Abstract
Changes in the contents and composition of polyphenolics and resulting antioxidant activities of S. verbenaca by-products were investigated at three phenological stages (flowering, early fruiting and late fruiting stages). The highest accumulation of total phenolics was detected at the flowering stage (58.36 mg GAE/g DW). HPLC analysis of methanolic extracts showed the prevalence of methyl carnosate (821.45–919.82 μg/g DW) and rosmarinic acid (544.51–649.26 μg/g DW). Phenolic diterpenes (1056.90–1148.42 μg/g DW) was the most represented class of compounds. Three complementary tests namely, DPPH• (IC50 value, 49.22 μg/mL) and ABTS•+-radical scavenging assays (146.86 μM TE/mg) and FRAP reducing power test (188.93 mM Fe(II)/mg) were used to evaluate the antioxidant capacity and showed the best performance at the early fruiting period. The current study evidenced the significant effect of phenophase on antioxidants and contributed to valorize S. verbenaca extracts as a source of functional phenolic compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of excessive quantities of reactive oxygen species in biological systems induce oxidative stress that may cause cell membrane dysfunction and DNA damage. Many degenerative disorders, namely cardiovascular and brain diseases, arthritis, diabetes, cancer and immune system decline involve cellular damage possibly caused by free radicals [1]. Plant antioxidants, particularly polyphenolics and antioxidant vitamins, exercise a protective action [2, 3] against several pathologies by acting as free radical scavengers and metal chelators [4], thus decreasing the extent of oxidative damage [1]. Polyphenols have been associated with several important functions in plants including protection from UV radiation, pigmentation, defense against invading pathogens, nodule production and attraction of pollinators and seed dispersers [5, 6]. These secondary metabolites contribute to improve the public health by exhibiting antibacterial, antiviral, antifungal, antiinflammatory, antidiarrheal and antiulcer properties, among other pharmacological activities [1, 7]. So, plant extracts and their components gained attention and were considered for extensive investigations.
Species of the genus Salvia are widespread in the Mediterranean region, South-East Asia and Central America. They constitute an important genus of the Lamiaceae family, reputed for their medicinal properties since they have been used in folk medicine for a long time. Salvia species are well known for possessing many polyphenolic compounds with significant benefits to human nutrition and health [1, 8–11]. Plants of the genus are particularly rich in diterpenoids, triterpenoids, phenolic acids and flavonoids.
Variations in climate and environmental stress imposed on plants during their growth cycle could have significant impact on concentrations of polyphenolics and consequently on the antioxidant performance. To our knowledge, there are no published reports about the influence of phenophase on antioxidants of S. verbenaca extracts. The present study aimed to determine the total phenolic content, the phenolic composition and the antioxidant capacity of S. verbenaca post-distilled aerial parts at different phenological periods.
Materials and Methods
Plant Material and Experimental Field
Salvia verbenaca L. seeds were collected in the Northeastern Tunisia, in the coastal region of Chott Meriem in May 2007. The collection site (35°53′ N, 10°35′ W, 8 m above the sea) was characterised by a lower semi-arid climate with an annual average temperature of 18 °C and rainfall of 300 mm.
S. verbenaca seeds were germinated and the plantlets were grown under greenhouse conditions for 3 months. Then, the plants were transplanted in the experimental field of the IMIDA (Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario; 37°47′N, 0°54′W, 30 m above the sea), in the region of Torreblanca (Murcia, Spain). The experimental area was characterised by an annual average temperature of 18.2 °C and rainfall of 308.3 mm in a semi-arid bioclimatic stage.
Plant aerial parts were harvested in 2009 and the flowering, early fruiting and late fruiting phenological stages were considered for the evaluation of the phenolic contents and antioxidant capacities of cultivated S. verbenaca plants.
Sample Preparation and Extraction
Aerial parts of S. verbenaca were dried in the oven at 35 °C until it reached a constant weight. The dried samples were submitted to hydrodistillation for 3 h using a Clevenger-type apparatus. Distilled plant material was recuperated, dried at 35 °C and subsequently finely ground to pass a 2 mm sieve. The ground aerial parts (0.5 g) were homogenised with 30 mL of petroleum ether under magnetic stirring for 5 min and taken to dryness at room temperature. The polyphenolic constituents of S. verbenaca were extracted by using 150 mL of methanol in a Soxhlet extractor (B-811) (Büchi, Flawil, Switzerland), for 2 h under an atmospheric nitrogen. Methanolic extracts were dried at 40 °C under vacuum conditions in an evaporator system (Syncore Polyvap R-96) (Büchi, Flawil, Switzerland). The dried residues were redissolved in methanol and made up to 5 mL. The extracts were kept in vials at −80 °C until their corresponding analysis.
Estimation of Total Phenolic Contents
The total phenolic content of the extracts was measured using the Folin–Ciocalteu colorimetric method according to the procedure of Singleton and Rossi [12]. Quantitative determination of phenolic contents was performed using a standard calibration curve of concentrations ranging from 25 to 300 mg/L of gallic acid and results were expressed as gallic acid equivalents (GAE) in milligrams per gram of dry plant material weight. The method was performed by adding to an aliquot of 15 μL of plant methanolic extract, 1185 μL of distilled water and 75 μL of Folin–Ciocalteu reagent. A vigorous stirring was performed and 225 μL of a solution of sodium carbonate (20 %) were added. The mixture was allowed to stand for 2 h and the absorbance of the resulting blue-coloured solution was measured at 765 nm and 25 °C with a Shimadzu (UV-2401PC, Japan) spectrophotometer. The experiment was carried out in triplicate.
HPLC Analysis of Phenolic Compounds
The most representative polyphenolic compounds in S. verbenaca aerial parts were determined using a reversed-phase high-performance liquid chromatography (RP-HPLC) according to a procedure adapted from Zheng and Wang [13]. the analysis was carried out on a Zorbax SB-C18 column (4.6 × 250 mm, 5 μm pore size, Hewlett Packard, USA) using a guard column (Zorbax SB-C18 4.6 × 125 mm, 5 μm pore size, Hewlett Packard, USA) at ambient temperature. Plant extracts were passed through a 0.45 μm filter (Millipore SAS, Molsheim, France) and 20 μL was injected in a Hewlett Packard (Germany) system equipped with a G1311A quaternary pump and G1315A photodiode array UV–vis detector. The mobile phase was acetonitrile (A) and acidified water containing 5 % formic acid (B). The gradient was as follows: 0 min, 5 % A; 10 min, 15 % A; 30 min, 25 % A; 35 min, 30 % A; 50 min, 55 % A; 55 min, 90 % A; 57 min, 100 % A and then held for 10 min before returning to the initial conditions. The flow rate was 1.0 mL/min and the wavelengths of detection were set at 280 and 330 nm. The phenolic constituents were characterised by comparison of retention times and spectra with those of commercially available standard compounds and quantitative measurements were made using linear regression models.
DPPH• Radical-Scavenging Activity
Free radical scavenging ability of S. verbenaca methanolic extracts was measured by bleaching of the purple-coloured solution of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), according to the method described by Brand-Williams et al. [14]. Methanolic extracts (500 μL) at different concentrations (5 to 120 μL/mL) were added to 1 mL of DPPH• methanolic solution (0.1 mM). The decrease in absorbance was measured using a Shimadzu (UV-2401PC, Japan) spectrophotometer at 517 nm after incubation for 20 min at room temperature in the dark. Absorbance was measured against a blank of 500 μL of sample plus 1 mL of methanol. The absorbance of the control consisting of 500 μL of methanol and 1 mL of DPPH• solution was measured daily against a blank of 1.5 mL of methanol. The antiradical activity was expressed as IC50, the inhibitory concentration of the extract necessary to decrease 50 % of the DPPH• absorbance. A lower IC50 value corresponds to a higher antioxidant activity of plant extract. Triplicate measurements were performed and the ability to scavenge the DPPH• radical was calculated using the following formula:
ABTS•+ Radical Cation Decoloration Assay
The method described by Re et al. [15] was adopted to evaluate the ABTS•+ free radical-scavenging activity of S. verbenaca methanolic extracts. ABTS•+ radical cation was produced by reacting 7 mM ABTS•+ solution with 2.45 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 16 h before use. The solution was diluted by using ethanol to an absorbance of 0.70 (±0.02) nm (constant initial absorbance value used for standard and samples) at 734 nm and 30 °C. An aliquot (15 μL) of each sample (with appropriate dilution) or Trolox standard was mixed with the solution (1.5 mL) of ABTS•+, and the decrease of absorbance was measured after 6 min at 734 nm using a Shimadzu (UV-2401PC, Japan) spectrophotometer. Triplicate measurements were performed and the ABTS•+ scavenging rate was calculated to express the antioxidant ability of the herbal extracts.
Ferric Reducing Antioxidant Power (FRAP)
The reducing powers of S. verbenaca methanolic extracts were estimated by using the method of Benzie and Strain [16]. The FRAP reagent was freshly prepared by using 300 mM acetate buffer (pH 3.6), 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) made up in 40 mM HCl and 20 mM FeCl3 ∙ 6H2O solution. All three solutions were mixed together in the ratio of 10:1:1(v/v/v). An aliquot of 40 μL of each sample (with appropriate dilution) was added to 1.2 mL of FRAP reagent. The absorption of the reaction mixture was measured at 593 nm after 2 min incubation at 37 °C. Measurements were performed in triplicate. The fresh solutions of known Fe (II) concentrations (FeSO4 ∙ 7H2O) of (0–2 mM) were used for calibration. The antioxidant capacity based on the ability to reduce ferric ions of samples was calculated from the linear calibration curve.
Statistical Analysis
Statistical analysis was processed with the computer programs Excel and STATISTICA software version 5.1. Results were reported as a mean ± standard deviation of at least three experiments. The significance of the differences between various experiments was analysed by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests. Pearson’s correlation coefficients were calculated to assess the relationships between individual and grouped phenolics. A p value less than 0.05 was considered to be statistically significant.
Results and Discussion
Variations of Total Phenolic Contents
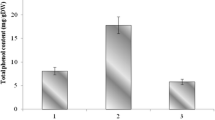
As shown in electronic supplementary material Fig. 1, concentrations of total phenols of S. verbenaca extracts were measured as gallic acid equivalents in milligrams per gram of dry plant material weight (mg GAE/g DW). The results revealed significant differences (p < 0.05) in total phenols content in S. verbenaca methanolic extracts depending on the harvesting time. The highest total phenolic content was detected at the flowering period (58.36 mg GAE/g DW). A significant (p < 0.05) decrease of total phenols, approximatively by the half, characterised the early fruiting (29.78 mg GAE/g DW). At late fruiting, polyphenolic content showed an increase by almost 25 % (41.36 mg GAE/g DW). It is well documented that Salvia species are a rich source of polyphenols. Besides, plant extracts of essential oil distillation gained attention with their high phenolic content. Previously, Ben Farhat et al. [17, 18] reported a good total phenolic content in post-distilled plant extracts with values ranging from 55.03 to 136.33 mg GAE/g DW in S. verbenaca post-distilled material and from 41.47 to 48.90 mg GAE/g DW in S. argentea by-products.
Changes in the Contents of Phenolic Compounds
Methanolic extract yields were expressed in terms of milligrams of dry methanolic extract weight per gram of dry plant weight (electronic supplementary material Fig. 1). S. verbenaca extract yields increased significantly (p < 0.05) from the flowering to reach the highest value at late fruiting (130.61 mg/g DW).
Polyphenol qualitative and quantitative determination in S. verbenaca extracts were assessed by RP-HPLC coupled with an UV-visible multi-wavelength detector. The analysis revealed a chemical profile composed of 18 individual phenolic compounds (electronic supplementary material Table 1). In S. verbenaca methanolic extracts, identified polyphenols exist as phenolic acids (gallic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid and rosmarinic acid), phenolic diterpenes (carnosic acid, carnosol, methyl carnosate), flavanones (naringenin, naringin), flavones (luteolin, cirsiliol, apigenin, cirsilineol, genkwanin) and flavone glycosides (apigenin-7-glucoside). In general, previous investigations on polyphenolics of Salvia species indicated the existence of polyphenolic flavonoids and phenolic acids [9]. The majority of flavonoids were classified into flavones, flavonols and their glycosides and phenolic acids were exclusively those based on caffeic acid building block with compounds formed from two to four or more caffeic acid units [9]. The abietane-type diterpenoids were also reported in Salvia species [8, 10].
The concentrations of the identified phenolic compounds were expressed as micrograms per gram of dry plant material (electronic supplementary material Table 1). The total phenolics assessed by HPLC was calculated as the sum of the identified individual phenolics. Its amount was the highest at flowering (2214.66 μg/g DW) and early fruiting (2180.42 μg/g DW) and showed a significant (p < 0.05) decrease at late fruiting (2009.57 μg/g DW). Such variation could be partly explained by the physiological and ecological functions ensured by polyphenolics. At flowering, accumulation of these secondary metabolites ensure the protection of the flowering parts and the attraction of pollinators [19, 20]. Methyl carnosate and rosmarinic acid were determined as major phenolics in S. verbenaca extracts at the three analyzed harvesting times. Methyl carnosate was detected in amounts ranging from 821.45 to 919.82 μg/g DW with the highest value showed at flowering. Rosmarinic acid displayed its lowest content at first harvest period (544.51 μg/g DW), then increased significantly (p < 0.05) to reach the highest amount (649.26 μg/g DW) at early fruiting. In a previous study, Ben Farhat et al. [17] established rosmarinic acid (349.60–2560.37 μg/g DW) followed by naringenin (254.82–2432.55 μg/g DW) and methyl carnosate (220.58–1159.73 μg/g DW) as the main phenolics, with different ranges of concentrations, in S. verbenaca post-distilled materiel collected from several tunisian regions. Non-treated plants of S. verbenaca were characterised by much larger concentrations of rosmarinic acid (26,120 μg/g DW) [21]. Studies on harvesting time influence on phenolic composition of S. officinalis revealed significant variation in contents of rosmarinic acid showing concentrations of 15,148 mg/L in February and 19,347 mg/L in May, representing amounts more than 2.5 times higher than August and November extracts [7]. Sellami et al. [22] reported the highest content of rosmarinic acid (49.30 mg/100 g DW) at late vegetative stage for a member of the Lamiaceae namely, Origanum majorana. Also, Munné-Bosch et al. [23] studied seasonal variations of S. officinalis diterpenes and revealed that carnosic acid, methyl carnosate and carnosol contents decreased gradually from June to reach their lowest concentrations in November. According to the authors, the diterpenes decrease is concomitant to the progress of the drought period and is related to the antioxidative protection conferred by these compounds to S. officinalis plants [23].
Contents of the remaining phenolic acids, phenolic diterpenes and flavonoids were significantly smaller than that of methyl carnosate and rosmarinic acid. The highest levels of carnosic acid (199.60 μg/g DW) and p-hydroxybenzoic acid (83.62 μg/g DW) were found at early fruiting. Vanillic acid, caffeic acid, p-coumaric acid and cirsilineol contents of S. verbenaca post-distilled aerial parts at flowering were 24.63, 68.75, 16.42 and 24.65 μg/g DW, respectively, and then displayed a progessive significant (p < 0.05) decrease during the fruiting period. In contrast, the content of carnosol approximatively doubled at the early fruiting (49.87 μg/g DW) and reached its highest level at late fruiting (66.50 μg/g DW). Several flavonoids namely, apigenin-7-glucoside, naringenin, luteolin, cirsiliol and apigenin showed an initial decrease from the flowering period to reach the lowest amounts at early fruiting but then increased their contents at the late fruiting. Both gallic acid and genkwanin were not detected at the flowering stage, however, naringin revealed an amount of 16.84 μg/g DW at the same period and observed a decline in the course of the fruiting period.
As shown in electronic supplementary material Table 1, phenolic acids and phenolic diterpenes showed the highest amounts at early fruiting with the values of 867.81 μg/g DW and 1148.42 μg/g DW, respectively, whereas, flavonoids showed its highest contents at flowering (403.79 μg/g DW). In order to analyze the relationships between grouped phenolic compounds, linear correlation coefficients were established (Table 1). Solely, the association between flavonoids and phenolic diterpenes was deemed to be significant (p < 0.05). Such negative correlation (r = −0.68) revealed an opposite evolution of the amounts of the above mentioned phenolic classes and suggested that possibly, the activation of biosynthesis of flavonoids was concomitant with a decrease of phenolic diterpenes production and vice versa in the course of the analyzed harvesting times.
It is worth noting that the early fruiting stage was characterised by the largest amounts of phenolic diterpenes and rosmarinic acid. This fact is relevant, since the mentioned compounds are known to possess great antioxidant activity [8, 21].
In comparison with earlier study, extracts of non-treated S. verbenaca plants showed qualitative and quantitative differences with our results. Much higher amounts of rosmarinic acid (4432.59–12,838.88 μg/g DW) and naringenin (350.16–1806.17 μg/g DW) were found in non-distilled plant material, however, contents of carnosic acid were lower [10]. In addition, gallic acid, apigenin-7-glucoside and cirsilineol were not detected in non-treated S. verbenaca [10]. The distillation process could be responsible for the differences in phenolic profiles of non-treated and post-distilled plants. As proved previously, Rosmarinus officinalis [24], Thymus zygis ssp. Gracilis [25], S. verbenaca [17] and S. argentea [18] phenolic compositions were significantly affected by the distillation treatment. Jordán et al. [25] reported that contents of hydrophilic components may decrease in post-distilled plant material, whereas, the distilled plants have been found to contain a higher amount of phenolic substances than the non-distilled, according to Parejo et al. [26]. In particular, Almela et al. [24] revealed a higher concentration of carnosol (0.61 g/100 g DW) in distilled Rosmarinus officinalis. This result could be explained by the liberation of polyphenolic compounds (bound forms of polyphenolics) as a consequence of exposure to heat during the distillation process [27].
The differential accumulation of phenolic compounds in S. verbenaca extracts at different phenological stages could be related to physiological changes during growth in response to environmental stress. The season, sunlight duration, UV radiation and temperature are known to influence the plant metabolism since some compounds may be accumulated at a particular period to respond to environmental changes [7, 28].
Phenophase Effect on Antioxidant Activities
The DPPH• and ABTS•+ radical-scavenging tests and the FRAP reducing power assay were used to evaluate the antioxidant capacity of S. verbenaca plant material collected at different harvesting times. The best antioxidant activity of S. verbenaca extracts was observed at early fruiting, as assessed by DPPH• (IC50, 49.22 μg/mL), ABTS•+ (146.86 μM TE/mg) and FRAP (188.93 mM Fe(II)/mg) assays, followed by the late fruiting stage and the lowest activity was detected at the flowering period (Table 2). Detected significant (p < 0.05) variation in antioxidant capacities between S. verbenaca plant material collected at various phenological stages could be related to differences in polyphenolic composition of analyzed extracts. Plants polyphenols are known as powerful antioxidants. Kontogianni et al. [11] advanced that the antioxidant activity of S. officinalis extracts is due to the content of phenolic abietane diterpenes namely, carnosic acid and its derivatives. Also, Cuvelier et al. [29] indicated that carnosol, rosmarinic acid and carnosic acid had the greatest antioxidant activity in S. officinalis plant material. In a previous study, Tepe [21] reported that rosmarinic acid and its derivatives are more likely to be responsible for most of the observed antioxidant activity of the non-diterpenoid components in S. virgata, S. staminea and S. verbenaceae.
S. verbenaca harvested at early fruiting stage was characterised by the highest levels of phenolic acids, in particular rosmarinic acid, a caffeic acid dimer, known as a powerful antioxidant [21]. Also, the maximal contents of carnosic acid were reported at early fruiting (electronic supplementary material Table 1). The potent antioxidant activity of rosmarinic acid and carnosic acid is due to the redox properties of their hydroxyl groups and the increase in activity has been observed to depend mainly on the position and/or pattern of hydroxylation rather than on the number of hydroxyl groups. According to Munné-Bosch and Alegre [30] carnosic acid may function as a “cascading” antioxidant, in which oxidation products are further oxidized, thus enhancing antioxidative protection by this phenolic diterpene.
Conclusions
The current study characterised the variations in polyphenolic contents and antioxidant properties of S. verbenaca extracts as influenced by the phenological stage for the first time. Post-distilled plants have significant antioxidant activity against various antioxidant systems in vitro with the best performance detected at the early fruiting stage. This phenological stage was the richest in phenolic compounds recognized for their great antioxidant activities [2], namely phenolic diterpenes and rosmarinic acid. Results are valuable in valorizing S. verbenaca by-products as good natural antioxidants, that could provide a chemical basis in food and therapeutics.
Abbreviations
- ABTS:
-
2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- DW:
-
Dry weight
- FRAP:
-
Ferric reducing antioxidant power
- GAE:
-
Gallic acid equivalents
- HPLC:
-
High-performance liquid chromatography
- IC50 :
-
Inhibitory concentration
- TE:
-
Trolox equivalent
- TEAC:
-
Trolox equivalent antioxidant capacity
- TPTZ:
-
2,4,6-tripyridyl-s-triazine
References
Rauter AP, Dias C, Martins A, Branco I, Neng NR, Nogueira JM, Goulart M, Silva FVM, Justino J, Trevitt C, Waltho JP (2012) Non-toxic Salvia sclareoides Brot. extracts as a source of functional food ingredients: phenolic profile, antioxidant activity and prion binding properties. Food Chem 132:1930–1935
López V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI (2007) In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum Nutr 62:151–155
Quispe C, Viveros-Valdez E, Schmeda-Hirschmann G (2012) Phenolic constituents of the Chilean herbal tea Fabiana imbricata R. et P. Plant Foods Hum Nutr 67:242–246
Sreelatha S, Padma RP (2009) Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 64:303–311
Croteau RKT, Lewis NG (2000) Natural products (secondary metabolites). American Society of Plant Biologists, Rockville
Knaggs AR (2003) The biosynthesis of shikimate metabolites. Nat Prod Rep 20:119–136
Generalić I, Skroza D, Ljubenkov I, Katalinić A, Burčul F, Katalinic V (2011) Influence of the phenophase on the phenolic profile and antioxidant properties of Dalmatian sage. Food Chem 127:427–433
Cuvelier ME, Berset C, Richard H (1994) Antioxidant constituents in sage (Salvia officinalis). J Agric Food Chem 42:665–669
Lu Y, Foo LY (2002) Polyphenolics of Salvia–a review. Phytochemistry 59:117–140
Ben Farhat M, Landoulsi A, Chaouch-Hamada R, Sotomayor JA, Jordán MJ (2013) Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind Crop Prod 49:904–914
Kontogianni VG, Tomic G, Nikolic I, Nerantzaki AA, Sayyad N, Stosic-Grujicic S, Stojanovic I, Gerothanassis IP, Tzakos AG (2013) Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem 136:120–129
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49:5165–5170
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 28:25–30
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. J Anal Biochem 239:70–76
Ben Farhat M, Landoulsi A, Chaouch-Hamada R, Sotomayor JA, Jordán MJ (2013) Phytochemical composition and in vitro antioxidant activity of by-products of Salvia verbenaca L. growing wild in different habitats. Ind Crop Prod 49:373–379
Ben Farhat M, Landoulsi A, Chaouch-Hamada R, Sotomayor JA, Jordán MJ (2013) Profiling of essential oils and polyphenolics of Salvia argentea and evaluation of its by-products antioxidant activity. Ind Crop Prod 47:106–112
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Macheix JJ, Fleuriet A, Jay-Allemand C (2005) Les composés phénoliques des végétaux: un exemple de métabolites secondaires d’importance économique. Presses polytechniques et universitaires romandes, Lausanne
Tepe B (2008) Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia virgata (Jacq), Salvia staminea (Montbret & Aucher ex Bentham) and Salvia verbenaca (L.) from Turkey. Bioresour Technol 99:1584–1588
Sellami IH, Maamouri E, Chahed T, Aidi Wannes W, Kchouk ME, Marzouk I (2009) Effect of the growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind Crop Prod 30:395–402
Munné-Bosch S, Mueller M, Schwarz K, Alegre L (2001) Diterpenes and antioxidative protection in drought-stressed Salvia officinalis plants. J Plant Physiol 158:1431–1437
Almela L, Sánchez-Muňoz B, Fernández-López JA, Roca MJ, Rabe V (2006) Liquid chromatography-mass spectrometry analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A 1120:221–229
Jordán MJ, Martínez RM, Martínez C, Moňino I, Sotomayor JA (2009) Polyphenolic extract and essential oil quality of Thymus zygis ssp gracilis shrubs cultivated under different watering levels. Ind Crop Prod 29:145–153
Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codina C (2002) Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled mediterranean herbs and aromatic plants. J Agric Food Chem 50:6882–6890
Chohan M, Forster-Wilkins G, Opara EI (2008) Determination of the antioxidant capacity of culinary herbs subjected to various cooking and storage processes using the ABTS*+ radical cation assay. Plant Foods Hum Nutr 63:47–52
Koenen EV (2001) Medicinal poisonous and edible plants in Namibia. Klaus Hess, Berlin
Cuvelier ME, Berset C, Richard H (1996) Antioxidant activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. JAOCS 73:645–652
Munné-Bosch S, Alegre L (2003) Drought-induced changes in the redox state of α-tocopherol, ascorbate, and the diterpene carnosic acid in chloroplasts of Labiatae species differing in carnosic acid contents. Plant Physiol 131:1816–1825
Acknowledgments
This work was supported by the Tunisian Ministry of Higher Education, Scientific Research and Technology and the European Social fund. The authors are thankful to Prof. Jalila Chérif for English language corrections.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Farhat, M., Chaouch-Hamada, R., Sotomayor, J.A. et al. Antioxidant Properties and Evaluation of Phytochemical Composition of Salvia verbenaca L. Extracts at Different Developmental Stages. Plant Foods Hum Nutr 70, 15–20 (2015). https://doi.org/10.1007/s11130-015-0466-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-015-0466-9