Abstract
Background
We hypothesized early post-stress left ventricular ejection fraction reserve (EFR) on solid-state-SPECT is associated with major cardiac adverse events (MACE).
Methods
151 patients (70 ± 12 years, male 50%) undergoing same-day rest/regadenoson stress 99mTc-sestamibi solid-state SPECT were followed for MACE. Rest imaging was performed in the upright and supine positions. Early stress imaging was started 2 minutes after the regadenoson injection in the supine position and followed by late stress acquisition in the upright position. Total perfusion deficit (TPD) and functional parameters were quantified automatically. EFR, ∆end-diastolic volume (EDV), and end-systolic volume (ESV) were calculated as the difference between stress and rest values in the same position. EFR < 0%, ∆EDV ≥ 5 ml, or ∆ESV ≥ 5 ml was defined as abnormal.
Results
During the follow-up (mean 3.2 years), 28 MACE occurred (19%). In Kaplan–Meier analysis, there was a significantly decreased event-free survival in patients with early EFR < 0% (P = 0.004). Similarly, there was a decreased event-free survival in patients with ∆ESV ≥ 5 ml at early stress (P = 0.003). However, EFR, ∆EDV, and ∆ESV at late stress were not associated with MACE-free survival. Cox proportional hazards model adjusting for clinical information and stress TPD demonstrated that EFR, ∆EDV, and ∆ESV at early stress were significantly associated with MACE (P < 0.05 for all).
Conclusions
Reduced early post-stress EFR on vasodilator stress solid-state SPECT is associated with MACE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although evidence supporting the use of single-photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) for risk assessment and clinical decision making has accumulated over the last 30 years,1 regional perfusion alone may underestimate the extent of coronary artery disease (CAD).2,3 In particular, relative perfusion defects may not identify patients with left main or multi-vessel disease due to balanced ischemia.2,3 However, such high-risk patients are the most important to identify since they have poor cardiac outcomes and may benefit from revascularization.4,5,6 Traditionally, high-risk nonperfusion parameters such as transient ischemic dilation 7,8 and post-ischemic stunning 9 have been used as aids to identify patients with more severe CAD than would be predicted by relative perfusion alone on SPECT or positron emission computed tomography (PET) MPI.
One advantage of PET is the ability to rapidly measure LV function during peak hyperemia, which is not possible with conventional SPECT camera systems. Previous PET studies demonstrated that early post-stress EF reserve is a useful diagnostic and prognostic marker.10,11,12
The recent emergence of solid-state SPECT camera systems resulted in significant improvements in count sensitivity 13 and diagnostic accuracy for CAD 14,15,16,17 allowing shorter acquisition times to facilitate early post-stress imaging. Previously, our group evaluated a novel imaging protocol with early gated acquisitions after regadenoson stress using a solid-state SPECT camera system and demonstrated that patients with significant ischemia were more likely to have negative early post-stress EF reserve.17 In the current study, we sought to establish if early post-stress EF reserve or change in end-systolic volume (ESV) and end-diastolic volume (EDV), measured using solid-state SPECT MPI, is associated with major adverse cardiac events (MACE).
Methods
Study Population
Patients referred for clinically indicated rest/regadenoson stress SPECT MPI at the Nuclear Medicine Department, Sacred Heart Medical Center, Springfield, Oregon, between November 2013 and May 2016, were considered for the protocol. Final patient selection depended on the ability to accommodate additional imaging during this protocol and, in particular, the ability to have stress lab and nursing staff available during the entire procedure. Out of 1224 patients who underwent rest/regadenoson stress SPECT MPI during the above time period, a total of 151 patients were selected for the study protocol. The study protocol, including a waiver of informed consent, was approved by the PeaceHealth Institutional Review Board.
Rest/Early and Late Regadenoson Stress Imaging Protocol
The study protocol is shown in Figure 1. Rest and stress 1-day 99mTc-sestamibi imaging was performed using high -efficiency, solid-state SPECT camera system (D-SPECT, Spectrum-Dynamics, Haifa, Israel).18 Patients were instructed to abstain from caffeine-containing drinks and foods for 24 hours before the study. Beta-blockers and calcium channel antagonists were discontinued for 24 hours and nitrates were withheld for 6 hours before the test.19 During the pre-imaging stress lab evaluation and procedures, standard 12 leads for electrocardiogram (ECG) monitoring and leads for image gating were applied, and a venous catheter was inserted into an antecubital vein. Patients were injected with 222-333 MBq (6-9mci) of 99mTc-sestamibi, according to a body mass index (BMI)-related dose schedule (6-7 mCi for BMI ≤ 35, and 8-9 mCi for BMI range of 36-50) at rest. A 10-12 minute rest acquisition in the upright position was obtained 15-30 minutes after the rest injection, followed by a 10-12 minute rest acquisition in the supine position. After the rest acquisition, regadenoson 0.4 mg was injected over 10 seconds with 5 ml saline flush, and 10-20 seconds later followed by an IV bolus of 888-1332 MBq (24-36 mCi) 99mTc-sestamibi, according to the BMI-related dose schedule (24-28 mCi for BMI ≤ 35, 32-36 mCi for BMI range of 36-50) and 10 ml saline. A 6-8 minute early stress acquisition was obtained 2 minutes after the regadenoson injection in the supine position, followed by 6-8 minute late stress acquisition in the upright position. The perfusion results were derived from the late stress acquisitions since our previous work demonstrated that 96% of the stress images performed 5 minutes after regadenoson/tracer injection demonstrated good, excellent, or fair quality with low or no extra-cardiac tracer activity, allowing for confident clinical interpretation.17 The late post-stress images were performed at 8-10 minutes post-injection, and therefore would be of adequate quality, and were in the default position for this camera. Heart rate, blood pressure, and ECG were monitored and recorded during the imaging protocol for 10 minutes after stress injection.
Early Post-Regadenoson Stress Solid-state SPECT MPI Protocol. Rest and stress 1-day 99mTc-sestamibi imaging was performed using high -efficiency, solid-state SPECT camera system (D-SPECT, Spectrum-Dynamics, Haifa, Israel).18 Patients were injected with 6-9mci of 99m Tc-sestamibi at rest. A 10-12 minute rest acquisition in the upright position was obtained 15-30 minutes after the rest injection, followed by a 10-12 minute rest acquisition in the supine position. After the rest acquisition, regadenoson 0.4 mg over 30 second was injected, immediately followed by an IV bolus of 24-36 mci 99m Tc-MIBI and 10 ml saline. A 6-8 minute early stress acquisition was obtained 2 minutes after the regadenoson injection in the supine position, followed by 6-8 minute late stress acquisition in the upright position
Transaxial images were created by a reconstruction algorithm based on a maximum likelihood maximization method.18 No attenuation or scatter correction was applied. Images were automatically re-oriented into short-axis, and vertical and horizontal long-axis slices with Quantitative Perfusion SPECT (QPS)/ Quantitative Gated SPECT (QGS) software (Cedars-Sinai Medical Center, Los Angeles, California).
Automated Quantification of Perfusion and Function Parameters
Automatically generated myocardial contours by QPS/QGS were evaluated, and when necessary, contours were adjusted to correspond to the myocardium by an experienced technologist. Rest and stress perfusion images were analyzed in the upright position (default position for this camera) as previously described using total perfusion deficit (TPD).20,21 Functional parameters including EF, EDV, and ESV at rest and stress were quantified separately for each acquisition from 16 frames per cardiac cycle. EF reserve, ∆EDV, and ∆ESV were calculated as the absolute difference between stress and rest values from the same position.10 Abnormal post-stress functional parameters were defined as EF reserve < 0%, ∆EDV ≥ 5 ml, or ESV ≥ 5 ml as previously reported.12,22
MACE
MACE consisted of all-cause mortality, nonfatal myocardial infarction, unstable angina, or coronary revascularization (PCI or CABG after SPECT MPI).13 All-cause mortality was determined from the Social Security Death Index. All information related to myocardial infarction, unstable angina, and late revascularization including symptoms, ECG changes, cardiac biomarkers, and imaging modalities such as echocardiography, stress test, computed tomography angiography, and invasive angiography were obtained by reviewing medical records, a patient questionnaire, or patient interview. A board-certified cardiologist adjudicated events based on all available information. The first event which occurred among the above components of MACE was considered as the main event for this analysis.
Statistical Analysis
All data were tested for normal distribution using the Kolmogorov–Smirnov test. Categorical variables are presented as frequencies and continuous variables as mean ± SD or median and interquartile ranges. Variables were compared using a χ2 statistic for categorical variables and Student’s t test or Wilcoxon rank-sum test for continuous variables. Kaplan–Meier analysis and log-rank test were used to assess any differences in event risk between groups with and without EF reserve < 0%, and ∆ESV ≥ 5 ml and EDV ≥ 5 ml.
The annualized event rates based on events per patient per year were calculated and compared between patients with normal and abnormal EF reserve, ∆ESV, and ∆EDV. Univariable Cox proportional hazards analysis was performed to assess associations with MACE. The baseline multivariable Cox proportional hazards analysis included age, history of prior PCI, and stress TPD (Model 1). Then, three multivariable Cox proportional hazards analyses were performed considering age, history of prior PCI, and stress TPD to determine the association between early post-stress functional parameters (Model 2: early EF reserve, Model 3: early ∆ EDV, and Model 4: early ∆ ESV) and MACE. A parsimonious multivariable model, including only significant predictors from the univariable analysis, was used to maintain statistical power.23 A hazard ratio (HR) and 95% confidence interval (CI) were calculated from the Cox models. Global chi-square analyses utilized cox models and a likelihood ratios test to evaluate the incremental prognostic value of early post-stress functional parameters. Two-tailed P value < 0.05 was considered statistically significant. STATA version 13 was used for all analyses (Stata Corp, College Station, TX).
Results
Outcome Events
During the mean follow-up of 3.2 ± 1.1 years, 28 MACE (19%) occurred including 13 deaths, 9 myocardial infarctions, and 6 coronary revascularizations.
Clinical Characteristics and Hemodynamic Results
Clinical characteristics and hemodynamic results are shown in Table 1. Patients with MACE were older (mean age 77 vs 68, P = 0.0005) and more likely to have a history of prior PCI (43% vs 17%, P = 0.003). There was no significant difference in hemodynamic results between patients with and without MACE.
Stress and Rest Quantitative MPI Results
SPECT imaging results are shown in Table 2. The automatic segmenting of the left ventricle was successful without need for manual adjustment of contours in 97%, 99%, 83%, 87%, 81%, and 91% on static stress, static rest, early stress gated, late stress gated, rest supine gated, and rest upright gated data. There was no significant difference in the need for manual adjustment between early stress gated and late stress gated data (P = 0.26). Stress and rest TPD were significantly higher in patients with MACE (median stress TPD: 12 vs 5, P = 0.0005) (median rest TPD:5 vs 3, P = 0.03). Compared to patients with no MACE, patients with MACE had higher ∆ESV, and lower EF reserve at early stress (P < 0.01 for both). The frequencies of patients with abnormal functional parameters are shown in Table 3. Early EF reserve < 0% and ∆ESV ≥ 5 ml were more frequent in patients who experienced MACE, while there was no significant difference in frequencies of abnormal late stress functional parameters between those with and without MACE.
Kaplan–Meier Analysis and Annualized MACE Rates
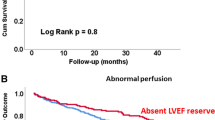
Kaplan–Meier analyses to compare MACE-free survival in patients with and without abnormal functional parameters at early and late stress are shown in Figures 2, 3, and 4 (Figures 2A and B: EF reserve, Figure 3A, and 3B: ∆ESV and Figure 4A and 4B: ∆EDV). In the group with early EF reserve < 0%, there was a significantly decreased event-free survival compared to patients with early EF reserve ≥ 0% (P = 0.004, Figure 2A). Similarly, in the group with ∆ ESV ≥ 5 ml at early stress, there was a significant decreased event-free survival (P = 0.003, Figure 3A) while there was no significant difference in the event risk between the group with ∆EDV ≥ 5 ml and those with ∆EDV < 5 ml at early stress (P = 0.24, Figure 4A). At late stress, EF reserve, ∆ EDV, and ∆ ESV did not stratify MACE-free survival (P for all ≥ 0.41, Figures 2B, 3B, and 4B). Unadjusted annualized MACE rate was significantly higher in patients with early EF reserve < 0 compared to those with early EF reserve ≥ 0 (Figure 2C). Similarly, patients with ∆ ESV ≥ 5 ml had higher annualized MACE rates compared to those with ∆ ESV < 5 ml at early stress (Figure 3C) while it was similar between patients with ∆ EDV ≥ 5 ml and those with ∆ EDV < 5 ml at early stress (Figure 4C).
Survival Curves by EF Reserve at Early and Late Stress. Kaplan–Meier curves to compare MACE-free survival in patients with and without abnormal EF reserve and at early and late stress are shown in A (early EF reserve), and B (late EF reserve). Annual MACE rates in patients with and without abnormal EF reserve at early and late stress are shown in C (early EF reserve), and D (late EF reserve). EF ejection fraction, MACE major adverse cardiac events
Survival Curves by ∆ESV at Early and Late Stress. Kaplan–Meier curves to compare MACE-free survival in patients with and without abnormal EF reserve and at early and late stress are shown in A (early ∆ ESV), and B (late ∆ ESV). Annual MACE rates in patients with and without abnormal EF reserve at early and late stress are shown in C (early ∆ ESV), and D (late ∆ ESV). ESV end-systolic volume, MACE major adverse cardiac events
Survival Curves by ∆EDV at Early and Late Stress. Kaplan–Meier curves to compare MACE-free survival in patients with and without abnormal EF reserve and at early and late stress are shown in A (early ∆ EDV), and B (late ∆ EDV). Annual MACE rates in patients with and without abnormal EF reserve at early and late stress are shown in C (early ∆ EDV), and D (late ∆ EDV). EDV end-diastolic volume, MACE major adverse cardiac events
Cox Proportional Hazards Analyses
Univariable Cox proportional hazards analysis are shown in Table 4. By univariable analysis, age, history of prior PCI, stress TPD, rest TPD, and early post-stress functional parameters were significantly associated with MACE (P for all < 0.05, Table 4). Multivariable Cox proportional hazards models are shown in Table 5. Model 1 demonstrated that stress TPD was significantly associated with MACE adjusting for age and history of prior PCI. Models 2 to 4 demonstrated that EFR, and change in EDV and ESV at early stress were significantly associated with MACE adjusting for age, history of prior PCI, and stress TPD. Figure 5 shows that the addition of EFR and change in EDV and ESV at early stress (Models 2 to 4) incrementally enhanced the combined model of age, prior history of percutaneous coronary intervention, and stress TPD (p for all < 0.05 compared to Model 1). Multivariable Cox proportional hazards models adjusting for age, history of prior PCI, and rest TPD are shown in Supplemental Materials.
Incremental MACE Prediction by the Addition of Early Post-stress Functional Parameters. The addition of each early stress functional parameter (Models 2: early EF reserve, Model 3: early ∆EDV and Model 4: early ∆ ESV) incrementally enhanced the combined model (Model 1) of age, prior history of percutaneous coronary intervention, and stress TPD (p for all < 0.05, compared to Model 1). EDV end-diastolic volume, EF ejection fraction, ESV end-systolic volume, PCI percutaneous coronary intervention, TPD total perfusion deficit
Discussion
This is the first study to evaluate the utility of early post-stress imaging for MACE on vasodilator stress solid-state SPECT MPI. Abnormal early post-stress EF reserve and change in ESV were more commonly observed in patients who experienced MACE and stratified MACE-free survival, while late post-stress imaging did not. Multivariable analysis demonstrated that early post-stress functional parameters were independently associated with MACE after adjusting for clinical and perfusion variables. The addition of early post-stress functional parameters improved risk stratification compared to a model considering other important SPECT MPI variables. The proposed protocol includes all image acquisitions used clinically and could potentially be modified to also allow myocardial blood flow estimation.
Due to the short half-life of PET radiotracers, PET stress images are routinely collected during peak hyperemia. In comparison, 99m Tc-sestamibi anger SPECT imaging in a standard protocol is performed 15-45 minutes after the stress tracer injection.20 Therefore, PET imaging is able to capture early functional changes during the post-stress period. In a cohort of 510 patients who underwent vasodilator stress 82Rubidium PET MPI, Dorbala et al. demonstrated that patients with severe ischemia and those with 3 vessel/left main disease had significantly lower EF during peak vasodilator stress and a lower EF reserve.10 Similarly, Brown et al. found that the magnitude of ischemia on 82Rubidium PET MPI was inversely correlated with EF Reserve.11 The results of these studies suggest that the EF reserve during vasodilator 82Rubidium PET is inversely related to the extent of myocardium at risk. Furthermore, in a cohort of 985 patients followed for 1.7 years, patients with EF reserve < 0 had a higher risk of annualized cardiac events compared to patients with an EF reserve ≥ 0 after accounting for differences in clinical factors and perfusion findings.12 Similarly, reduced EF reserve provided incremental prognostic information in patients with systolic dysfunction.24 Our findings suggest that SPECT measurement of early EF reserve provides the capability for detecting this potentially useful prognostic parameter.
Recently, Gomez et al. 25 demonstrated that LVEF reserve following regadenoson stress was not associated with increased risk of adverse cardiac events in 929 patients who underwent conventional Anger SPECT. The most important difference from our study is that peak stress imaging was not performed by Gomez et al. In our study, late post-stress functional parameters were not associated with MACE but early post-stress parameters were. Therefore, it seems that early post-stress gated acquisitions may yield valuable additional diagnostic and prognostic information. We previously demonstrated that significantly lower EF reserve was obtained 5 to 9 minutes after regadenoson injection in patients with significant ischemia compared to patients without ischemia, while with later post-stress imaging this difference was no longer significant.17 In the current study, we demonstrated that early post-stress EF reserve and change in ESV were independently associated with increased MACE. Additionally, both parameters significantly improved upon a prognostic model with other important SPECT variables. Conversely, late post-stress functional variables were not associated with MACE.
The current study also suggests that early ∆ESV may be a stronger predictor of cardiac events than early EF reserve and ∆EDV. This is consistent with a previous stress echocardiogram study demonstrating an independent association between ∆ESV and all-cause mortality in 934 patients with stable CAD after accounting for rest imaging variables.26 The authors of the echocardiogram study concluded that ESV may more accurately assess contractile reserve since it is less sensitive to cardiac loading and varies greatly in response to changes in contractility.
The early post-stress imaging could potentially be a dynamic acquisition in order to add the ability to estimate absolute MBF (33). SPECT MBF estimates correlate well with values from PET (33) and have high diagnostic accuracy for obstructive CAD.24,26 Therefore, implementing early post-stress imaging could allow centers to improve disease diagnosis and risk stratification by assessing both EF reserve and MBF; markers which have dramatically improved the clinical utility of PET. Larger studies are required to determine if there is additive diagnostic or prognostic value to considering both early post-stress EF reserve and ∆ESV from MPI, in combination with other perfusion parameters.
Study Limitations
There were several limitations to this study. This was a retrospective study with a small sample size from a single center; however, early post-stress SPECT MPI imaging is not currently a routine clinical protocol. The sample size limited our ability to adjust for other SPECT MPI variables in multivariable modeling. The analysis of incremental prognostic value in specific perfusion categories was not possible due to limited statistical sample and larger prospective studies are needed. Similar to previous studies,27,28,–29 we relied on all-cause mortality rather than cardiovascular mortality which may be more closely related to post-stress EF reserve. All-cause mortality is often chosen as an endpoint in cardiovascular studies because physician determined cause-of-death is frequently discrepant with autopsy findings and methods to determine cardiovascular mortality retrospectively may underestimate the true proportion of events.30 Lastly, since the acquisition time for rapid stress was limited, only supine imaging could be performed at rapid stress.
Conclusion
Ejection fraction reserve, ∆ EDV, and ESV measured after early post-stress by solid-state SPECT MPI provide significant, independent, and incremental value for predicting the risk of adverse cardiovascular events. The measurement of LVEF reserve is possible using a solid-state SPECT system and prospective validation of the potential value of this information is warranted.
New Knowledge Gained
Early ejection fraction reserve, measured using solid-state SPECT MPI, was significantly decreased in patients who experienced MACE. Early post-stress (but not late) ejection fraction reserve was independently associated with MACE and improved risk stratification compared to a model with other important SPECT MPI variables.
Abbreviations
- CABG:
-
Coronary artery bypass graft
- CAD:
-
Coronary artery disease
- ECG:
-
Electrocardiogram
- EF:
-
Ejection fraction
- MPI:
-
Myocardial perfusion imaging
- PCI:
-
Percutaneous coronary intervention
- PET:
-
Positron emission tomography
- QPS:
-
Quantitative perfusion SPECT
- SPECT:
-
Single-photon emission computed tomography
- TPD:
-
Total perfusion deficit
References
Doukky R, Frogge N, Balakrishnan G, Hayes K, Collado FM, Rangel MO, et al. The prognostic value of cardiac SPECT performed at the primary care physician’s office. J Nucl Cardiol. 2013;20:519–28.
Berman DS, Kang X, Slomka PJ, Gerlach J, de Yang L, Hayes SW, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14:521–8.
Lima RS, Watson DD, Goode AR, Siadaty MS, Ragosta M, Beller GA, et al. Incremental value of combined perfusion and function over perfusion alone by gated SPECT myocardial perfusion imaging for detection of severe three-vessel coronary artery disease. J Am Coll Cardiol. 2003;42:64–70.
Campeau L, Corbara F, Crochet D, Petitclerc R. Left main coronary artery stenosis: the influence of aortocoronary bypass surgery on survival. Circulation. 1978;57:1111–5.
Wynne J, Cohn LH, Collins JJ Jr, Cohn PF. Myocardial revascularization in patients with multivessel coronary artery disease and minimal angina pectoris. Circulation. 1978;58:I92–5.
Emond M, Mock MB, Davis KB, Fisher LD, Holmes DR Jr, Chaitman BR, et al. Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation. 1994;90:2645–57.
Alama M, Labos C, Emery H, Iwanochko RM, Freeman M, Husain M, et al. Diagnostic and prognostic significance of transient ischemic dilation (TID) in myocardial perfusion imaging: A systematic review and meta-analysis. J Nucl Cardiol. 2018;25:724–37.
Miller RJH, Hu LH, Gransar H, Betancur J, Eisenberg E, Otaki Y, et al. Transient ischaemic dilation and post-stress wall motion abnormality increase risk in patients with less than moderate ischaemia: analysis of the REFINE SPECT registry. Eur Heart J Cardiovasc Imaging. 2020;21:567–75.
Emmett L, Iwanochko RM, Freeman MR, Barolet A, Lee DS, Husain M. Reversible regional wall motion abnormalities on exercise technetium-99 m-gated cardiac single photon emission computed tomography predict high-grade angiographic stenoses. J Am Coll Cardiol. 2002;39:991–8.
Dorbala S, Vangala D, Sampson U, Limaye A, Kwong R, Di Carli MF. Value of vasodilator left ventricular ejection fraction reserve in evaluating the magnitude of myocardium at risk and the extent of angiographic coronary artery disease: a 82Rb PET/CT study. J Nucl Med. 2007;48:349–58.
Brown TL, Merrill J, Volokh L, Bengel FM. Determinants of the response of left ventricular ejection fraction to vasodilator stress in electrocardiographically gated (82)rubidium myocardial perfusion PET. Eur J Nucl Med Mol Imaging. 2008;35:336–42.
Dorbala S, Hachamovitch R, Curillova Z, Thomas D, Vangala D, Kwong RY, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. 2009;2:846–54.
Slomka PJ, Miller RJH, Hu LH, Germano G, Berman DS. Solid-State Detector SPECT Myocardial Perfusion Imaging. J Nucl Med. 2019;60:1194–204.
Imbert L, Poussier S, Franken PR, Songy B, Verger A, Morel O, et al. Compared performance of high-sensitivity cameras dedicated to myocardial perfusion SPECT: a comprehensive analysis of phantom and human images. J Nucl Med. 2012;53:1897–903.
Sharir T, Slomka PJ, Hayes SW, DiCarli MF, Ziffer JA, Martin WH, et al. Multicenter trial of high-speed versus conventional single-photon emission computed tomography imagingquantitative results of myocardial perfusion and left ventricular function. J Am Coll Cardiol. 2010;55:1965–74.
Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol. 2007;14:645–58.
Brodov Y, Fish M, Rubeaux M, Otaki Y, Gransar H, Lemley M, et al. Quantitation of left ventricular ejection fraction reserve from early gated regadenoson stress Tc-99 m high-efficiency SPECT. J Nucl Cardiol. 2016;23:1251–61.
Gambhir SS, Berman DS, Ziffer J, Nagler M, Sandler M, Patton J, et al. A novel high-sensitivity rapid-acquisition single-photon cardiac imaging camera. J Nucl Med. 2009;50:635–43.
Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol. 2016;23:606–39.
Nakazato R, Tamarappoo BK, Kang X, Wolak A, Kite F, Hayes SW, et al. Quantitative upright-supine high-speed SPECT myocardial perfusion imaging for detection of coronary artery disease: Correlation with invasive coronary angiography. J Nucl Med. 2010;51:1724–31.
Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol. 2005;12:66–77.
Usui Y, Chikamori T, Nakajima K, Hida S, Yamashina A, Nishimura T, et al. Prognostic value of post-ischemic stunning as assessed by gated myocardial perfusion single-photon emission computed tomography: a subanalysis of the J-ACCESS study. Circ J. 2010;74:1591–9.
Austin PC, Steyerberg EW. Events per variable (EPV) and the relative performance of different strategies for estimating the out-of-sample validity of logistic regression models. Stat Methods Med Res. 2017;26:796–808.
Agostini D, Roule V, Nganoa C, Roth N, Baavour R, Parienti JJ, et al. First validation of myocardial flow reserve assessed by dynamic (99 m)Tc-sestamibi CZT-SPECT camera: Head to head comparison with (15)O-water PET and fractional flow reserve in patients with suspected coronary artery disease. The WATERDAY study. Eur J Nucl Med Mol Imaging. 2018;45:1079–90.
Gomez J, Golzar Y, Fughhi I, Olusanya A, Doukky R. The significance of post-stress decrease in left ventricular ejection fraction in patients undergoing regadenoson stress gated SPECT myocardial perfusion imaging. J Nucl Cardiol. 2018;25:1313–23.
Bailly M, Thibault F, Brana Q, Courtehoux M, Metrard G, Angoulvant D, et al. Myocardial Blood Flow measurement during SPECT perfusion imaging for coronary artery disease screening: improving diagnostic performance. J Nucl Med. 2020;61:1625.
Otaki Y, Betancur J, Sharir T, Hu LH, Gransar H, Liang JX, et al. 5-year prognostic value of quantitative versus visual MPI in subtle perfusion defects: results from REFINE SPECT. JACC Cardiovasc Imaging. 2019;13(3):774–85.
Knott KD, Seraphim A, Augusto JB, Xue H, Chacko L, Aung N, et al. The prognostic significance of quantitative myocardial perfusion: An artificial intelligence-based approach using perfusion mapping. Circulation. 2020;141:1282–91.
Lloyd-Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–6.
Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease: A review of global methodologies of mortality measurement. Circulation. 2013;127:749–56.
Disclosure
Dr. Slomka participates in software royalties for QPS software at Cedars-Sinai Medical Center and has received research grant support from Siemens Medical Systems. All other authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Otaki, Y., Fish, M.B., Miller, R.J.H. et al. Prognostic value of early left ventricular ejection fraction reserve during regadenoson stress solid-state SPECT-MPI. J. Nucl. Cardiol. 29, 1219–1230 (2022). https://doi.org/10.1007/s12350-020-02420-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02420-w