Abstract
The aim of this study is to investigate physiological responses and root characteristics of four sugarcane genotypes under drought stress and recovery conditions. The experiment was conducted in rhizoboxes under greenhouse conditions from February to May of 2019. A factorial experiment in completely randomized design with two replications was employed. Factor A contained three water regimes (full irrigation, drought, and recovery) and factor B consisted of the four sugarcane genotypes; Biotec 1, PR3067, UT6, and UT12. Data were recorded on physiological parameters, root characteristics and biomass yield. Early-drought led to a reduction in the relative water content (RWC), SPAD chlorophyll meter reading (SCMR), chlorophyll fluorescence (Fv/Fm), stomatal conductance (gs), transpiration rate (E), net photosynthetic rate (Pn), root length (RL), root surface area (RSA), root diameter (RD), root volume (RV), and biomass. Conversely, water use efficiency (WUE) increased. Biotec 1 showed low reductions on Fv/Fm, SCMR, Pn, gs and E whereas PR3067 and UT 12 showed high reductions for all traits. After rewatering, four parameters (SCMR, Fv/Fm, Pn, and WUE) illustrated how the sugarcane genotypes recovered from drought. We concluded that the ability of sugarcane to recover from drought was genotype dependent. Genotype Biotec 1 and UT 6 had the best drought recovery among the other sugarcane genotypes in each parameter and recover to levels equal to that of full recovery. Genotype Biotec 1 showed good recovery for SCMR, Fv/Fm and RL but Pn and WUE cannot reach to the full irrigation. Interestingly, UT 6 displayed good recovery for SCMR, Fv/Fm, Pn, RL and WUE. Additionally, the Biotec 1 and UT 6 genotypes demonstrated a high recovery efficiency for biomass by 85% and 79%, respectively. Despite rewatering, genotypes PR3067 and UT 12 produced low recovery efficiency at 56% and 49%, respectively. Our study proposes Biotec 1 and UT 6 as drought-tolerant genotypes capable of maintaining satisfactory photosynthetic rates, as well as suitable root systems, leading to high biomass after recovery. Furthermore, breeders can utilize this genotype as a parent in sugarcane breeding for drought resistance, and photosynthetic and root traits could be used as selection criteria for enhancing sugarcane drought resistance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugarcane is an important industrial crop, as well as one of the main sources of sugar and bioethanol (Souza et al. 2014) worldwide. Additionally, waste, and other by-products generated in sugar production may be used in bioelectricity and fertilizer production (Singh et al. 2021). Sugarcane is largely cultivated in tropical and subtropical regions where erratic and insufficient rainfall significantly limits crop productivity. In Thailand, sugarcane occupies more than 1.8 million hectares (FAO, 2022), where 90 percent of those growing areas exist under rainfed conditions (OCSB., 2022). Sugarcane is commonly cultivated in Thailand’s late rainy season from October to January where drought occurs at the early growth or tillering stage.
Sugarcane is frequently exposed to early-season and mid-season drought. This stress can reduce plant growth, resulting in plant stunting, tillering suppression, and defective and low-millable cane; eventually leading to both cane and sugar yield losses (Robertson et al. 1999; Dinh et al. 2017; Devi et al. 2018). Roughly 75% of the reduction of sugarcane yields are due to drought stress (Robertson et al. 1999). In sugarcane, genotype selection for drought resilience emphasizes the necessity of adaptability in drought-prone areas and high yield retentions under water deficits. Understanding drought-resistant mechanisms are, therefore, important in sugarcane breeding programs. Plants have several adaptive mechanisms in response to drought stress. One mechanism, for example, involves the root’s ability to increase water uptake during drought conditions and enhances the root’s ability to grow to deeper soil levels allowing plants to extend below ground moisture (Fang et al. 2017; Zhang et al. 2017) and compensate for the lack of water (Puangbut et al. 2018; Gano et al. 2021). The adapted root system promotes the uptake of water and nutrients to maintain the water balance in the plant in anticipation of upcoming dehydration.
Water is an integral part of the physiological processes in plants, particularly photosynthesis. When the ability to uptake water decreases, stomatal conductance will reduce to avoid excess water loss from the plant. This action limits the amount of carbon dioxide entering through the stomata. Drought decreases several of the sugarcane’s physiological traits; such as relative water content (RWC), stomatal conductivity (gs), PSII photochemical efficiency (Fv/Fm), and photosynthetic rate (Pn) (Silva et al. 2013; Graça et al. 2010; Devi et al. 2018; Khonghintaisong et al. 2018). Today, breeding drought-tolerant sugarcane involves several important physiological traits as selection criteria; including leaf area, chlorophyll content, stomatal conductance, transpiration, and photosynthetic rate (Silva et al. 2007; Pirnajmedin et al. 2015). Devi et al (2018) reported that drought-tolerant sugarcane genotypes possess high photosynthetic activity and yield retention. However, information on the photosynthetic response of sugarcane during drought and recovery periods is still lacking.
The physiological and photosynthetic mechanisms of drought resistance in sugarcane are determined by the roots under drought conditions (Namwongsa et al. 2019). Previous studies revealed that root length at deeper layers contribute to the photosynthetic rate in maize and Jerusalem artichoke (Lavinsky et al. 2016; Puangbut et al. 2018). Such information is lacking in the study of sugarcane. This study aimed to investigate the root traits and physiological responses of sugarcane under drought stress and recovery conditions. Sugarcane genotypes with favorable photosynthesis and yield retention are expected to be promoted in regions under water deficits.
Materials and Methods
Plant Materials and Crop Management
The experiment was conducted in rhizoboxes under greenhouse conditions from February to May 2019 at the Agronomy Field Crop Research Station, Khon Kaen University, Khon Kaen, Thailand (16°28′N, 102°48′E, 200 m above sea level). A factorial experiment in completely randomized design with two replications was used. Factor A was three water regimes: full irrigation (control treatment), drought (withholding water beginning from 15 to 90 days after transplanting: DAT), and recovery (rewatering beginning from 76 to 90 DAT) and factor B were four sugarcane genotypes. Biotec 1, an interspecific hybridization crossed between Chainat 1 (Saccharum officinalum) and wild species (Saccharum spontaneum) (Department of Agriculture (Thailand), 2022), PR3067 is drought susceptible genotype with high reduction for root length has been reported by Namwongsa et al., (2019). The variety UT6, a commercial genotype and UT12, a drought susceptible genotype from Thailand (Khonghintaisong et al. 2020; Nawae et al. 2020).
At three days after bud germination, a single bud of each genotype was planted at the center of each box at the depth of 5 cm below the soil’s surface. Fertilizer (15-15-15) was applied at the rate of 1.56 g per rhizobox at 1 DAT, whereas the fertilizer grade 46-0-0 was applied at the rate of 1.56 g per rhizobox at 60 DAT.
Rhizobox Preparation and Irrigation Management
The measurements of root distribution and root architecture were conducted in the modified box-pinboard method (needle-board) previously described by Namwongsa et al (2019) and Thangthong et al (2017). Briefly, rhizoboxes 50 cm in width, 10 cm in thickness, and 120 cm in height were filled evenly with dry soil to a height of 115 cm. The packed soil was then divided into 11 layers at 10 cm intervals, starting from the bottom of the box to the top. The boxes contained a needle grid at the back of the box, spaced 5 × 5 cm, in which to hold the roots in their original position after washing. A transparent window at the front of each box allowed users to observe and photograph root growth and development.
Prior to transplanting, water was applied to all the rhizoboxes at field capacity (FC) to a depth of 5 cm for crop establishment for 14 days. Soil moisture content at FC were determined to be 12%, using the pressure plate method. At 15 DAT, drought treatment was imposed by withholding the water at 50% evapotranspiration (ET) through 90 DAT. Recovery treatment was imposed by withholding the water at 50% ET from 15 to 75 DAT, and the plants were rewatered to FC conditions through 90 DAT. The full irrigation treatment required water to be applied to the growing media at FC throughout the entire experiment.
The water requirements of sugarcane were calculated on a daily basis based on the crop water requirement. Water losses through plant transpiration and soil evaporation were determined by the Namwongsa et al (2019) procedure.
Soil Moisture Content
Soil moisture content was measured using the micro-auger gravimetric method at 14, 30,60, and 90 DAT at the soil depths of 15 and 15–30 cm (14 DAT); 0–15, 15–30, 30–45, 45–60, and 60–75 cm (30 and 60 DAT); and 10, 25, 45, 65, 85, and 105 cm (90 DAT), respectively. Soil moisture content was calculated through the following equation:
Physiological and Photosynthetic Traits
Physiological traits; including relative water content (RWC), SPAD chlorophyll meter reading (SCMR), and chlorophyll fluorescence (Fv/Fm) were measured at 60 (drought period) and 90 (recovery period) DAT. These parameters were recorded on the second or third fully expanded leaf from the top of the main stalk.
Relative water content (RWC) was determined as the weight difference between a harvested fresh leaf and a water saturated leaf (Chumphu et al. 2019), calculated through Eq. 2, below. Leaf samples were taken and recorded for leaf fresh weight. The leaf sample was then cut into three or four 2 × 2 cm pieces and then imbibed in distilled water for 24 h at a room temperature (25 °C). The water saturated leaves were dried with blotting paper and weighed to determine their saturated weights. The leaf samples were oven-dried at 80 °C for 72 h to obtain the leaf dry weight.
SCMR was measured via a SPAD-502 chlorophyll meter (Minolta SPAD-502 m, Tokyo, Japan). Chlorophyll fluorescence (Fv/Fm) was measured using a chlorophyll fluorescence meter (MINI-PAM-2000, Heinz Walz GmbH, Germany) between 09:00 and 12:00. The measured leaves were dark-adapted for 15 min using leaf clips (FL-DC, Opti-Science) before fluorescence measurements were taken. The chlorophyll fluorescence was determined following the procedures of Maxwell and Johnson (2000).
Net photosynthetic rate (Pn), stomatal conductance (gs), and transpiration rate (E) were measured at 90 DAT using a LI-6400 Portable Photosynthesis System (LI-COR Inc., Lincoln, Nebraska, USA). Light intensity and carbon dioxide concentration were set at 1,500 µmol m-2 s-1 and 400 µmol mol-1, respectively. Water use efficiency (WUE) for each treatment was calculated as the net photosynthetic rate divided by the transpiration rate (Pn/E) at 90 DAT.
Root Traits
Root traits were observed at 90 DAT and the roots harvested from the rhizoboxes were washed carefully with tap water. After washing, the needles were carefully removed from the roots. The root samples were then divided into smaller sizes (10 × 10 cm) and scanned with an Epson scanner (Perfection V700, Japan). Analyses of root length (RL), root surface area (RSA), root diameter (RD), and root volume (RV) were done through the WinRHIZO program (WinRHIZO Pro(s) V.2004a, Regent Instruments Inc).
Biomass Production
Sugarcane plant shoots were cut at the soil surface and separated into leaves and stems. Each sample was oven-dried at 80 ºC for 48 h or a constant weight was reached and, weighed. Similarly, the fresh roots which had been scanned were oven-dried at 80 ºC for 48 h and recorded as root dry weight. Biomass was calculated by adding the total shoot dry weight with the root dry weight.
Statistical Analysis
Data was compiled using Microsoft Excel 2019. Statistical software SigmaPlot 10.0 was utilized for data visualization.
Results
Soil Moisture Contents and Relative Leaf Water Contents of Four Sugarcane Genotypes following Full Irrigation, Drought Stress, and Recovery Treatments
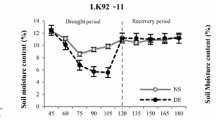
Soil moisture contents under full irrigation (FI), drought stress (DS), and recovery (RC) treatments are presented in Fig. 1. The soil moisture contents showed clear distinctions between full irrigation and drought treatments at 30 and 60 days after transplanting (DAT). When the imposed drought was applied, soil moisture content reduced from 11.00% to 7.70% at 30 DAT and declined even more to 3.39% at 60 DAT. Meanwhile under recovery treatment, the values decreased to 7.17% and 4.24%, while withholding water up to 30 and 60 DAT, respectively. However, following the recovery treatment beginning at day 76, the values recovered to field capacity by 90 DAT.
Relative water content (RWC) decreased as the imposed drought occurred for all tested genotypes at 90 DAT (Fig. 2). Drought reduced RWC by 24.3% compared to the full irrigation treatment. However, in some genotypes, the RWC recovered to levels like what was achieved with full irrigation. Interestingly, when the plant of genotype PR3067 exposed to drought was given water, the RWC recovered to levels as high as that under full irrigation, whereas plants of genotype Biotec 1, UT 6 and UT 12 did not.
Root Distribution Patterns of Four Sugarcane Genotypes following Full Irrigation, Drought Stress, and Recovery Treatments
Figure 3 displays photographs depicting the root distribution patterns of the whole root system on a black sheet of four sugarcane genotypes under full irrigation (FI), drought stress (DS), and recovery (RC) treatments. Drought reduced root growth in all four sugarcane genotypes, compared to the full irrigation treatment. In response to drought stress, all genotypes, particularly genotype UT 12, showed greatly reduced root growth in the upper soil layers (0–30 cm). However, root growth in the recovery treatment increased in both the upper soil layers (0–30 cm) and in the lower soil layers (30–110 cm) after recovery. For PR3067 and UT 12, root mass was found to increase in the lower soil layers rather than the upper soil layers. Additionally, the root growth of Biotec 1 and UT 6 fully recovered from drought, whereas that of UT 12 did not completely recover.
Responses of Root Traits of Four Sugarcane Genotypes following Full Irrigation, Drought Stress, and Recovery Treatments
Drought decreased root length (RL), root surface area (RSA), root diameter (RD), and root volume (RV) by 53%, 61.4%, 62.7%, and 74.2%, respectively; compared to that of the control treatment (Fig. 4). Those reductions varied among sugarcane genotypes. Genotype UT 12, for example, showed relatively high reductions in RL, RSA, RD, and RV under drought condition, whereas the UT6 and PR3067 genotypes showed less reduction in those traits under drought condition. Genotype Biotec 1 presented the highest reductions in RL, RSA, and RV, whereas RD decreased only slightly.
However, all root traits after rewatering showed a recovery to the control level (Fig. 4). The level of drought recovery illustrated by the root traits was genotype dependent. The Biotec 1 genotype had higher RL and RSA values than that under the full irrigation treatment. The RD and RV values were similar across both treatments. For genotype UT6, only two traits; RL and RD produced the increments required to reach values as high as that of the full irrigation treatment. In the remaining two genotypes, PR3067 and UT 12, the values of all root traits post drought recovery was less than those under the full irrigation treatment.
Responses of Physiological and Photosynthetic Traits of Four Sugarcane Genotypes following Full Irrigation, Drought Stress, and Recovery Treatments
SPAD chlorophyll meter reading (SCMR) and chlorophyll fluorescence (Fv/Fm) in all genotypes decreased under the drought treatment at 60 DAT. The lowest Fv/Fm and SCMR values were observed in genotypes PR3067 and UT 12, whereas the Biotec 1 genotype had the highest Fv/Fm and SCMR under drought conditions (Fig. 5). Both SCMR and Fv/Fm increased during the recovery period, corresponding to those under full irrigation at 90 DAT. Whereas he SCMR of all genotypes under recovery treatment was higher than that under full irrigation treatment at 90 DAT, for the Fv/Fm, all genotypes displayed levels similar to that in the full irrigation treatment.
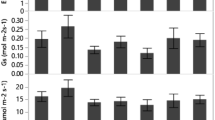
Net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (E), and water use efficiency (WUE) under the full irrigation, drought, and recovery treatments are presented in Fig. 6. Under full irrigation, genotypes UT6 and UT12 reported higher values of Pn, gs, and E than genotypes Biotec 1 and PR3067. Drought stress decreased the Pn, gs, and E of all genotypes. Genotype Biotec 1 under drought treatment revealed the lowest reductions among all genotypes. However, the Pn, gs, and E of all genotypes increased after rewatering.
Net photosynthetic rate (Pn), stomatal conductance (gs), transpiration rate (E), and water use efficiency (WUE) of four sugarcane genotypes under full irrigation (FI), drought stress (DS), and recovery (RC) conditions at 90 days after transplanting (DAT). The bars represent the standard error (SE) for different means
Water use efficiency (WUE) increased when early drought was imposed at 90 DAT for all sugarcane genotypes, except genotype PR3067 (Fig. 6). The WUE under drought treatment of genotypes UT6, Biotec 1, and UT12 increased to 17.4%, 8.2% and 4.3%, respectively; surpassing the values achieved under full irrigation. The WUE of genotypes UT6 and PR3067 in the recovery period was higher than that under full irrigation. Notably, the value of genotype UT12 in the recovery treatment declined after re-watering.
Biomass Production of Four Sugarcane Genotypes following Full Irrigation, Drought Stress, and Recovery Treatments
Shoot dry weight (SDW), root dry weight (RDW), and biomass at 90 DAT under full irrigation were higher than those under drought and recovery treatments for all sugarcane genotypes (Fig. 7). Genotype PR3067 had the highest SDW and biomass among sugarcane genotypes under full irrigation.
Drought stress reduced SDW, RDW, and biomass by 59.0%, 73.9%, and 62.6%, respectively. Two genotypes, PR3067 and UT6, showed smaller reductions than the average for biomass (46.0% and 47.4%), SDW (44.4% and 44.4%), and RDW (53.6%, 56.7%), respectively; whereas genotype UT 12 presented the highest reductions for biomass (89.4%), SDW (86.4%), and RDW (97.7%).
Recovery treatment led most genotypes to increase SDW, RDW, and biomass by 39.6%, 62.3%, and 44.5%, respectively, compared to the drought treatment. The recovery efficiency on those traits were highest in genotypes Biotec 1 and UT 6 and lowest in genotypes PR3067 and UT 12. However, all genotypes previously subjected to drought were unable to reach the same values for these traits as those in the full irrigation treatment.
Discussion
Plant Water Status of Four Sugarcane Genotypes in Response to Drought and Recovery
Soil moisture content indicates the amounts of actual water in the soil and how much water is lost when plants are subjected to drought. In this study, soil moisture content declined when severe drought was imposed at 60 DAT. Subsequently, soil moisture content increased to the control level (FI) after the recovery treatment at 90 DAT. Similarly, relative water content (RWC) decreased with increasing periods of drought. The RWC among genotypes in this study reduced only by approximately 24% following exposure to drought indicating the possibility of some level of drought tolerance among these genotypes. Silva et al (2011) noticed that highly drought-resistant genotypes maintain a higher RWC value under water-limited conditions. Genotype UT12 was susceptible to drought, because it showed a greater reduction in RWC during the drought period. Conversely, the Biotec 1 genotype was tolerant to drought indicated by a low reduction in RWC. These findings support the previous results of Namwongsa et al (2019) stating that drought-resistance sugarcane genotypes contain a high RWC. RWC was the physiological attribute that best differentiated resistant and susceptible genotypes (Silva et al. 2011). Additionally, the RWC value in the recovery treatment increased after rewatering. However, the RWC of stressed plants were unable to reach the level of the control. Marcos et al (2018) observed that RWC partially recovered after a few days of plant rehydration applied consecutively for four days. Thus, a complete recovery indicated by RWC may depend upon the pre-drought severity and the duration of the recovery.
Physiological and Photosynthetic Effects of Four Sugarcane Genotypes in Response to Drought and Recovery
Photosynthesis is an important physiological process for plant growth, development, and crop productivity. However, the process is limited by drought. Previous studies had reported that several physiological traits were suppressed when plants were subjected to drought during the early growth stage (Zhou et al. 2015; Silva et al. 2013; Devi et al. 2018). In our study, drought significantly altered sugarcane photosynthesis by reducing the SPAD chlorophyll meter reading (SCMR) and chlorophyll fluorescence (Fv/Fm), net photosynthetic rate (Pn), stomatal conductance (gs), and transpiration rate (E) of all sugarcane genotypes. Silva et al (2013) and Verma et al (2020) found that drought stress reduced stomata conductance, CO2 diffusion, photosynthetic pigments (SCMR), and photosynthetic rates in sugarcane. Moreover, the levels of reduction of photosynthetic traits varied among genotypes. Genotype Biotec 1, possessing low reductions in Fv/Fm, SCMR, Pn, gs, and E; maintained a high Pn under drought, although the stomatal conductance was limited by the water deficit. Still, partial stomatal closure led the plants under the water deficit to fix carbon and regulate photosynthesis; thereby increasing WUE under drought conditions (Yordanov et al. 2000; Lawlor and Tezara 2009; Pirasteh-Anosheh et al., 2016; Li et al. 2017). We, therefore, concluded that plants possessing a better control of the stomatal function were more drought-tolerant.
Lack of water directly affected the photosynthesis system, as water is the major donor of electrons to the Photosystem II (PSII); thus, any reduction in water content may decrease the electrochemical potential of ATP synthase (Pimentel et al. 2014). Photosystem II photochemical efficiency was determined by the Fv/Fm, in which values below 0.79 indicated that the PSII was damaged, leading to reduced photosynthetic potential (Maxwell and Johnson 2000). Our findings indicated that Photosystem II was inhibited by drought, as the values of Fv/Fm in all sugarcane genotypes were lower than 0.79, except genotype Biotec 1. The Biotec 1 genotype maintained a high photosynthetic rate, as its photosynthetic apparatus remained intact when the plants were exposed to drought (Devi et al. 2018). In contrast, genotypes PR3067 and UT 12 showed high reductions in Fv/Fm below 0.70 during the drought period, thus those genotypes were susceptible to drought. The genotype with a low Fv/Fm value was identified as a drought susceptible genotype (Silva et al. 2007; Devi et al. 2018).
After rewatering, the functions of photosynthesis and other physiological traits were restored. The SCMR fully recovered to the level reached in the full irrigation treatment. The Fv/Fm values also recovered, but could not be equal to the full irrigation treatment. The increases of SCMR and Fv/Fm may enhance photosynthesis after rehydration since those two parameters had highly positive correlations with the photosynthetic rates in sugarcane (Silva et al. 2013; Vilela et al. 2017; Devi et al. 2018). Our study revealed that the photosynthetic rate (Pn) fully recovered, and that the value was greater than that under the full irrigation treatment in certain genotypes. For example, the Pn of PR3067 was 30% higher than that under full irrigation. The ability of plants to recover from drought stress depends on the sugarcane genotype and traits (Devi et al. 2018). Our results indicated that different sugarcane genotypes had different physiological responses to drought and recovery. In addition, the capacity to maintain a high Fv/Fm ratio during drought and rehydration periods would likely optimize the Pn, resulting in high yield retention.
Root Distribution Patterns and Root Traits of Four Sugarcane Genotypes in Response to Drought and Recovery
The result indicated that water stress had more impact in altering the root pattern of each sugarcane genotype. We observed that root growth was distributed primarily at the lower soil layers where adequate soil moisture remained in contrast to the top soil layer where the soil moisture had diminished; corroborating the former studies of Puangbut et al (2018), Namwongsa et al (2019) and Set-Tow et al (2020). Sugarcane roots penetrate deeper into soil layers to absorb water (Namwongsa et al. 2019; Set-Tow et al. 2020); thus, the root architecture was further adjusted following the soil moisture gradients at different soil depths as a plant mechanism to avoid drought (Khonghintaisong et al. 2018; Namwongsa et al. 2019; Chumphu et al. 2019; Set-Tow et al. 2020). However, the root responses under drought conditions were genotype dependent. Genotype UT12 was more sensitive to drought stress as indicated by large reductions in all observed root traits and, therefore, was identified as a susceptible genotype according to the previous study by Nawae et al (2020) and Khonghintaisong et al (2020). Genotypes UT6 and PR3067 produced high root lengths and root surfaces, indicating that those genotypes had well-developed root systems essential for plants to absorb available water at deeper soil layers. Similarly, Hamedani et al (2020) noted that when water was scarce during the early growth stage, there was an increase in vertical root penetration and root length in deeper soil layers which facilitated better water uptake. We determined that genotypes UT6 and PR3067 were the most drought-tolerant genotypes based on the low reductions observed in the root characteristics. Increased root depth is a drought response that allows plants to obtain more accessible water. The study herein confirmed prior observations that improving a plant’s root system increased its potency to extract nutrients and increase water uptake capacity from lower soil layers under drought conditions (Comas et al. 2013; Paz1 et al. 2015; Khonghintaisong et al. 2018; Namwongsa et al. 2019).
After rewatering, the sugarcane roots of all tested genotypes were evenly distributed in both the upper and lower soil layers. Interestingly, the root growths of Biotec 1 and UT 6 were greatly enhanced in the upper and lower soil layers after recovery, whereas PR3067 and UT 12 produced increased root growth in only the lower soil layer. Superficial roots regenerated in the upper soil layer, as indicated by their white roots, allowed plants to absorb water and nutrients at that soil layer (Laclau and Lacla, 2009; Namwongsa et al. 2019). Root growth stimulated both root length and root distribution of all genotypes, except genotype UT 12, which did fully respond to the full irrigation treatment. The sugarcane root’s ability to recovery after experiencing drought depends upon the genotype (Khonghintaisong et al. 2018; Leanasawat et al. 2022). The recovery treatment’s findings revealed that both the root length and root surface area of genotype Biotec 1 increased, surpassing that under the full irrigation treatment. This demonstrated Biotec 1’s overcompensation response in root characteristics after a short period of rewatering. Increases in root length and root surface area due to rewatering would promote greater water uptake from the upper and lower soil layers, and eventually increase the biomass. Jangpromma et al (2012) reported that root length and root surface were important traits associated with biomass after drought recovery. Ohashi et al (2015) demonstrated that roots had high plasticity regarding their forms and sizes following dynamic soil conditions.
Water Use Efficiency and Total Dry Matter of Four Sugarcane Genotypes in Response To Drought and Recovery
Our findings showed that water use efficiency (WUE) increased with early drought, corroborating the previous study of Leanasawat et al (2022). When the supply of water was limited, drought-tolerant sugarcane genotypes had higher WUE than that grown under well-watered conditions, but this will not happen for drought-susceptible genotypes which consistently had lower WUE under both conditions (Silva et al. 2013). The reason for this was that the stomatal closure was enhanced under drought conditions which prevented excessive water loss via transpiration, but may also reduce CO2 uptake for photosynthesis, thereby adversely influencing plant productivity and water use efficiency (Lawson and Vialet-Chabrand 2019; Puangbut et al. 2022). However, drought-tolerant plant species can establish a balance level between water loss and carbon gain by optimizing their CO2 uptake for photosynthesis, while minimizing water loss and improving water use efficiency (Silva et al. 2013; Li et al. 2017; Wu et al. 2018; Puangbut et al. 2022). Our results indicated that the balance between carbon gain in photosynthesis and water loss via transpiration contributed to water use efficiency (Xu and Zhou, 2008; Bertolino et al. 2019). A significant increment of WUE was noticed under rainfed conditions, and stomatal adjustment played an important role in stabilizing CO2 fixation and transpiration (Leanasawat et al. 2022; Pirasteh‐Anosheh et al., 2018); thus, the photosynthetic capacity could be retained (Lawson and Vialet-Chabrand 2019).
WUE also increased in all sugarcane genotypes when the drought was relieved. The improved WUE after rewatering may be due to a high photosynthetic rate and low transpiration (Leanasawat et al. 2022; Puangbut et al. 2022). Furthermore, during and after rewatering, sugarcane roots can absorb more water and nutrients to promote plant growth while minimizing water loss via transpiration (Khonghintaisong et al. 2018). The responses of the roots and photosynthetic traits after rewatering were important to determine water use efficiency and total biomass.
We observed that biomass decreased due to early drought in all sugarcane genotypes, corroborating the works of Khonghintaisong et al (2018) and Leanasawat et al (2022) that reported that early drought might adversely influence sugarcane growth, biomass and cane production under both greenhouse and field conditions. Our findings also indicated that sugarcane biomass previously subjected to early drought and followed by rewatering could not grow and develop optimally as that under the full irrigation treatment, due to the short 14-day recovery period. Previous research had indicated that the period of recovery and drought duration impacted the partitioning of assimilates between roots and shoots (Jangpromma et al. 2012; Khonghintaisong et al. 2018). Given the short recovery period, the assimilates were most likely translocated for root growth rather than stalk and leaf growth in order to increase the root’s ability to absorb water from the soil and promote plant recovery. However, due to the short recovery period, root characteristics may not fully recover, causing severe disruption to the biomass. This confirmed previous studies that sugarcane biomass could not fully recover from drought stress despite rewatering (Khonghintaisong et al. 2018; Leanasawat et al. 2022). However, Biotec 1 and UT 6 showed higher biomass recovery ability, whereas UT 12 had the lowest. These results supported the previous findings of Nawae et al (2020) that reported that the UT 12 genotype was drought susceptible, significantly reducing yields. The results herein also agreed with previous reports illustrating that the recovery period length and the severity of early drought may share significant contributions in the plant’s ability to recover.
SCMR, root length, and chlorophyl fluorescence were identified as photosynthetic traits affected by drought whose regulation when under drought condition can affect biomass and cane yield (Silva et al. 2011, 2013; Khonghintaisong et al. 2018; Namwongsa et al. 2019; Khonghintaisong et al., 2020). Our study also determined that sugarcane genotypes with high SCMR paired with improved root growth and root length could maintain high photosynthetic values and biomass, as well as a higher recovery ability for those traits. The Biotec 1 and UT 6 herein were found to have good recovery in drought resistant traits. This suggested that there was a relationship between photosynthesis and root traits in sugarcane genotypes.
Conclusion
Early drought reduced the physiological, photosynthetic, and root traits in all sugarcane genotypes. However, Biotec 1 and UT 6 displayed low reductions in all traits. After recovery, the mean values of all observed traits increased, yet were still below those under full irrigation, except for SCMR, Pn, and WUE. Response of sugarcane to drought stress and recovery from drought stress is genotype dependent. The Biotec 1 genotype presented good recovery potential in SCMR, Fv/Fm and RL which recovered to reach the levels of full irrigation but Pn and WUE were unable to achieve the level of the control. Notably, UT 6, SCMR, Fv/Fm, Pn, RL and WUE fully recovered to the level achieved in the full irrigation. While the PR3067 and UT 12 genotypes showed good recovery for SCMR, Fv/Fm, and Pn; they did not completely recover for all root traits and WUE. Additionally, Biotec 1 and UT 6 had high recovery efficiency for biomass, whereas PR3067 and UT 12 displayed low recovery efficiency. Furthermore, sugarcane genotypes with high SCMR and increased root length maintained good photosynthetic and total biomass. As a result, selection based on physiological, photosynthetic, and root traits may be helpful in enhancing drought resistance and recovery potential in sugarcane. In a large-scale breeding effort, these traits could be used as selection criteria for drought resistance in sugarcane germplasm under early drought conditions.
References
Bertolino, L.T., R.S. Caine, and J.E. Gray. 2019. Impact of stomatal density and morphology on water-use efficiency in a changing world. Frontiers in Plant Science 10: 225. https://doi.org/10.3389/fpls.2019.00225.
Chumphu, S., N. Jongrungklang, and P. Songsri. 2019. Association of physiological responses and root distribution patterns of ratooning ability and yield of the second ratoon cane in sugarcane elite clones. Agronomy 9 (4): 200. https://doi.org/10.3390/agronomy9040200.
Comas, L.H., S.R. Becker, V.V. Cruz, P.F. Byrne, and D.A. Dierig. 2013. Root traits contributing to plant productivity under drought. Frontiers in Plant Science 4: 442. https://doi.org/10.3389/fpls.2013.00442.
Department of Agriculture (Thailand). 2022. Sugarcane breeding manual. https://www.doa.go.th/fcri/wp-content/uploads/2022/smart-box/sugar-cane2565.pdf. Accessed 18 April 2023.
Devi, K., R. Gomathi, R.A. Kumar, R. Manimekalai, and A. Selvi. 2018. Field tolerance and recovery potential of sugarcane varieties subjected to drought. Indian Journal of Plant Physiology 23 (2): 271–282. https://doi.org/10.1007/s40502-018-0367-7.
Dinh, H.T., K. Watanable, H. Takaragaw, and Y. Kawamitsu. 2017. Effects of drought stress at early growth stage on response of sugarcane to different nitrogen application. Sugar Tech 20: 420–430. https://doi.org/10.1007/s12355-017-0566-y.
Fang, Y., Y. Du, J. Wang, A. Wu, S. Qiao, B. Xu, S. Zhang, K.H.M. Siddique, Y. Chen, and Y. 2017. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Frontiers in Plant Science 8: 672–680. https://doi.org/10.3389/fpls.2017.00672.
Food and Agriculture Organization (FAO). 2022. Available online: http://www.fao.org (accessed on 18 May 2022).
Gano, B., J.S.B. Dembele, A. Ndour, D. Luquet, G. Beurier, D. Diouf, and A. Audebert. 2021. Using UAV borne, multi-spectral imaging for the field phenotyping of shoot biomass, leaf area index and height of West African sorghum varieties under two contrasted water conditions. Agronomy 11 (5): 850. https://doi.org/10.3390/agronomy11050850.
Graça, J.P., F.A. Rodrigues, J.R.B. Farias, M.C.N. Oliveira, C.B. Hoffmann-Campo, and S.M. Zingaretti. 2010. Physiological parameters in sugarcane cultivars submitted to water deficit. Brazilian Journal of Plant Physiology 22 (3): 189–197. https://doi.org/10.1590/S1677-04202010000300006.
Hamedani, N.G., M. Gholamhoseini, F. Bazrafshan, B. Amiri, and F. Habibzadeh. 2020. Variability of root traits in sesame genotypes under different irrigation regimes. Rhizosphere 13: 100190. https://doi.org/10.1016/j.rhisph.2020.100190.
Jangpromma, N., S. Thammasirirak, P. Jaisil, and P. Songsri. 2012. Effects of drought and recovery from drought stress on above ground and root growth, and water use efficiency in sugarcane Saccharum officinarum L. Australian Journal of Crop Science 6 (8): 1298–1304.
Khonghintaisong, J., P. Songsri, B. Toomsan, and J. Jongrungklang. 2018. Rooting and physiological trait responses to early drought stress of sugarcane cultivars. Sugar Tech 20: 396–406. https://doi.org/10.1007/s12355-017-0564-0.
Khonghintaisong, J., P. Songsri, and N. Jongrungklang. 2020. Root characteristics of individual tillers and the relationships with above-ground growth and dry matter accumulation in sugarcane. Pakistan Journal of Botany 52: 101–109.
Laclau, P.B., and J.P. Laclau. 2009. Growth of the whole root system for a plant crop of sugarcane under rainfed and irrigated environments in Brazil. Field Crops Research 114 (3): 351–360. https://doi.org/10.1016/j.fcr.2009.09.004.
Lavinsky, A.O., P.C. Magalhães, M.M. Diniz, C.C. Gomes-Jr, E.M. Castro, and R. Ávila. 2016. Root system traits and its relationship with photosynthesis and productivity in four maize genotypes under drought. Cereal Research Communications 44 (1): 1–9. https://doi.org/10.1556/0806.43.2015.029.
Lawlor, D.W., and W. Tezara. 2009. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: A critical evaluation of mechanisms and integration of processes. Annals of Botany 103 (4): 561–579. https://doi.org/10.1093/aob/mcn244.
Lawson, T., and S. Vialet-Chabrand. 2019. Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist 221: 93–98. https://doi.org/10.1111/nph.15330.
Leanasawat, N., M. Kosittrakun, W. Lontom, and P. Songsri. 2022. Physiological and agronomic traits of certain sugarcane genotypes grown under field conditions as influenced by early drought stress. Agronomy 11 (11): 2319. https://doi.org/10.3390/agronomy11112319.
Li, Y., H. Li, Y. Li, and S. Zhang. 2017. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought resistant wheat. The Crop Journal 5: 231–239. https://doi.org/10.1016/j.cj.2017.01.001.
Marcos, F.C., N.M. Silveira, P.E. Marchiori, E.C. Machado, G.M. Souza, M.G.A. Landell, and R.V. Ribeiro. 2018. Drought tolerance of sugarcane propagules is improved when origin material faces water deficit. PLoS ONE 13 (12): e0206716. https://doi.org/10.1371/journal.pone.0206716.
Maxwell, K., and G.N. Johnson. 2000. Chlorophyll fluorescence-a practical guide. Journal of Experimental Botany 51 (345): 659–668. https://doi.org/10.1093/jexbot/51.345.659.
Namwongsa, J., N. Jongrungklang, and P. Songsri. 2019. Genotypic variation in root distribution changes and physiological responses of sugarcane induced by drought stress. SABRAO Journal of Breeding and Genetics 51: 470–493. https://doi.org/10.1101/503912.
Nawae, W., J.R. Shearman, S. Tangphatsornruang, P. Punpee, T. Yoocha, D. Sangsrakru, C. Naktang, C. Sonthirod, W. Wirojsirasak, K. Ukoskit, K. Sriroth, P. Klomsa-ard, and W. Pootakham. 2020. Differential expression between drought-tolerant and drought-sensitive sugarcane under mild and moderate water stress as revealed by a comparative analysis of leaf transcriptome. Peer, J 8: e9608. https://doi.org/10.7717/peerj.9608.
Office of the cane and sugar board, (OCSB). 2022. Available online: http://www.ocsb.go.th/ (accessed on 18 May 2022).
Ohashi, A.Y.P., R.C.D.M. Pires, R.V. Ribeiro, and A.L.B.D.O. Silva. 2015. Root growth and distribution in sugarcane cultivars fertigated by a subsurface drip system. Bragantia 74: 131–138. https://doi.org/10.1590/1678-4499.0295.
Paz, H., F. Pineda-García, and L.F. Pinzón-Pérez. 2015. Root depth and morphology in response to soil drought: comparing ecological groups along the secondary succession in a tropical dry forest. Oecologia 179: 551–561. https://doi.org/10.1007/s00442-015-3359-6.
Pimentel, P., R.D. Almada, A. Salvatierra, G. Toro, M.J. Arismendi, M.T. Pino, B. Sagredoa, and M. Pinto. 2014. Physiological and morphological responses of Prunus species with different degree of tolerance to long-term root hypoxia. Scientia Horticulturae 180: 14–23. https://doi.org/10.1016/j.scienta.2014.09.055.
Pirasteh‐Anosheh, H., A. Saed‐Moucheshi, H. Pakniyat, and M. Pessarakli. (2016). Stomatal responses to drought stress, In Water Stress and Crop Plants: A Sustainable Approach, 1st ed; Ahmad, P., Ed; John Wiley & Sons, New Jersey USA 3: 24–40. https://doi.org/10.1002/9781119054450.ch3.
Pirnajmedin, F., M.M. Majidi, and M. Gheysari. 2015. Root and physiological characteristics associated with drought tolerance in Iranian tall fescue. Euphytica 202: 141–155. https://doi.org/10.1007/s10681-014-1239-5.
Puangbut, D., S. Jogloy, N. Vorasoot, and K. Craig. 2018. Root distribution pattern and their contribution in photosynthesis and biomass in Jerusalem artichoke under drought conditions. Pakistan Journal of Botany 50: 879–886.
Puangbut, D., S. Jogloy, N. Vorasoot, and P. Songsri. 2022. Photosynthetic and physiological responses to drought of Jerusalem artichoke genotypes differing in drought resistance. Agricultural Water Management 259: 107252. https://doi.org/10.1016/j.agwat.2021.107252.
Robertson, M.J., N.G. Inman-Bamber, R.C. Muchow, and A.W. Wood. 1999. Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crops Research 64: 211–227. https://doi.org/10.1016/S0378-4290(99)00042-8.
Set-Tow, S., P. Songsri, and N. Jongrungklang. 2020. Variations in root distribution patterns and cane yield of 16 elite sugarcane clones grown under varied soil conditions. Sugar Tech 22 (6): 1018–1031. https://doi.org/10.1007/s12355-020-00834-x.
Silva, M.A., J.L. Jifon, J.A.G.D. Silva, and V. Sharma. 2007. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Brazilian Journal of Plant Physiology 19 (3): 193–201. https://doi.org/10.1590/S1677-04202007000300003.
Silva, M.D.A., J.A.G.D. Silva, J. Enciso, V. Sharma, and J. Jifon. 2008. Yield components as indicators of drought tolerance of sugarcane. Scientia Agricola 65: 620–627. https://doi.org/10.1590/S0103-90162008000600008.
Silva, M.D.A., J.L. Jifon, V. Sharma, J.A.G.D. Silva, M.M. Caputo, M.B. Damaj, E.R. Guimarães, and M.I.T. Ferro. 2011. Use of physiological parameters in screening drought tolerance in sugarcane genotypes. Sugar Tech 13 (3): 191–197. https://doi.org/10.1007/s12355-011-0087-z.
Silva, M.A., J.L. Jifon, C.M. Santos, C.J. Jadoski, and J.A.G.D. Silva. 2013. Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Brazilian Archives of Biology and Technology 56 (5): 735–748. https://doi.org/10.1590/S1516-89132013000500004.
Singh, S.P., M. Jawaid, M. Chandrasekar, K. Senthilkumar, B. Yadav, N. Saba, and S. Siengchin. 2021. Sugarcane wastes into commercial products: Processing methods production optimization and challenges. Journal of Cleaner Production 328: 129453. https://doi.org/10.1016/j.jclepro.2021.129453.
Souza, A.P., A. Grandis, D.C.C. Leite, and M.S. Buckeridge. 2014. Sugarcane as a bioenergy source: History performance and perspectives for second-generation bioethanol. BioEnergy Research 7 (1): 24–35. https://doi.org/10.1007/s12155-013-9366-8.
Thangthong, N., S. Jogloy, N. Jongrungklang, C.K. Kvien, V. Pensuk, T. Kesmala, and N. Vorasoot. 2017. Root distribution patterns of peanut genotypes with different drought resistance levels under early-season drought stress. Journal of Agronomy and Crop Science 204: 111–122. https://doi.org/10.1111/jac.12249.
Verma, K.K., X. Song, Y. Zeng, D. Li, D. Guo, V.D. Rajput, G. Chen, A. Barakhov, T.M. Minkina, and Y. Li. 2020. Characteristics of leaf stomata and their relationship with photosynthesis in Saccharum officinarum under drought and silicon application. ACS Omega 5 (37): 24145–24153. https://doi.org/10.1021/acsomega.0c03820.
Vilela, R.D., B.K.L. Bezerra, A. Froehlich, and L. Endres. 2017. Antioxidant system is essential to increase drought tolerance of sugarcane. Annals of Applied Biology 171: 451–463. https://doi.org/10.1111/aab.12387.
Wu, G., H. Liu, L. Hua, Q. Luo, Y. Lin, P. He, S. Feng, J. Liu, and Q. Ye. 2018. Differential responses of stomata and photosynthesis to elevated temperature in two co-occurring subtropical forest tree species. Frontiers in Plant Science 9: 467. https://doi.org/10.3389/fpls.2018.00467.
Xu, Z., and G. Zhou. 2008. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. Journal of Experimental Botany 59 (12): 3317–3325. https://doi.org/10.1093/jxb/ern185.
Yordanov, I., V. Velikova, and T. Tsonev. 2000. Plant responses to drought acclimation and stress tolerance. Photosynthetica 38 (2): 171–186. https://doi.org/10.1023/A:1007201411474.
Zhang, Z., H. Ding, T. Kang, L. Daia, D. Cia, F. Qina, and W. Song. 2017. Rooting traits of peanut genotypes differing in drought tolerance under drought stress. International Journal of Plant Production 5: 349–360.
Zhou, L., S. Wang, Y. Chi, Q. Li, K. Huang, and Q. Yu. 2015. Responses of photosynthetic parameters to drought in subtropical forest ecosystem of China. Scientific Reports 5 (1): 1–11. https://doi.org/10.1038/srep18254.
Acknowledgements
This research was fund by the Northeast Thailand Cane and Sugar Research Center (NECS), Faculty of Agriculture, Khon Kaen University, Thailand.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.S., D.P. and N.J.; performed the analyses and collected the data, P.C.; writing original draft preparation, P.C. and D.P.; writing review and editing, P.S. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chanaphai, P., Jongrungklang, N., Puangbut, D. et al. Response of Photosynthetic and Root Traits of Sugarcane Genotypes Under Drought and Recovery Conditions. Sugar Tech 25, 1102–1114 (2023). https://doi.org/10.1007/s12355-023-01288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-023-01288-7