (26°25′N, 127°45′E; altitude 126 m). The two root-boxes (RB-A and RB-B, 93 cm × 8 cm × 96 cm) were filled up to 90 cm of height by 2 mm sieve mixture of Shimajiri Mahji red soil: sea sand: peat moss (1: 1: 1, v v−1). In each root-box, volume water content sensors (5TE soil moisture and temperature, Decagon Devices Inc., USA) were installed at 5, 25 and 50 cm depths; a soil matric potential sensor (pF sensor, MPS-6, Decagon Devices Inc., USA) was also installed at 25 cm depth. A vertical soil temperature sensor (VTS-1, ADS), which can record data in each 1 cm, was also installed from ground to 30 cm depth (Fig. 1). The 2-month-old seedling of the commercial sugarcane variety NiF8 was transplanted, and then, water was irrigated from the topsoil to fill up the box. After that, water was supplied every day to replace daily water loss. Until 4 November, water was withdrawn until the photosynthetic rate almost ceased. (It took 22 days.) Prolonging by 2 days of the most severe drought period to see more clearly, the effect of water deficit, as well as the sensitivity of leaf photosynthesis to re-watering, was done with RB-B. After stress period, re-irrigation was done until the end of the experimental period. Plant in each root-box was fertilized weekly by replacing irrigation with 500 mL of the modified Hoagland’s nutrient solution with a composition of 6 mM Ca(NO3)2·4H2O, 4 mM KNO3, 2 mM KH2PO4, 2 mM MgSO4·7H2O, 25 µM H3O3, 10 µM MnSO4·5H2O, 2 µM ZnSO4·7H2O, 0.5 µM CuSO4·5H2O, and 0.1 mM C10H12FeN2NaO8·3H2O.
Data Collection
The outdoor and indoor climatic parameters including air temperature and relative humidity, vapor pressure, solar radiation, and precipitation were recorded at 10-min interval by weather systems (Harusa View, ADS) installed beside and inside the glasshouse. Soil physical parameters including moisture content, temperature, and electric conductivity were recorded by MPS-6, 5TE, and VTS-1 sensors for every 10-min interval.
Photosynthetic parameters including potential photosynthetic rate (A), stomatal conductance (gs), transpiration rate (E), and internal CO2 concentration (Ci) were determined daily at the same first (L1) and second (L2) fully expanded leaves (which became the third and fourth leaves at the later period) from the first day before stress period until the end of experimental period by a LI-6400 portable photosynthesis system (Li-COR, Lincoln, Nebraska, USA) equipped with a 2 × 3 cm2 LED chamber between 900 and 1500 h at a photon flux density of 2000 µmol m−2 s−1, leaf temperature of 33 ± 2 °C, and CO2 concentration of 450 µmol mol−1, which is close to glasshouse air condition. SPAD values were also recorded as soon as photosynthesis measurement was done at the same positions using a SPAD meter (SPAD- 502, Minolta, Japan).
Results
Overall, the large differences in environmental parameters between indoor and outdoor conditions were shown at midday. In fact, the indoor air temperature and relative humidity fluctuated from 16.4 to 37.3 °C and from 29.5 to 94.5% which were higher than outdoor air temperature from − 2.3 to 13.7 °C and relative humidity from − 10.3 to 41.1%, respectively. The solar radiation changed from 0.0 to 645.0 W m−2, lower than that recorded outdoor from 0.0 to 630.9 W m−2. The indoor vapor pressure fluctuated between 13.3 to 31.5 hPa. In open-air condition, precipitation was recorded from 0.0 to 1.8 mm. The solar radiation, air temperature, and vapor pressure tended to reach the peak at midday and bottomed at midnight, whereas the air relative humidity had the opposite trend (Fig. 2).
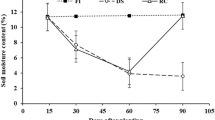
As can be seen from Fig. 3, the daily soil moisture increased whenever irrigation started and then decreased during the day. It is interesting to note that soil moisture recovered at nighttime despite withholding water during a drought period. Before stress period, volume water content (VWC) which was recorded by 5TE sensors ranged around 25% at three depths of 5, 25, and 50 cm in RB-A. Meanwhile, in RB-B, the same VWC values were recorded at depths of 25 and 50 cm, but at the 5 cm depth, VWC fluctuated more widely in comparison with others. During the stress period, in general, VWC changed at the most rapid rate during the first 10 days of drought period (DDP). After that, VWC steadily declined up to 10%. The change of VWC at deeper layers was slower in comparison with other layers. During the recovery period, VWC immediately returned to the initial condition at upper layers, whereas it took 4 days at 50 cm of depth. On the contrary, pF values which were recorded by MPS-6 sensor increased from 2.0 at the beginning up to 4.2 at the most severe drought stress, then rapidly dropped to non-stress value (around 2.0) as soon as re-watering happened (Fig. 4).
The daily change in soil moisture content during the experimental period. Air Temp and SR, air temperature and solar radiation at indoor condition; VWC, volume water content (recorded by 5TE sensors), pF, pF value (recorded by MPS-6 sensor); 5 cm, 25 cm and 50 cm, moisture content recorded by 5TE sensors at depth of 5, 25 and 50 cm, respectively
Time-out of daily soil moisture during the experimental period. RB-A and RB-B, root-boxes A and B, respectively; VWC, volume water content (recorded by 5TE sensors), pF, pF value (recorded by MPS-6 sensor); 5 cm, 25 cm and 50 cm, moisture content recorded by 5TE sensors at depths of 5, 25 and 50 cm, respectively
SPADs of the plant in RB-A fluctuated around 50 during 14 DDP before declining to 42 at 22 DDP. SPAD of the upper leaf, then, rapidly recovered to 45 at 3 days after re-irrigation (DAR), while SPAD of lower leaf continuously decreased to 40 and then recovered to 45 one day later. Similarly, SPAD of the plant in RB-B declined to 40 at 24 DDP, and then, SPAD of upper leaf recovered 3 days sooner in comparison with that of lower leaf. Photosynthetic parameters including A and gs decreased moderately during the first 10 DDP before rapidly declining to zero at 21 DDP. From this day on, the photosynthetic activity almost ceased. However, after re-watering photosynthesis immediately recovered. Interestingly, leaf Ci steadily decreased during the first 15 DDP and then rapidly increased to reach the peak at the most severe stress levels, but it also rapidly decreased to the normal value as before stress when water was again supplied (Fig. 5).
There were same tendencies in the responses of sugarcane for photosynthetic parameters on the changes of VWC at three levels of soil depth, but the effect of moisture stress on photosynthesis seemed to be indicated earlier at 5 cm depth and later at 50 cm depth in comparison with 25 cm depth (data not shown). In this study, therefore, we showed only the relationship of photosynthetic parameters with the change of VWC at 25 cm depth as the representative of the photosynthetic response of sugarcane to change of soil moisture (Fig. 6). A of the plant in RB-A reduced moderately from over 30 µmol m−2 s−1 at pF/VWC of approximately 2.0/23% to around 27 µmol m−2 s−1, whereas A of the plant in RB-B decreased more quickly to around 22 µmol m−2 s−1 during the first 4 DDP. After that, A of these plants seemed to become stable when soil moisture dropped from 2.8/15% to approximately 3.8/10%; then, it suddenly fell down to nearly 0.0 µmol m−2 s−1 when VWC was lower than 10% and pF was higher than 3.8. There were similar trends in the relationship between the change of soil moisture with the change of photosynthetic parameters including A, gs, and Ci until pF and VWC reached 4.0 and 10.0%, respectively. From these soil moisture values, Ci changed by an opposite way and went up from below 100 µmol mol−1 to close to 300 µmol mol−1. After re-watering, the recovery of all investigated parameters was recorded.
Relationships between soil moisture content (recorded by pF and 5TE at 25 cm of depth sensors) with photosynthesis rate (A), stomatal conductance (gs) and internal CO2 concentration (Ci). Note: RB-A and RB-B, photosynthetic parameters from root-boxes A and B, respectively; L1 and L2, first and second leaf, respectively; VWC, volume water content (recorded by 5TE sensors) at 25 cm of depth; pF, pF value (recorded by MPS-6 sensor)
Discussion
The air temperature, air humidity, solar radiation, wind speed, and precipitation are climatic factors that control soil evaporation and plant transpiration. In fact, reference evapotranspiration (ET) could be calculated by these factors (Zotarelli et al. 2015). Moreover, solar radiation has a positive correlation with ET and often can be calculated by the daily air temperature. Increasing solar radiation and air temperature and decreasing air humidity lead to increase vapor pressure that stimulates photosynthetic activity following a higher water loss in the plant. Therefore, on a sunny day, soil moisture decreases rapidly. Meanwhile, on a cloudy or rainy day, photosynthetic rate and transpiration rate are lower, and soil moisture decreases more slowly than that on the sunny day. Soil moisture in the upper layer from 0 to 25 cm depth reduced faster than that at the lower layer (50 cm depth) (Figs. 3, 4). It is because the secondary roots of sugarcane, which is often concentrated in soil surface zone, suck water from subsurface layer first. This leads to water shortage stimulating the role of lower roots to find water from deeper soil layer to help the plant escape temporarily from drought stress.
Interestingly, during drought stress period, soil moisture had a tendency to recover at the nighttime. This was clearer in the recovery of soil moisture at depths of 5 and 25 cm (Fig. 3). It could be explained that with nighttime conditions (no sunlight, low air temperature, and high air humidity), the plant stops photosynthetic activities, which lead to cessation of water loss from leaf (transpiration) as well as from the soil. Moreover, water always moves in the direction from higher potential energy region (deeper soil layer) to lower potential region (upper layer) (Davidson 1989). These reasons led to the increase in soil moisture in upper soil surface. However, the osmotic water from deeper layer was not enough to compensate for a large water loss that is absorbed by the root system to support leaf photosynthesis in the daytime; hence, overall soil moisture still declined. Furthermore, Richards and Caldwell (1987) described the upward movement of water from deep wet to shallow dry soil layer in terms of “hydraulic lift.” This could also explain the recovery of soil moisture at upper layers. During the daytime, plant transpiration forces water inflow from the soil through the stem and out to atmosphere via open stomata. At night, the close of stomata suppresses transpiration which leads to equilibrium between plant water potential and that of the soil where most active roots are found, which results in water potential gradients between the plant and the drier soil points; hence, water moves from roots to these dry soil layers (Kramer and Boyer 1995; Prieto et al. 2011). The hydraulic lift could be one of the mechanisms that help the plant living in waterless environments to mitigate the harmful effects of water deficit.
The result showed that there were quick reductions in A and gs during the first four DDP when soil moisture dropped in moderate-deficit threshold (pF from 2.0 to 2.8 and VWC from 23 to 15%, respectively). After that, they became stable 6 days later (Figs. 5, 6). Meanwhile, relative chlorophyll content (SPAD values) maintained during the first 14 DDP. It could be explained that during the first 3 DDP, leaf photosynthesis of sugarcane plant was sensitive to drought stress. Stomata closed very fast to restrict water loss and interrupted CO2 exchange which was a reason for the decrease of A and Ci. In the following days, when the plant is adapted to water deficit, the water from root system at deeper layer along with water stored in the stalk supplied water to maintain the amount of water transported to leaves to support photosynthesis, gs followed by A, was maintained. However, since 10 DDP, soil moisture decreased to a more severe-deficit threshold of pF from 3.8 to 4.2 and VWC from 10 to 8%, which could reach the soil permanent wilting point. At this time, the senescence of lower leaves was observed, chlorophyll content in top leaves was reduced, stomata closed more rapidly leading to the reduction of A close to zero and plant growth seemed to stop. Likewise, Rodrigues et al. (2009) observed the very low A at moderate (8 days) and severe (10 days) stress when sugarcane was subjected to dehydration condition. Under a rainout shelter conditions, Koonjah et al. (2006) found difference in A between the well-watered and water-stressed when the leaf water potential of the latter reached to − 0.7 MPa at 15 days of water stress and reached to the lowest level of 2.2 µmol m−2 s−1 at leaf water potential of − 1.6 MPa at 25 days of water stress. Zhao et al. (2013) reported that SPAD did not significantly differ between the well-watered and water-stressed plants when soil moisture slightly decreased during the first 10 to 15 days after initiation of the water stress treatment, but later when plants were already exposed to severe water stress, SPAD of water stress plants sharply declined. Similarly but sooner, A and gs of stressed plants declined sharply and significantly lower than that of the well-watered plants from 7 to 10 days after initiation of the water stress period. It confirmed that because leaf chlorophyll content is less sensitive and more stable during the first drought period than A and gs. In the small size pot experiments by withdrawing steadily 50% of daily water loss, Dinh et al. (2017a, 2018) found that sugarcane could maintain growth during the first 2 weeks, but later, when soil moisture reached to severe stress level, plant growth stopped with very low increase in rate of plant height and total number of leaves in drought stress treatments. Meanwhile, in the smaller pot, because severe stress was advanced, Graça et al. (2010) found a decrease in photosynthesis after just 5 days of water-deficit initiation. Although pot size or root zone size causes a difference in the rate to subject to severe drought stress, we could confirm that since photosynthetic parameters are susceptible to the change of soil moisture, it should be used as an indicator to detect time for starting irrigation.
It is interesting that after sharply decreasing from 200 to below 100 µmol mol−1, Ci suddenly increased close to 300 µmol mol−1 when soil moisture still continuously reduced to VWC below 10% and pF above 4.0. This was in accordance with Du et al. (1996) that Ci was decreased when leaf water potential decreases from − 0.37 MPa to − 0.85 MPa, but below − 0.85 MPa, with further decrease in leaf water potential, Ci increased rapidly. Plant maintains open stomata to uptake CO2; then, CO2 is translocated and fixed by photosynthetic enzymes such as ribulose-1,5-bisphosphate carboxylase/oxygenase, phosphoenolpyruvate carboxylase, NADP malic enzyme, etc. (Zingaretti et al. 2012). Water deficit caused stomatal closure as a result of decreasing CO2 uptake into leaf tissue. However, the reduction of photosynthetic enzymes under the effects of water stress (Du et al. 1996; Parry et al. 2002; Barbosa et al. 2015) led the decline of CO2 translocation and fixation. Along with stomatal closure, it caused the stagnation and increase in CO2 concentration in leaf tissue. We consider that because of severe stress, almost at the permanent wilting point, plant expresses disorders in physiological function, namely in the photosynthetic apparatus. If continuing to prolong this status, it will lead to plant death or cessation of growth. A supplemental irrigation has to be done to rescue the plant. Nevertheless, there is an argument that increase of Ci could be from error calculation of Ci when stomata close. Ci is routinely calculated from the outward diffusive behavior of water vapor (Caemmerer and Farquhar 1981; Boyer and Kawamitsu 2011). This calculation seems to be reasonably accurate just in case of open stomata because the effect of gas exchange through the cuticle is minor in comparison with gas exchange via stomata (Boyer et al. 1997). When stomata close, the calculation becomes more affected because of increasing the error from cuticle effects (Tominaga and Kawamitsu 2015). Hence, a direct measurement of Ci to confirm our result as well as to clear the change of Ci when stomata close under the effect of moisture stress should be conducted in further study.
Our result confirmed that photosynthetic parameters recovered when re-watered. Previous studies found the recovery of photosynthetic parameters such as A, chlorophyll content or SPAD with the values equal or even higher than that in the non-stress condition or that before water stress (Radha et al. 2015; Dinh et al. 2017b). However, in our finding, those parameters could recover but not as before stress. It may be because previous studies just focused on the first or second leaf, whereas in our study photosynthesis was measured at the same leaves. (These first leaves became third and fourth leaf at the later period.) Leaf nitrogen, which positively correlates with SPAD as well as A, depends on the leaf age (Allison et al. 1997). Moreover, water and nutrient rather than nitrogen are priority to support younger leaves. Therefore, upper or younger leaves often recover sooner and have higher photosynthetic ability than lower or older leaves. The earlier and stronger recovery for SPAD and photosynthetic parameters in third than those in fourth leaf in this study could demonstrate the effect of leaf age on the recovery of SPAD and leaf A (Fig. 5). Similarly, measuring on the same leaves, Pedrozo et al. (2015) found the reduction of leaf photosynthesis after 20 days. They also found the recovery of A, gs, E and SPAD from water deficit was equivalent to those in well-watered condition.
In conclusion, sugarcane photosynthetic parameters changed with the change in soil moisture, except for Ci which increased when soil moisture stress reached the most severe levels. Soil moisture decreasing to severe levels led to disorder and standstill in photosynthesis. However, photosynthesis recovered with soil moisture’s recovery. Therefore, photosynthetic parameters could be important indicators to evaluate drought stress effect as well as to determine the time to re-irrigate to help plant maintain normal growth. From this study, we observed that VWC of 15% (recorded by the 5TE sensor) or pF of 2.8 (recorded by the MPS-6 sensor) should be the initial time to start irrigation to maintain acceptable photosynthesis. Moreover, irrigation should not be delayed when soil moisture content reaches VWC of 10% and pF of 3.8, respectively, to avoid any disorder that may happen in photosynthetic activity. The research was conducted under root-box conditions where the restricted root zone was the scope of this study. Furthermore, the response of leaf photosynthesis to soil moisture change may be different in different sugarcane varieties; the change in soil moisture could vary among soil types. Further studies under field conditions with various sugarcane varieties and soil should be conducted to validate the results before applying to the actual farmer field conditions.
Abbreviations
- DAR:
-
Days after re-irrigation
- DDP:
-
Days of drought period
- VWC:
-
Volume water content
- A :
-
Potential photosynthetic rate
- C i :
-
Internal CO2 concentration
- E :
-
Transpiration rate
- g s :
-
Stomatal conductance
References
Allison, J.C.S., H.T. Williams, and N.W. Pammenter. 1997. Effect of specific leaf nitrogen content on photosynthesis of sugarcane. Annals of Applied Biology 131: 339–350.
Bahrani, M.J., M. Shomeili, S.H. Zande-Parsa, and A. Kamgar-Haghighi. 2009. Sugarcane responses to irrigation and nitrogen in subtropical Iran. Iran Agricultural Research 27(1–2) & 28(1): 17–26.
Barbosa, A.M., K.A. Guidorizi, T.A. Catuchi, T.A. Marques, R.V. Ribeiro, and G.M. Souza. 2015. Biomass and bioenergy partitioning of sugarcane plants under water deficit. Acta Physiologiae Plantarum 37: 142. https://doi.org/10.1007/211738-015-1887-7.
Basnayake, J., P.A. Jackson, N.G. Inman-Bamber, and P. Lakshmanan. 2012. Sugarcane for water-limited environments. Genetic variation in cane yield and sugar content in response to water stress. Journal of Experimental Botany 63: 6023–6033. https://doi.org/10.1093/jxb/ers251.
Boyer, J.S., and Y. Kawamitsu. 2011. Photosynthesis gas exchange system with internal CO2 directly measured. Environment Control Biology 49: 193–207.
Boyer, J.S., S.C. Wong, and G.D. Farquhar. 1997. CO2 and water vapour exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiology 114: 185–191.
Caemmerer, S.V., and G.D. Farquhar. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387.
Davidson, J.M. 1989. Principles of water and nutrient movement in soil. http://irrec.ifas.ufl.edu/flcitrus/pdfs/short_course_and_workshop/second_international_citrus/Davidson-Principles_of_Water_and_Nutrient_Movement.pdf. Accessed 17 Apr 2018.
Dinh, T.H., H. Takaragawa, and Y. Kawamitsu. 2018. Nitrogen use efficiency and drought tolerant ability of various sugarcane varieties under drought stress at early growth stage. Plant Production Science 22: 250–261. https://doi.org/10.1080/1343943X.2018.1540277.
Dinh, T.H., K. Watanabe, H. Takaragawa, and Y. Kawamitsu. 2017a. Effects of drought stress at early growth stage on response of sugarcane to different nitrogen application. Sugar Tech 20: 420–430. https://doi.org/10.1007/s12355-017-0566-y.
Dinh, T.H., K. Watanabe, H. Takaragawa, M. Nakabaru, and Y. Kawamitsu. 2017b. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Production Science 20: 412–422. https://doi.org/10.1080/1343943X.2017.1371570.
Du, Y.C., Y. Kawamitsu, A. Nose, S. Hiyane, S. Murayama, K. Wasano, and Y. Uchida. 1996. Effect of water stress on carbon exchange rate and activities of photosynthetic enzymes in leaves of sugarcane (Saccharum sp.). Australian Journal of Plant Physiology 23: 719–726.
FAO-Food and Agriculture Organization of the United Nations. Chapter 2: Crop water needs. http://www.fao.org/docrep/s2022e/s2022e02.htm.
Ferreira, T.H.S., M.S. Tsunada, D. Bassi, P. Araújo, L. Mattiello, G.V. Guidelli, G.L. Righetto, V.R. Gonçalvesm, P. Lakshmanan, and M. Menossi. 2017. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Frontiers in Plant Science 8: 1–18. https://doi.org/10.3389/fpls.2017.01077.
Ghannoum, O. 2009. C4 photosynthesis and water stress. Annals of Botany 103: 635–644. https://doi.org/10.1093/aob/mcn093.
Global Agriculture. Agriculture at a Crossroads. Findings and recommendations for future farming. https://www.globalagriculture.org/report-topics/water.html.
Graça, J.P.D., F.A. Rodrigues, J.R.B. Farias, M.C.N.D. Oliveira, C.B. Hoffmann-Campo, and S.M. Zingaretti. 2010. Physiological parameters in sugarcane cultivars submitted to water deficit. Brazilian Society of Plant Physiology 22: 189–197.
Ibrahim, H.S. 1978. Effects of irrigation sugarcane at different soil moisture deficits in the Sudan. Experimental Agriculture 14: 151–156. http://cambridge.org/core/journals/experimental-agriculture/article/effects-of-irrigating-sugarcane-at-different-soil-moisture-deficits-in-the-sudan/8B8A2BA95F682114EA28A561125B31D1. Accessed 3 May 2018.
Ibrahim, H.S. 2006. Determination of irrigation interval for sugarcane by monitoring changes in soil moisture in the field at the Guneid Scheme. http://agris.fao.org/agrissearch/search.do?recordID=SD2006000030. Accessed 3 May 2018.
Inman-Bamber, N.G. 2004. Sugarcane water stress criteria for irrigation and drying off. Field Crops Research 89: 107–122. https://doi.org/10.1016/j.fcr.2004.01.018.
Inman-Bamber, N.G., and D. Smith. 2005. Water relations in sugarcane and response to water deficits. Field Crops Research 92: 185–202. https://doi.org/10.1016/j.fcr.2005.01.023.
Koonjah, S.S., S. Walker, A. Singel, R.V. Antwerpen, and R. Nayamuth. 2006. A quantitative study of water stress effect on sugarcane photosynthesis. Proceeding of South African Sugar Technologists’ Association 80: 140–158.
Kramer, P.J., and J.S. Boyer. 1995. Water relations of plants and soils. Orlando: Academic Press.
Parry, M.A.J., P.J. Andralojc, S. Khan, P.J. Lea, and A.J. Keys. 2002. Rubisco activity: Effects of drought stress. Annals of Botany 89: 833–839.
Pedrozo, C.A., J. Jifon, M.H.P. Barbosa, J.A.D. Silva, J. Park, and N.S. Gracia. 2015. Differential morphological, physiological, and molecular responses to water deficit stress in sugarcane. Journal of Plant Breeding and Crop Science 7: 226–232. https://doi.org/10.5897/JPBCS2015.0500.
Prieto, I., C. Armas, and F.I. Pugnaire. 2011. Water release through plant roots: New insights into its consequences at the plant and ecosystem level. New Phytologist 193: 830–841.
Radha, J., A. Chandara, K.V. Visha, and S. Solomon. 2015. Physiological changes and expression of SOD and P5CS genes in response to water deficit in sugarcane. Sugar Tech 17: 276–282.
Richards, J.H., and M.M. Caldwell. 1987. Hydraulic lift: Substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia 73: 486–489.
Rodrigues, F.A., M.L.D. Laia, and S.M. Zingaretti. 2009. Analysis of gene expression profiles under water stress in tolerant and sensitive sugarcane plants. Plant Science 176: 286–302. https://doi.org/10.1016/j.plantsci.2008.11.007.
Singh, P.N., and S.C. Mohan. 1994. Water use and yield response of sugarcane under different irrigation schedules and nitrogen levels in a subtropical region. Agricultural Water Management 26: 253–264.
Tominaga, J., and Y. Kawamitsu. 2015. Cuticle affects calculations of internal CO2 in leaves closing their stomata. Plant and Cell Physiology 56: 1900–1908. https://doi.org/10.1093/pcp/pcv109.
Wiedenfeld, B., and J. Enciso. 2008. Sugarcane responses to irrigation and nitrogen in semiarid South Texas. Agronomy Journal 100: 665–671.
Zhao, D., G. Barry, and J.C. Comstock. 2013. Sugarcane leaf photosynthesis and growth characters during development of water deficit stress. Crop Science 52: 1066–1075. https://doi.org/10.2135/cropsci2012.09.0554.
Zingaretti, S.M., F.A. Rodrigues, J.P.D. Graça, L.D.M. Pereira, and M.V. Lourenço. 2012. Sugarcane responses at water deficit conditions. In Water stress, ed. P.M.M. Rahman. Shanghai: InTech. https://doi.org/10.5772/30986.
Zotarelli, L., M.D. Dukes, C.C. Romero, K.W. Migliaccio, and K.T. Morgan. 2015. Step by step calculation of the Penman–Monteith evapotranspiration (FAO-56 method). UF/IFAS extension. The University of Florida. http://edis.ifas.ufl.edu/pdffiles/ae/ae45900.pdf. Accessed 18 Nov 2016.
Zuurbier, P., and J.V.D. Vooren. 2008. Introduction to sugarcane ethanol contributions to climate change mitigation and the environment. In Sugarcane ethanol. Contributions to climate change mitigation and the environment, ed. P. Zubbier and J.V.D. Vooren, 19–27. Wageningen: Wageningen Academic Publishers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dinh, T.H., Takaragawa, H., Watanabe, K. et al. Leaf Photosynthesis Response to Change of Soil Moisture Content in Sugarcane. Sugar Tech 21, 949–958 (2019). https://doi.org/10.1007/s12355-019-00735-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00735-8