Abstract
The present study has been conducted to investigate the effects of vinasse and press mud (PM) (sugar industry by-products) applications on sugarcane soil microbial population, its enzyme activities and biomass carbon and nitrogen in order to work out its potential to be used as bio-friendly fertilizer. The treatments were compared with traditional chemical fertilizer (CF) applications. Vinasse and PM amended soils were found to have slightly lower pH and higher total N and C content, however, the difference was insignificant with CF amended soils. Both the treatments increased sugarcane yield compared to chemical fertilizer, but this increase was not significant in PM amended soils. The populations of fungi, bacteria and actinomycetes increased in vinasse and PM amended soils. This increase in former one was significant in PM amended soils only, and that of the latter in vinasse amended soil. The biomass C and N contents were also higher in both the treatments, however, only the former one had significant difference with CF. Amongst the soil enzymes, the activities of cellulase, phosphatase and aminopeptidase were significantly higher in PM treatment while these were at par in vinasse and CF treatments. These results showed a potential possibility of substituting chemical fertilizers with vinasse and press mud which besides improving soil health and enhancing sugarcane productivity, can also solve the problem of their disposal in free environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since 1993, the Guangxi province is the top sugarcane and sugar producer of China and accounts for more than 65 % sugar production of nation (Li 2004). During the year 2009–2010, the sugarcane crop was cultivated on 103.14 million ha area and yielded 71.2 million tons of sugarcane. Besides producing sugar and alcohol, the sugarcane industries release a large amount of by-products, the disposal of which has become a great problem and threat to the environment. The sugar industry in Guangxi produces approximately more than 5 million tons of vinasse (a liquid by-product of alcohol industry) every year (Ou et al. 2002). During the past decades, the vinasse was discharged directly into the river which has caused serious water pollution and threat to the aquatic organisms. Since the year 2003, the researchers began to use the diluted concentrations of vinasse as liquid fertilizer in sugarcane fields. The results showed significant improvement in yield of sugarcane, vis-à-vis, soil’s physico-chemical properties without any hardening symptoms (Li et al. 2007; Zhu et al. 2009). There have been some reports about the effects on soil properties including physical, chemical and microbial aspects (Su et al. 2008, 2009a, 2009b), however, more evidences are needed to illustrate the mechanisms of vinasse application on promotion of plant growth. Microflora and fauna are of immense importance to soils and any changes made to them would affect soils health as well as the yield of crops. In view of this, an experiment has been conducted to elucidate the effects of vinasse application in sugarcane fields on changes in bacterial and fungal composition of soil and activities of certain enzymes, and growth and yield of sugarcane.
Materials and Methods
Treatments
The experiment was conducted in the sugarcane fields of Qianjiang Farm (109°36′S, 23°36′E), Guangxi, China, the variety of sugarcane was used in “ROC 22”, and consisted of three treatments. In the first treatment (T1), the suitably diluted (1:5; vinasse:water) vinasse @ (120 t ha−1) was applied in furrows before planting sugarcane, in the month of February. In the second treatment (T2), press mud (obtained after cane juice filtration) was applied in the sugarcane fields @150,000 kg ha−1. The third treatment (T3) included the application of N, P and K (in 8:5:7 ratios) fertilizer in the form of urea, P2O5 and K2O of potash. Vinasse and press mud were supplied by the Qianjiang Sugar Mill Limited, Qianjiang, China. The soil samples from each treatment were collected at 6 months after treatment to analyze soil characteristics, number of bacteria and fungi, and the activities of some soil specific enzymes. Every sample was a mixture of soils taken from 5 to 10 locations from 0 to 30 cm depth. Each soil sample was sieved through stainless steel mesh (2 mm) and stored at °C in refrigerator for analysis.
Soil Analysis
The solution of air dried soil with distilled water (1:2.5) was shaken for 1 h and measured with a glass pH electrode. Total C and N were analyzed with air dried soil samples using NC-analyzer (Sumigraph NC-80 AUTO, Sumitomo Chemical Co., Ltd., Japan). The amount of available phosphate (Truog-P) was determined using the vanado-molybdate method after extraction with 0.001 mol L−1 H2SO4 at a ratio of 1:200 (w/v) (Truog 1930).
Microbial Numbers
Microbial number was determined using the method of agar plate dilution amended with cycloheximide (100 μg L−1), as described by Martin (1950). Rose bengal-streptomycin agar medium and starch casein medium were used to determine the number of fungi and actinomycetes in fresh soil samples as described by Miyashita (1997). The pH of medium was adjusted to 6.8 with HCl or NaOH. The microbial, fungi and actinomycetes were counted in 5 replicates.
Microbial Biomass
The contents of soil microbial biomass N (MBN) and soil microbial biomass C (MBC) were determined using the chloroform fumigation-extraction method as described by Brookes et al. (1985) and Vance et al. (1987).
Activity of Soil Enzymes
Cellulase Activity
β–Glucosidase (EC.3.2.1.21) and exocellulase (EC.3.2.1.91) assays were based on p-nitrophenol ( pNP) release after cleavage of a synthetic substrate ( p-nitrophenyl-β-D-glucoside and p-nitrophenyl-β-D-cellobioside, respectively). The color of released p-NP was measured at 400 nm in a spectrophotometer (UV-1700, Shimadzu, Japan). A standard curve was plotted using 0–80 μg mL−1 concentrations of p-NP. The enzyme activities were expressed as n moles pNP released per g dry soil per minute (n mol pNP g−1 min−1).
Phosphatase Activity
Phosphatase (Phosphodiesterase and phosphomonoesterase) activity in soils was estimated by measuring the amount of p-NP released after incubating the samples with p-nitrophenyl-phosphate (Alef et al. 1995). In a reaction tube, 0.25 mL toluene, 4.0 mL modified universal buffer (5× MUB; pH 6.0; made by dissolving 12.1 g tris, 11.6 g maleic acid, 14.0 g citric acid and 6.3 g boric acid in 500 mL 1 M NaOH and making the volume 1 L) and 1.0 mL p-nitrophenyl-phosphate (15 mM) were added to 1.0 g soil sample and the mixture was incubated at 37 °C for 1 h. The reaction was terminated by adding 1.0 mL of 0.5 M CaCl2 and 4.0 mL of 0.5 M NaOH to the mixture prior to filtration. The absorbance of released pNP was taken at 400 nm in a spectrophotometer (UV-1700, Shimadzu, Japan) and the phosphatase activity was expressed in mg p-NP g−1 h−1.
Protease Activity
Aminopeptidase activity was measured by the method as described by Pansombat et al. (1997) using 0.002 M N-benzyl-oxycarbonyl glycyl l-phenylalanine (ZGP). The absorbance was measured in spectrophotometer at 570 nm wavelength. All the analyses were conducted in 5 replicates.
Statistical Analyses
Statistical analyses were carried out using multiple range test at a 0.95 level of probability) to determine significant differences (p ≤ 0.05) between the treatments.
Results and Discussion
Soil Properties and Cane Yield
Both the vinasse and press mud treatments significantly decreased the pH of soil, however, this decrease was more pronounced in press mud treatment. Generally, both of these by-products have significant amount of nutrients and organic matter, which is useful for soil health. Slightly acidic soils facilitate the availability of micronutrients to the plants and enhance the growth and development processes. Both the treatments also recorded higher C and N content in the soils compared to T3, however, no significant differences were observed between treatments T1 and T2. The soils treated with T1 and T2 recorded lower C/N ratios which indicate greater N availability and its solubilization in the treated soils. Vinasse treatment enhanced available P content of the soil, but it decreased in T2 compared to T3. The improvement in soil mineral and organic matter content and facilitated nutrient availability due to application of vinasse and press mud resulted in slightly higher cane yield in both treatments compared to T3, however, the increase in cane yield was not significant in the press mud treatment (Table 1). These results suggested a potential possibility of vinasse and press mud to be used as fertilizers which besides improving soil health and enhancing sugarcane productivity, can also solve the problem of their disposal in free environment.
Microbial Numbers
Fungi along with bacteria and actinomycetes play a vital role in the decomposition of organic matter in soil, thus releasing the nutrients locked up in the dead organic matter of plant, animal and microbial matter and bringing about the recycling of nutrients in nature. In soil, microbes oxidize organic carbon to CO2 and liberate bound materials. All the treatments showed similar trend of the bacterial and fungi population. The soils treated with press mud recorded maximum population of these two microorganisms which was significantly highest amongst all the treatments. Vinasse was not found to alter the bacterial or fungal population as it showed no significant difference with the soils treated with chemical fertilizer. On the other hand, actinomycete population differed significantly amongst all the treatments. Application of vinasse recorded the highest number of actinomycete followed by the press mud and chemical fertilizer treatments. The population of microorganism in soil was affected greatly by its organic matter content (Pansombat et al. 1996). The organic residues or substances of soil are first attacked by bacteria and fungi and later by actinomycetes, because they are slow in activity and growth than bacteria and fungi. The presence of high number of actinomycetes in soils treated with vinasse might be due to its complex organic compounds.
Microbial Biomass
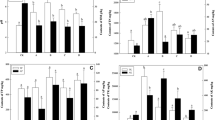
Soil microorganisms are the main participant in soil formulation and nutrient recycling (Spedding et al. 2004). Size and activity of microbial biomass are greatly stimulated by the addition of manure. Soil microbial biomass is the living component of soil organic matter (Zhang and Zhang 2003; Gil-Stotres et al. 2005). And it generally comprises 1–5 % of total organic matter content (Nsabimana et al. 2004). Microbial biomass measurements have been used to give an early indication of the changes in the organic matter content of a soil as a result of variation in soil management (Garcia-Gil et al. 2000; Hargreaves et al. 2003; Insam et al. 1989; De Varies et al. 2007; Zhang et al. 2004). Amongst all the treatments, application of press mud resulted in the highest microbial C and N, followed by the soils treated with vinasse and chemical fertilizer (Fig. 1). However, no significant difference was observed between the vinasse and press mud treatments. This might be due to the microbial activities during long time storage duration of vinasse and press mud. Higher total C and N contents in the soils with T1 and T2 treatments (Table 1) acted as an energy source for the autochthonous microorganisms (Perucci 1992) and significantly increased the microbial numbers (fungi, bacteria and actinomycetes) (Fig. 2). The results of the present study are in accordance with those of Leita et al. (1999) who reported that the soil microbial biomass, which represents approximately 1–4 % of total soil organic C, is a more sensitive indicator of changing soil conditions than direct analysis of the organic C content.
Soil Enzyme Activities
Cellulases Activity
β-Glucosidase and exocellulase (C1 enzyme) activities play an important role in carbon metabolism in soils. Especially, the β-glucosidase, an enzyme involved in the carbon cycle, has been widely used in quality evaluation of soils subjected to different management procedures (Gil-Stotres et al. 2005). In the present study, the highest activity of β-glucosidase was found in press mud treated soils (0.320–0.339 μg p-NP g−1 h−1), followed by the chemical fertilizer treated soils (0.312–0.334 μg p-NP g−1 h−1) (Fig. 3a). The soils treated with vinasse recorded the lowest activity. However, no significant difference was observed in exocellulase activity amongst different treatments. The difference between T1 and T2 was significant at 0.05 as well as 0.01 P level, however, T2 and T3 differed significantly at 0.05 P. Furthermore, contrary to the findings of Chang et al. (2008), significant negative correlations of β-glucosidase activity with biomass C (r2 = −0.32) and biomass N (r2 = −0.20) were observed (Table 2) which suggested higher activity of this enzyme was related with the decomposition of organic material (Dinesh et al. 2004), and difference in enzyme activity was due to different types of fertilizers (Chang et al. 2008). Besides this, positive correlations were also found between the enzyme activity and the number of fungi (r2 = 0.73) and bacteria (r2 = 0.68), suggesting their role in promotion on this enzyme. However, the β-glucosidase activity was found negatively correlated with the actinomycetes population.
Protease Activity
Within organic farming in particular, increasing amounts of waste products from the food industry are being used in addition to manure to cover crop nitrogen (N) needs. These different organic fertilizers release N at different and often unknown rates. To achieve high N use efficiency with maximized yield return and minimized N loss to the environment, the availability of N from organic fertilizers must be synchronized with crop uptake. By documenting the course of mineralization under field conditions, it could be possible to estimate when as yet non-mineralized nitrogen becomes plant-available in relation to the time of fertilization. It needs to determine when to fertilize to get the maximum N utilization efficiency. The course of mineralization for a number of organic fertilizers has been studied under controlled laboratory conditions (Griffin and Honeycutt 2000; Raupp 2005; Cordovil et al. 2007). In the present study, it was found that the different fertilizers application differently affected the soil aminopeptidase activity (Fig. 3b). Similar to phosphatase activity, the activity of aminopeptidase was also found the highest in press mud treated soils, followed by the vinasse treated soils. Loll and Bollag (1983) reported that soil amended with organic compounds, such as straw, increase the protease activity. Our results of total N contents (Table 1) in the soils are in accordance with the enzyme activity. The present study also revealed a significant correlation of biomass N content with the aminopeptidase activity. A strong positive correlation was also observed between the enzyme activity with bacterial and fungal populations (Table 2).
Phosphatase Activity
In soil, phosphorus exists mostly in organic and inorganic forms, both of which are important sources of P for plant and microbial uptake. The organic form exists mostly in humus and other organic materials. P availability is also controlled by environmental conditions such as soil organic matter moisture content and aeration which influence microbial activity and eventually microbial transformations of phosphorus. Soil phosphatase, the enzyme that transforms organic P to inorganic P is mostly of plant and microbial origin and consists of alkaline and acid types. The alkaline phosphatase is mostly of microbial origin. Loss of moisture likely to influence the activity of the microbial populations and in turn influences the phosphorus mineralization process. Phosphatases are important in soils because these extracellular enzymes catalyze the hydrolysis of organic phosphate esters to orthophosphate, thus they form an important link between biologically unavailable and mineral P (Kanazawa et al. 2005; Amador et al. 1997). Phosphatase activity is sensitive to soil pH, organic amendments and fertilizer additions etc. In the present study, the activity of phosphodiesterase enzyme was found the highest in soils treated with press mud followed by vinasse treatments. It varied from 0.360 to 0.574, 0.421–0.603 and 0.310–0.397 μg p-NP g−1 h−1 in soils treated with vinasse, press mud and chemical fertilizer treatments, respectively (Fig. 3c). No significant variation was observed between the T1 and T2, however, both the treatments showed significant differences (P ≤ 0.05) with the T3. The enhancement in enzyme activity in the T1 and T2 might be related with the decrease in soil pH (Table 1) (Dick et al. 2000).
Conclusions
The results of the present study clearly showed that the application of vinasse and press mud in sugarcane fields significantly improved the soil fertility status by enhancing soil C and N contents. Lower C/N ratios in the amended soils indicated higher N mineralization by microbial activities. Vinasse treatment enhanced available P content in the soil, however, it decreased in the press mud amended soils. High millable cane yield under vinasse amended conditions might be attributed to the higher micronutrient availability under slightly acidic conditions caused by vinasse application. In addition, enhancement in fungal and bacterial populations by application of press mud and actinomycetes population in vinasse treated soils suggesting their roles in decomposition of organic materials to release nutrients for plants growth and development. Furthermore, the higher biomass C and N contents in the soils treated with vinasse and press mud showed changes in soil organic matter content caused by microbial enzymatic activities. The present study revealed higher cellulase, phosphatase and protease activities in the vinasse and press mud treated soils which facilitated the process of carbon, phosphorus and nitrogen mineralization in the soils and improved the fertility status. These results suggested a potential possibility of vinasse and press mud to be used as fertilizers which besides improving soil health and enhancing sugarcane productivity, can also solve the problem of their disposal in free environment. Decomposition of soil organic matter, as well as the organic matter presented in vinasse and press mud, is a slow process. These organic fertilizers besides adding nutrients to the soil, sustained the microbial growth and a medium for enzymatic activities which might be more useful for enhancing the productivity of the ratoon crop, and will reduce the application of chemical fertilizers.
References
Alef, K., P. Nannipieri, and C. Trazar-Cepeda. 1995. Phosphatase activity. In Methods in applied soil microbiology and biochemistry, ed. K. Alef, and P. Nannipieri, 335–3344. London: Academic Press.
Amador, J.A., A.M. Glucksman, J.B. Lyons, and J.H. Gores. 1997. Spatial distribution of soil phosphatase activity within a riparian forest. Soil Science 162: 808–825.
Brookes, P.C., A. Landman, G. Pruden, and D.S. Jenkinson. 1985. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biology and Biochemistry 17: 837–842.
Chang, E.H., R.S. Chung, and F.N. Wang. 2008. Effect of different types of organic fertilizers on the chemical properties and enzymatic activities of an oxisol under intensive cultivation of vegetables for 4 years. Soil Science and Plant Nutrition 54: 587–599.
Cordovil, C., F. Cabral, and J. Coutinho. 2007. Potential mineralisation of nitrogen from organic wastes to ryegrass and wheat crops. Bioresource Technology 98: 3265–3268.
De Varies, F.T., J. Bloem, N. van Eekeren, L. Brusaard, and E. Hoffland. 2007. Fungal biomass in pastures increase with age and reduced N input. Soil Biology and Biochemistry 39: 1620–1630.
Dick, W.A., L. Cheng, and P. Wang. 2000. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biology and Biochemistry 32: 1915–1919.
Dinesh, R., S.G. Chaudhuri, A.N. Ganeshamurthy, and S.C. Pramanik. 2004. Biochemical properties of soils of undisturbed and disturbed mangrove forests of South Andaman (India). Wetlands Ecology and Management 12: 309–320.
Garcia-Gil, J.C., C. Plaza, P. Soler-Rovira, and A. Polo. 2000. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biology and Biochemistry 32: 1907–1913.
Gil-Stotres, F., C. Trasar-Cepeda, M.C. Leiros, and S. Seoane. 2005. Different approaches to evaluating soil quality using biochemical properties. Soil Biology and Biochemistry 37: 877–887.
Griffin, T.S., and C.W. Honeycutt. 2000. Using growing degree days to predict nitrogen availability from livestock manures. New England Plant Soil and Water Lab. University of Maine. Forest Experiment Station Journal No. 2394.
Hargreaves, P.R., P.C. Brookes, G.J.S. Ross, and P.R. Poulton. 2003. Evaluating soil microbial biomass carbon as an indicator of long-term environmental change. Soil Biology and Biochemistry 41: 249–256.
Insam, H., D. Parkinson, and K.H. Domsh. 1989. Influence of macroclimate on soil microbial biomass. Soil Biology and Biochemistry 21: 211–221.
Kanazawa, S., G.H. An, and K. Toshiyuki. 2005. Effects curtailment of nitrogen fertilizer on biological properties and tea leaf yield in acid tea field soils. Soil Science and Plant Nutrition 51: 675–677.
Leita, L., M. De Nobili, C. Mondini, G. Mühlbachová, L. Marchiol, G. Bragato, and M. Contin. 1999. Influence of inorganic and organic fertilization on soil microbial biomass, metabolic quotient and heavy metal bioavailability. Biology and Fertility of Soil 28: 371–376.
Li, Y.R. 2004. China: an emerging sugar super power. Sugar Tech 6(4): 213–228.
Li, Y.R., Q.Z. Zhu, W.Z. Wang, and S. Solomon. 2007. Pre-emergence application of vinasse on sugarcane growth and sugar productivity in China. Sugar Tech 9(2&3): 160–165.
Loll, M.J., and J.M. Bollag. 1983. Protein transformation in soil. Advances in Agronomy 6: 351–382.
Martin, J.P. 1950. Use of acid, rose Bengal and streptomycin in the plate method for estimating soil fungi. Soil Science 69: 215–233.
Miyashita, S. 1997. Methods of soil microbiology, 55–61. Japan: Yokendo LTD.
Nsabimana, D., R.J. Haynes, and F.M. Wallis. 2004. Size, activity and catabolic diversity of the soil microbial biomass as affected by land use. Applied Soil Ecology 26: 81–92.
Ou, S.B., H. Yang, H.Q. Liang, H.Z. Zhou, and Z.M. Fang. 2002. Review and prospect of vinasse management technology of China. Guangxi Journal of Light Industry 4: 10–21.
Pansombat, K., S. Kanazawa, and T. Horiguchi. 1996. Microbial ecology in tea soils 1. Soil properties and microbial populations. Soil Science and Plant Nutrition 43: 317–327.
Pansombat, K., S. Kanazawa, and T. Horiguchi. 1997. Microbial ecology in tea soils 2. Soil protease activity. Soil Science and Plant Nutrition 43: 431–438.
Perucci, P. 1992. Enzyme activity and microbial biomass in a field soil amended with municipal refuse. Biology and Fertility of Soils 14: 54–60.
Raupp, J. 2005. Nitrogen mineralisation of farmyard manure, faba bean meal, alfalfa meal and castor meal under controlled conditions in incubation tests. Ende der Nische, Beiträge zur 8. Wissenschaftstagung Ökologischer Landbau.
Spedding, T.A., C. Hamel, G.R. Mehuys, and C.A. Madramootoo. 2004. Soil microbial dynamics in maize growing soil under different tillage and residue management systems. Soil Biology and Biochemistry 36: 499–512.
Su, T.M., Y.R. Li, Y.L. Mo, C.Y. Lu, G.P. Wei, and Z.P. Jiang. 2009a. Effect of vinasse application on the agronomic characters of sugarcane. Chinese Journal of Soil Science 40(2): 276–278.
Su, T.M., Y.R. Li, G.P. Wei, and Z.P. Jiang. 2009b. Effect of sugarcane vinasse on soil physicochemical properties and oxidoreductase enzymes. Chinese Journal of Eco-Agriculture 17(6): 1106–1110.
Su, T.M., Y.L. Mo, Y.R. Li, G.P. Wei, and Z.P. Jiang. 2008. Effect of vinasse application on hydrolytic enzyme activity of soil. Acta Agriculturae Boreali-occidentalis Sinica 17(3): 199–204.
Truog, E. 1930. The determination of the readily available phosphorus of soils. Journal of American Society of Agronomy 22: 874–882.
Vance, E.D., P.C. Brookes, and D.S. Jenkison. 1987. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry 19: 703–707.
Zhang, H., and G.L. Zhang. 2003. Microbial biomass carbon of soils as affected by rubber cultivation. Pedosphere 13: 353–357.
Zhang, T.Q., C.F. Drury, and B.D. Kay. 2004. Soil dissolved organic carbon: influences of water-filled pore space and red clover addition and relationships with microbial biomass carbon. Canadian Journal of Soil Science 84: 151–158.
Zhu, Q.Z., Y.R. Li, W.Z. Wang, and J. Liao. 2009. Effect of continual quantitative rational application of vinasse in sugarcane field. Sugar Crops of China 2: 10–13.
Acknowledgments
This research was supported by Postdoctoral Research Grant (No.77686), National Scientific R&D Program (No. 2007BAD30B00), International Scientific Cooperation Program projects (2008DFA30600, 2009DFA30820), 948 Program (No. 2009-Z8), Guangxi Scientific R&D Program (No. 0782004) and Guangxi Educational Key Program (No. 201102ZD003). The authors are grateful to Mr. Guang-Po Wei, Guangxi Academy of Agricultural Sciences, for his assistance in sampling the soils.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, SD., Liu, JX., Wu, J. et al. Effects of Vinasse and Press Mud Application on the Biological Properties of Soils and Productivity of Sugarcane. Sugar Tech 15, 152–158 (2013). https://doi.org/10.1007/s12355-012-0200-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-012-0200-y