Abstract
Background
The effect of prasugrel over clopidogrel on myocardial salvage in ST-segment-elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (p-PCI) is not fully elucidated.
Methods
Among 854 consecutive STEMI patients who underwent p-PCI, 446 patients were evaluated by two-phase (7 days and 3 months) single-photo emission computed tomography (SPECT). Patients were divided into two groups based on the loading P2Y12 inhibitor. The clopidogrel group was further divided based on the result of platelet function testing. Thus, the prasugrel group included 227 patients; the clopidogrel without high-residual platelet reactivity (HRPR) group, 109 patients; and the clopidogrel with HRPR group, 107 patients. The primary endpoint was the Myocardial Salvage Index (MSI), determined by SPECT.
Results
The incidence of final TIMI 0/1 and TIMI myocardial perfusion grade 0/1 was higher in the clopidogrel with HRPR group (0.9%, 1.8%, and 7.5%, P = .002; 19.8%, 29.4%, and 41.1%, P = .0002, in the prasugrel, clopidogrel without HRPR, and clopidogrel with HRPR groups, respectively). The MSI was significantly lower in the clopidogrel with HRPR group (48% [27-66], 44% [30-72], and 36% [15-55], P = .006, respectively).
Conclusions
Prasugrel in STEMI patients was associated with an increased MSI compared with clopidogrel in the presence of HRPR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dual antiplatelet therapy with aspirin and P2Y12 inhibitor is the gold standard for treatment of acute coronary syndrome (ACS). Clopidogrel is the most widely used P2Y12 inhibitor, but has shown wide inter-individual variability in the concentration of active metabolite and in the magnitude of platelet inhibition, leading to higher risk of ischemic events after percutaneous coronary intervention (PCI) in patients with high-residual platelet reactivity (HRPR).1 However, it is currently unknown if the myocardial damage at the time of primary PCI (p-PCI) in patients with ST-segment-elevation myocardial infarction (STEMI) is influenced by the results of platelet function testing (PFT).
Prasugrel is a novel P2Y12 inhibitor that inhibits platelet aggregation more rapidly with less inter-individual variability; it has been associated with a lower risk of thrombotic events in patients with ACS than clopidogrel.2 Nevertheless, it is unknown if, in a context of p-PCI to treat STEMI, periprocedural myocardial injury might be attenuated by greater platelet inhibition with prasugrel, because an additional myocardial injury cannot be detectable in this setting, characterized by prior biomarker elevation and ongoing symptom of index myocardial infarction (MI).3 According to worldwide clinical databases, only one-fourth of STEMI patients have received prasugrel, because of a higher bleeding risk along with an unknown acute myocardial salvage effect. Owing to these limitations, clopidogrel still remains the most widely used P2Y12 inhibitor.4,5,6 Therefore, elucidating the effect of prasugrel on the extent of myocardial salvage in patients with STEMI is clinically relevant.
The purpose of this study was to compare the effect of prasugrel over clopidogrel, especially in patients with HRPR, on myocardial salvage in patients with STEMI undergoing p-PCI. To address this issue, we investigated the angiographic findings and Myocardial Salvage Index (MSI) calculated from single-photo emission computed tomography (SPECT).
Methods
Patient Population and Study Protocol
We conducted a single-center registry analysis at Ogaki Municipal Hospital from May 2012 to October 2018. We identified consecutive patients with STEMI that underwent p-PCI within 12 hours after the symptom onset. STEMI is defined as a recent ischemia lasting more than 20 minutes at rest, with one of the following electrocardiographic features: (1) ST-segment elevation (0.1 mV or higher) in two or more contiguous leads or (2) new or alleged new left bundle branch block.7 Exclusion criteria were as follows: (1) contraindication of clopidogrel, prasugrel, or both; (2) admission to the emergency department (ED) more than 12 hours after the symptom onset; (3) in-hospital death; (4) mechanical complications; (5) past history of MI; (6) patients who were not examined by SPECT as defined in this study protocol; and (7) inadequate quality of SPECT.
We designed the study protocol according to a previous study8 (Figure 1). Briefly, oral antiplatelet agents were administered in the ED, followed by coronary angiography (CAG) and p-PCI. We performed 123I-beta-methyl-p-iodophenyl pentadecanoic acid (BMIPP) SPECT within 7 days, PFT on days 7-14, and 99m Technetium (Tc)-tetrofosmin SPECT 3 months after p-PCI. SPECT was routinely performed in all patients except those with hemodynamical instability in the acute phase for BMIPP SPECT and those who were unable to come for a follow-up visit.
Flowchart detailing the study protocol. PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction; BMIPP, 123I-beta-methyl-p-iodophenyl pentadecanoic acid; SPECT, single-photo emission computed tomography; PFT, platelet function testing; ADP PATI, adenosine 5’-diphophate platelet aggregatory threshold index, Tc-TF, 99mTechnetium-tetrofosmin; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; Cath Lab., catheter laboratory; CCU, coronary care unit
On June 2015, long-term prescription of prasugrel was approved by the Japanese Health, Labor and Welfare Ministry. Therefore, since July 2015, we tried to use prasugrel for all patients who needed p-PCI for STEMI. In this study, we divided all patients into two groups, depending on the loading P2Y12 inhibitors in the ED, prasugrel or clopidogrel groups. Then, we further divided the clopidogrel group according to the result of PFT, because clopidogrel had shown a high inter-individual variability in platelet inhibition. Thus, patients were classified into three groups: prasugrel-treated, clopidogrel-treated without HRPR, or clopidogrel-treated with HRPR. As previously reported,9 we found that prasugrel showed less pharmacological variability and a lower HRPR frequency than clopidogrel. Hence, we did not divide patients in prasugrel group based on the PFT.
All the demographic, angiographic, and procedural data of the patients undergoing CAG and PCI were prospectively entered into a dedicated database maintained at our hospital. Analysis of CAG was performed using an automated edge detection system (QAngio XA, Medis Medical Imaging System, Leiden, the Netherlands) by experienced radiology technologists, who were unaware of the treatment allocation. Other clinical data not available in the database were collected from hospital charts. This study was approved by the research review board of Ogaki Municipal Hospital and conducted according to the Helsinki Declaration. Because of its observational nature, requirement of written informed consent from the patients was waived; however, we excluded those patients who refused to participate in the study when contacted at follow-up.
Treatment
We performed p-PCI according to the current standard technique. Indication for PCI after CAG and the decision to administer any adjunctive medication in support of PCI was left to the discretion of the treating physician. Unfractionated heparin was administered in boluses of 5000 IU at the ED and 3000 IU at the start of PCI, followed by additional doses to achieve an activated clotting time of 250–300 seconds. Intracoronary nitrate was administered in all patients before taking the final angiograms. Glycoprotein IIb/IIIa inhibitors are not available in Japan. All patients received a 200-mg loading dose of aspirin with a loading dose of P2Y12 inhibitor (300 mg clopidogrel or 20 mg prasugrel) immediately after the diagnosis of STEMI. After p-PCI, patients received a maintenance dose of aspirin (100 mg) and a maintenance dose of either clopidogrel (75 mg) or prasugrel (3.75 mg). Dosage and administration of prasugrel were according to the Japanese prescription label, whose adjustment was approved by the Japanese Health, Labor and Welfare Ministry to fit the Japanese population.10 Additionally, all patients were given a 20 mg loading dose of atorvastatin in the ED to reduce infarction size.11
Study Endpoint and Definitions
The primary endpoint of this study was the MSI, calculated from SPECT images. The secondary endpoints were the final Thrombolysis in Myocardial Infarction (TIMI) flow grade, the TIMI myocardial perfusion (TMP) grade, the incidence and degree of transient no-reflow phenomenon and left ventricular (LV) volume and function.
Epicardial coronary blood flow in the infarct-related artery was quantified using the TIMI flow grade criteria.12 Myocardial blush grade was assessed at the end of procedure according to the TMP grading.13 No-reflow phenomenon was defined as a reduced antegrade flow (TIMI flow grade ≤ 2) without mechanical obstruction after recanalization.14
SPECT Studies
As shown in Figure 1, BMIPP SPECT study was performed 7 days after p-PCI as acute phase imaging, while 99mTc SPECT was performed 3 months after p-PCI as follow-up imaging. BMIPP image acquisition was started 15 minutes after intravenous injection of 111 MBq of 123I-BMIPP (Cardiodine Injectable 123I-BMIPP; Nihon Medi-Physics). Tc image acquisition was started 30-60 minutes after intravenous injection of 740 MBq of 99mTc-tetrofosmin.
We used the total perfusion deficit (TPD) to calculate the area at risk (AAR) and infarct size, as previously described,15 using quantitative perfused SPECT (QPS) software (AutoQUANT 7.2, Cedars-Sinai Medical Center, Los Angeles, CA, USA) referring to the published normal database of myocardial SPECT.16 Regarding quantitative gated SPECT (QGS) data, QGS software (Cedars-Sinai Medical Center, Los Angeles, CA, USA) was used to calculate LV function and volume. The SPECT data were collected and calculated as follows: AAR (%) as TPD on 123I-BMIPP polar-map; infarct size (%) as TPD on 99mTc-tetrofosmin polar-map; MSI = (AAR − infarct size)/AAR; LV volume and function (LV end-systolic volume [LVESV], LV end-diastolic volume [LVEDV], and LV ejection fraction [LVEF]) based on 99mTc-tetrofosmin QGS data. All SPECT analyses were performed by experienced radiology technologists who were unaware of patients’ and angiographic characteristics.
PFT
PFT was performed with an aggregometer of the whole blood (Mebanix Co., Ltd., Yokohama, Japan) based on the screen filtration pressure method. It measures the flow resistance of whole-blood samples through a screen of micro-sieve with 30 μm2 openings and provides the pressure rate as an index of platelet aggregation. We measured the platelet aggregatory threshold index (PATI), defined as the concentration of adenosine 5′-diphophate (ADP) necessary to induce a 50% of pressure rate. The reproducibility of this method is verified in the literature.17 Four reaction tubes containing 200-μL aliquots of whole blood were prepared and 22.2-μL of ADP solution at four different concentrations (2, 4, 8, and 16-μM) was added. In case of non-achievement of 50% pressure rate even with 16-μM of ADP, the concentration of the added ADP solution was increased up to 80-μM. As PATI indicates the concentration of agonist (ADP) necessary to induce platelet aggregation, higher PATI (needs more agonist) indicates the resistance of blood to clot and lower PATI indicates high-platelet reactivity.
Because there was no definitive cut-off value of this assay, we adopted the median value of PATI in patients taking clopidogrel as the cut-off value to identify HRPR, based on published epidemiological data showing that approximately 50% of Japanese ACS patients were classified as intermediate- or poor-metabolizers of clopidogrel.18,19
Statistical Analyses
Continuous variables were shown as mean with standard deviation (SD) or as median with interquartile range (IQR), while categorical variables are shown as numbers and proportions. Continuous variables among the three groups were compared using the one-way analysis of variance or the Kruskal–Wallis test, based on the variable distributions. Categorical variables were compared using chi-squared test. We adapted continuous covariates into two qualitative categories using clinically significant reference.
To address the problem of multiple testing, we first compared the clinical outcomes among all three groups. If we found a significant difference, we then proceeded with a comparison between each group. We used the Holm method to adjust P values in multiple testing20
All statistical analyses were performed using JMP software version 13.1 (SAS Institute Inc., Cary, NC, USA). A P value < .05 was considered statistically significant.
Results
PFT and Patient Classification
Of 1202 patients who underwent emergency PCI during the study period, we identified 446 eligible patients who presented with STEMI within 12 hours from the symptom onset and did not meet the exclusion criteria (Figure 2). Of note, six patients were excluded because of the lack of available analysis of TPD on BMIPP SPECT, while five patients were excluded because of no accumulation of BMIPP. Of the eligible patients, 227 patients were initiating from prasugrel, while 219 were initiating from clopidogrel. Among those initiating from clopidogrel, PFT was not performed in three patients. The median value of ADP-PATI in the clopidogrel group was 4.0 (IQR = 3.1-9.7), which led to the definition of HRPR as patient having ADP-PATI ≤ 4.0. Accordingly, 227 patients were classified into the prasugrel group; 109 patients initiating from clopidogrel with ADP-PATI > 4, the clopidogrel without HRPR group; and 107 patients initiating from clopidogrel with ADP-PATI ≤ 4, the clopidogrel with HRPR group. The median value of ADP-PATI was significantly higher in the prasugrel group than in the clopidogrel with and without HRPR groups (13.2 [6.8-28], 9.7 [6.7-14.1], and 3.1 [2.3-3.5], P < .0001, respectively).
Patient flowchart. PCI, percutaneous coronary intervention; NSTE-ACS, non-ST-segment-elevation acute coronary syndrome; STEMI, ST-segment-elevation myocardial infarction; MI, myocardial infarction; IRA, infarct-related artery; BMIPP, 123I-beta-methyl-p-iodophenyl pentadecanoic acid; SPECT, single-photo emission computed tomography; Tc-TF, 99mTechnetium-tetrofosmin; PFT, platelet function testing
Baseline Characteristics
Clinical characteristics of the enrolled patients are summarized in Table 1. The peak value of creatine kinase (CK) and CK myocardial band were comparable among the three groups.
Regarding lesion and procedural characteristics, culprit vessel, number of diseased vessels, onset-to-balloon time were similar. Manual thrombectomy was more frequently used in the clopidogrel groups (58.6% in the prasugrel group, 74.3% in the clopidogrel without HRPR group, and 78.5% in the clopidogrel with HRPR group, P = .0003), whereas all but one patient who underwent ELCA was in the prasugrel group. Intra-aortic balloon pumping was less frequently used in the prasugrel group than in the clopidogrel with and without HRPR groups (17.6%; 34.9%; 39.3%, P < .0001, respectively). This difference was associated with an increased incidence of no-reflow phenomenon, thrombus protrusion into the implanted stents, side branch occlusion, and distal embolization in the clopidogrel groups. Clinical characteristics, excluding those patients who underwent ELCA, are shown in Supplemental Table 1.
Regarding medication at discharge, the prescription rate of beta-blocker was significantly larger in the prasugrel group than in the clopidogrel groups.
Angiographic Results
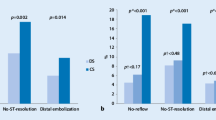
The angiographic results before-and-after PCI are shown in Table 2. The incidence of no-reflow phenomenon was higher in patients in the clopidogrel without HRPR group, while that of severe no-reflow (TIMI 0/1) was higher in the clopidogrel with HRPR group. Therefore, the incidence of worse final flow and myocardial perfusion was higher in the clopidogrel with HRPR group (final TIMI 0/1 flow: 0.9%, 1.8%, and 7.5%, P = .002; TMP 0/1: 19.8%, 29.4%, and 41.1%, P = .0002, in the prasugrel, in the clopidogrel without HRPR, in the clopidogrel with HRPR groups, respectively).
SPECT Analysis
The results of SPECT studies are shown in Table 3 and Figure 3. The MSI was numerically higher in the prasugrel group than in the whole clopidogrel group (48% [27-66] vs 41% [23-64], P = .2). Among the three groups, the MSI was significantly lower in the clopidogrel with HRPR group (48% [27-66] in the prasugrel group, 44% [30-72] in the clopidogrel without HRPR group, 36% [15-55] in the clopidogrel with HRPR group, P = .006) (Figure 3B). After excluding those patients who underwent ELCA, the result was still consistent with the main analysis (48% [25-69], 45% [31-72], and 36% [15-55], P = .007, respectively). When we divided the patients based on the onset-to-balloon time (≤ 180 and > 180 minutes), no significant difference were seen among the three groups in patients with onset-to-balloon time ≤ 180 minutes (45% [27-66], 53% [32-73], and 43% [21-64], P = .10, respectively); in contrast, there was a significant difference in patients with onset-to-balloon time > 180 minutes (49% [25-66], 40% [22-68], and 29% [12-49], P = .008, respectively). Each representative case of the three groups are shown in Figure 4.
Representative cases in the prasugrel (A), clopidogrel without high-residual platelet reactivity (HRPR) (B), and clopidogrel with HRPR groups (C). (A) A 65-year-old woman presented with a first anterior myocardial infarction (MI). The pre-angiogram revealed severe stenosis of proximal left anterior descending artery (LAD) with TIMI 2 flow (A-1). The final angiogram revealed good expansion of the culprit lesion with TIMI 3 flow (A-2). The onset-to-balloon time was 184 minutes. The total perfusion deficit (TPD) on BMIPP SPECT on the post-operative day (POD) 4 was 43% (A-3). After 3 months, the TPD on Tc SPECT was 13% (A-4). (B) A 54-year-old man presented with a first anterior MI. The pre-angiogram revealed severe stenosis of proximal LAD with TIMI 2 flow (B-1). The culprit lesion was well expanded with good flow (TIMI 3) but with moderate myocardial blush (TMP 2) (B-2). The onset-to-balloon time was 173 minutes. BMIPP SPECT on the POD 7 revealed TPD of 35% (B-3). After 3 months, Tc SPECT revealed the TPD of 19% (B-4). (C) A 66-year-old man presented with a first anterior MI. The emergent angiogram showed severe stenosis of proximal LAD with TIMI 2 flow (C-1). Finally, good expansion of the culprit lesion was obtained with good flow (TIMI 3) but moderate myocardial blush (TMP 2) (C-2). The onset-to-balloon time was 140 minutes. BMIPP SPECT on the POD 5 showed TPD of 34% (C-3). After 3 months, Tc SPECT showed the TPD of 29% (C-4)
Regarding QGS measurements, LVEDV and LVESV were smaller and LVEF was numerically higher in the prasugrel group (LVEDV: 90 mL [71-108], 95 mL [78-113], and 97 mL [81-127], P = .005; LVESV: 37 mL [26-50]; 41 mL [28-54]; 42 mL [27-57], P = .04; LVEF: 59% [50-66], 57% [51-66], and 57% [47-64], P = .68, in the prasugrel, in the clopidogrel without HRPR, in the clopidogrel with HRPR groups, respectively).
Bleeding Complications
The incidence of TIMI major or minor bleeding were comparable among the three groups (12 [5.3%], 6 [5.5%], and 6 [5.6%], P = .99, in the prasugrel, in the clopidogrel without HRPR, in the clopidogrel with HRPR groups, respectively). In the subgroup of patients with a high risk of bleeding, as reported in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet inhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction (TRITON-TIMI) 38 study,2 the incidence of TIMI major or minor bleeding among those aged ≥ 75 years or those with body weight (BW) < 60 kg was not different among the three groups (aged ≥ 75 years: 6 [8.8%], 2 [6.5%], and 1 [4.0%], P = .71; BW < 60 kg: 7 [9.3%], 4 [10.0%], and 3 [7.5%], P = .91, respectively).
Discussion
In this study, we assessed the effect of prasugrel and clopidogrel on myocardial salvage in patients with STEMI undergoing p-PCI, in the presence or absence of HRPR. The main findings of this study are as follows:
-
1.
The MSI was numerically higher in the prasugrel group than in the whole clopidogrel group.
-
2.
The prasugrel group demonstrated a similar degree of MSI to the clopidogrel without HRPR group; the clopidogrel with HRPR group showed worse myocardial salvage than the other two groups.
-
3.
A lower incidence of no-reflow phenomenon was noted in the prasugrel group, subsequently resulting in a more frequent good final TMP grade in this group.
This study highlighted the clinical importance of using prasugrel for myocardial salvage in STEMI patients undergoing p-PCI compared to that of clopidogrel, for which we observed a wide inter-individual variability in the platelet inhibitory response, as already reported. Consistent with our findings, prasugrel demonstrated a superior efficacy over clopidogrel in the TRITON-TIMI 38 study.2,21 However, the main targets of previous studies were patients with non-ST-segment-elevation ACS.2,4,21,22 Currently, there are only few studies investigating the efficacy of prasugrel and clopidogrel for myocardial salvage in patients with STEMI. Although a sub-study of the TRITON-TIMI 38 program has previously compared clinical outcomes between prasugrel and clopidogrel, the comparison of the extent of myocardial damage in patients with STEMI was limited, because an additional myocardial injury cannot be detected without measuring the MSI on paired SPECT or magnetic resonance imaging.3 The novelty of this study is the measurement MSI on SPECT, demonstrating the significant difference between the efficacy of two drugs by evaluating the impact of residual platelet reactivity. In this regard, the present study has important clinical implication.
One potential explanation for our findings is the degree of platelet inhibition. p-PCI for STEMI is an approach that needs a rapid and strong inhibition of platelet activation and aggregation to achieve therapeutic success. In TRITON-TIMI 38 study, glycoprotein IIb/IIIa inhibitors were used more frequently in patients treated with clopidogrel than in those with prasugrel.21 It was reported that this difference was resulted from the bailout use of glycoprotein IIb/IIIa for thrombosis. In this study, we observed a higher incidence of thrombus protrusion stents, side branch occlusion, and distal embolization in patients treated with clopidogrel. Because periprocedural myocardial injury is a partially platelet-dependent event,23 more potent and prompt platelet inhibition might explain the increased salvage index in this study. Given that a successful reperfusion greatly affects the reduction in infarct size and LV function,24 better LV volume and function in the prasugrel group further support this hypothesis.
Published pharmacodynamic data have shown that prasugrel can achieve platelet inhibition much faster than clopidogrel after loading dose administration (30 minutes vs 6 hours).25,26 Conversely, in a sub-study of TRITON-TIMI 38 investigating the relationship between CYP2C19 genetic polymorphisms and the response to prasugrel and clopidogrel, non-carriers of CYP2C19 loss-of-function mutation treated with clopidogrel had similar rate of major adverse cardiovascular events compared to those treated with prasugrel.9,27 Thus, it is plausible that the development of HRPR during clopidogrel treatment, in addition to the rate of clopidogrel metabolism, may also contribute to the clinical outcomes.
In this study, we used BMIPP SPECT as a substitute for AAR. In normal myocardium, BMIPP enters the myocardial cell as BMIPP-CoA according to regional blood flow and most of the fraction is subsequently retained in the lipid pool.28 Under ischemic condition, the myocardial energy metabolism shifts from fatty acid to glucose. In addition, back-diffusion of non-metabolized BMIPP from the myocardium increases.28 Therefore, BMIPP reflect AAR as an impaired fatty acid metabolism even after successful revascularization. Kawai et al. reported the ability of BMIPP imaging at 1 week after MI to identify AAR is similar to that of Tc perfusion frozen imaging before revascularization.29 Tanaka et al. also reported that the risk areas illustrated by frozen image with Tc before PCI were very close to risk areas on BMIPP image after seven days, though AAR in BMIPP image days was slightly smaller than that in frozen image.30 These data indicate that it is appropriate for this study to use BMIPP SPECT after 1 week as a substitute for AAR, though MSI may be slightly underestimated. Furthermore, the close correlations between physiological characteristics on BMIPP and perfusion SPECT image and microvascular obstruction on cardiac magnetic resonance imaging or coronary flow velocity by doppler wire were reported.31,32 Therefore, it was reasonable to use MSI calculated from BMIPP and perfusion SPECT imaging to investigate the difference of periprocedural myocardial injury partially mediated by thrombus embolization between prasugrel and clopidogrel.
Limitations
First, it was performed at a single medical center with observational design. To reduce the selection bias, we enrolled consecutive patients and determined the treatment according to the date of enrollment; still there might be a selection bias which is related to inclusion period. In addition, we did not examine the clinical outcome in relation to the MSI. Second, the doses of prasugrel used in Japan were approximately one-third of the dose approved in Western countries. Although Japanese phase III trial compared reduced dose of prasugrel with standard dose of clopidogrel, this prasugrel dose showed a similar efficacy to that used in the TRITON-TIMI 38 study, with a lower incidence of bleeding complications.2,33 It may be difficult to entirely translate the results of our study to clinical practice in Western countries because of the different usage of prasugrel dose; however, the fact that a lower dose of prasugrel than the standard dose showed effectiveness for myocardial salvage might have clinical relevance outside Japan. Third, beta-blocker was more frequently used in the prasugrel group than in the other groups. However, the effect of beta-blocker on CK rise or LV remodeling prevention is limited.34,35 Finally, the rate of manual aspiration and intra-aortic balloon pumping (IABP) was higher in the clopidogrel groups. However, manual thrombectomy has been proven not to reduce thrombus burden,36,37 leading to the absence of clinical benefit in randomized studies.38 Moreover, the concomitant use of IABP did not reduce the infarct size.39 Therefore, we think that the differences between the use of the manual aspiration or IABP among the three groups did not affect the results of this study.
Conclusion
In this study of STEMI patients, prasugrel in STEMI patients was associated with a higher MSI compared with clopidogrel in the presence of HRPR.
New Knowledge Gained
The prasugrel group demonstrated a similar degree of MSI to the clopidogrel without HRPR group; the clopidogrel with HRPR group showed worse myocardial salvage than the other two groups. Our findings suggest that prasugrel is an attractive alternative to clopidogrel in the management of patients with STEMI, especially considering that in this challenging clinical setting, we cannot use PFT to guide decision-making, as such urgent conditions need prompt drug treatment.
Abbreviations
- AAR:
-
Area at risk
- BMIPP:
-
123I-beta-methyl-p-iodophenyl pentadecanoic acid
- HRPR:
-
High-residual platelet reactivity
- MSI:
-
Myocardial Salvage Index
- PFT:
-
Platelet function testing
- p-PCI:
-
Primary percutaneous coronary intervention
- SPECT:
-
Single-photo emission computed tomography
- STEMI:
-
ST-segment-elevation myocardial infarction
- Tc:
-
99mTechnetium
- TPD:
-
Total perfusion deficit
References
Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: Response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003;107:2908-13.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15.
Bonaca MP, Wiviott SD, Braunwald E, Murphy SA, Ruff CT, Antman EM, et al. American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: Observations from the TRITON-TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38). Circulation 2012;125:577-83.
Baber U, Sartori S, Aquino M, Kini A, Kapadia S, Weiss S, et al. Use of prasugrel vs clopidogrel and outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention in contemporary clinical practice: Results from the PROMETHEUS study. Am Heart J 2017;188:73-81.
Danchin N, Lettino M, Zeymer U, Widimsky P, Bardaji A, Barrabes JA, et al. Use, patient selection and outcomes of P2Y12 receptor inhibitor treatment in patients with STEMI based on contemporary European registries. Eur Heart J Cardiovasc Pharmacother 2016;2:152-67.
Kim K, Lee TA, Touchette DR, DiDomenico RJ, Ardati AK, Walton SM. Contemporary trends in oral antiplatelet agent use in patients treated with percutaneous coronary intervention for acute coronary syndrome. J Manag Care Spec Pharm 2017;23:57-63.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237-69.
Ishihara M, Asakura M, Kimura K, Nakao K, Hamada C, Hirayama A. Trial design and rationale of TY-51924 as a novel Na+/H+ exchanger inhibitor in patients with ST-elevation acute myocardial infarction undergoing percutaneous coronary intervention. J Cardiol 2014;63:82-7.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: Relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009;119:2553-60.
Nakamura M, Iizuka T, Sagawa K, Abe K, Chikada S, Arai M. Prasugrel for Japanese patients with acute coronary syndrome in short-term clinical practice (PRASFIT-Practice I): A postmarketing observational study. Cardiovasc Interv Ther 2018;33:135-45.
Kim JS, Kim J, Choi D, Lee CJ, Lee SH, Ko YG, et al. Efficacy of high-dose atorvastatin loading before primary percutaneous coronary intervention in ST-segment elevation myocardial infarction: The STATIN STEMI trial. JACC Cardiovasc Interv 2010;3:332-9.
Group TS. The thrombolysis in myocardial infarction (TIMI) trial Phase I findings. N Engl J Med 1985;312:932-6.
Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, et al. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 2000;101:125-30.
Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, et al. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol 2000;36:1202-9.
Slomka PJ, Nishina H, Berman DS, Akincioglu C, Abidov A, Friedman JD, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol 2005;12:66-77.
Nakajima K. Normal values for nuclear cardiology: Japanese databases for myocardial perfusion, fatty acid and sympathetic imaging and left ventricular function. Ann Nucl Med 2010;24:125-35.
Ozeki Y, Sudo T, Toga K, Nagamura Y, Ito H, Ogawa T, et al. Characterization of whole blood aggregation with a new type of aggregometer by a screen filtration pressure method. Thromb Res 2001;101:65-72.
Nagashima Z, Tsukahara K, Morita S, Endo T, Sugano T, Hibi K, et al. Platelet reactivity in the early and late phases of acute coronary syndromes according to cytochrome P450 2C19 phenotypes. J Cardiol 2013;62:158-64.
Yamamoto K, Hokimoto S, Chitose T, Morita K, Ono T, Kaikita K, et al. Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy. J Cardiol 2011;57:194-201.
Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65-70.
Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): Double-blind, randomised controlled trial. Lancet 2009;373:723-31.
Chin CT, Wang TY, Anstrom KJ, Zhu B, Maa JF, Messenger JC, et al. Treatment with adenosine diphosphate receptor inhibitors-longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) study design: Expanding the paradigm of longitudinal observational research. Am Heart J 2011;162:844-51.
Steinhubl SR, Lauer MS, Mukherjee DP, Moliterno DJ, Lincoff AM, Ellis SG, et al. The duration of pretreatment with ticlopidine prior to stenting is associated with the risk of procedure-related non-Q-wave myocardial infarctions. J Am Coll Cardiol 1998;32:1366-70.
Ishii H, Amano T, Matsubara T, Murohara T. Pharmacological intervention for prevention of left ventricular remodeling and improving prognosis in myocardial infarction. Circulation 2008;118:2710-8.
Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA 2002;288:2411-20.
Brandt JT, Payne CD, Wiviott SD, Weerakkody G, Farid NA, Small DS, et al. A comparison of prasugrel and clopidogrel loading doses on platelet function: Magnitude of platelet inhibition is related to active metabolite formation. Am Heart J 2007;153(66):e69-76.
Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009;360:354-62.
Yoshinaga K, Naya M, Shiga T, Suzuki E, Tamaki N. Ischaemic memory imaging using metabolic radiopharmaceuticals: Overview of clinical settings and ongoing investigations. Eur J Nucl Med Mol Imaging 2014;41:384-93.
Kawai Y, Tsukamoto E, Nozaki Y, Kishino K, Kohya T, Tamaki N. Use of 123I-BMIPP single-photon emission tomography to estimate areas at risk following successful revascularization in patients with acute myocardial infarction. Eur J Nucl Med 1998;25:1390-5.
Tanaka R, Nakamura T. Time course evaluation of myocardial perfusion after reperfusion therapy by 99mTc-tetrofosmin SPECT in patients with acute myocardial infarction. J Nucl Med 2001;42:1351-8.
Mori H, Isobe S, Sakai S, Yamada T, Watanabe N, Miura M, et al. Microvascular obstruction on delayed enhancement cardiac magnetic resonance imaging after acute myocardial infarction, compared with myocardial (201)Tl and (123)I-BMIPP dual SPECT findings. Eur J Radiol 2015;84:1516-24.
Suzuki N, Hiasa Y, Takahashi T, Chen H, Miyazaki S, Mahara K, et al. Early prediction of myocardial salvage after primary coronary angioplasty: Comparative study of coronary flow velocity pattern immediately after primary coronary angioplasty and perfusion-metabolism mismatch. J Cardiol 2007;49:163-70.
Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: The PRASFIT-ACS study. Circ J 2014;78:1684-92.
Ellis SG, Brener SJ, Lincoff AM, Moliterno DJ, Whitlow PL, Schneider JP, et al. beta-blockers before percutaneous coronary intervention do not attenuate postprocedural creatine kinase isoenzyme rise. Circulation 2001;104:2685-8.
Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet 2001;357:1385-90.
Bhindi R, Kajander OA, Jolly SS, Kassam S, Lavi S, Niemela K, et al. Culprit lesion thrombus burden after manual thrombectomy or percutaneous coronary intervention-alone in ST-segment elevation myocardial infarction: The optical coherence tomography sub-study of the TOTAL (ThrOmbecTomy versus PCI ALone) trial. Eur Heart J 2015;36:1892-900.
Onuma Y, Thuesen L, van Geuns RJ, van der Ent M, Desch S, Fajadet J, et al. Randomized study to assess the effect of thrombus aspiration on flow area in patients with ST-elevation myocardial infarction: An optical frequency domain imaging study—TROFI trial. Eur Heart J 2013;34:1050-60.
Jolly SS, Cairns JA, Yusuf S, Meeks B, Pogue J, Rokoss MJ, et al. Randomized trial of primary PCI with or without routine manual thrombectomy. N Engl J Med 2015;372:1389-98.
Patel MR, Smalling RW, Thiele H, Barnhart HX, Zhou Y, Chandra P, et al. Intra-aortic balloon counterpulsation and infarct size in patients with acute anterior myocardial infarction without shock: The CRISP AMI randomized trial. JAMA 2011;306:1329-37.
Disclosures
Dr. Ishii received lecture fees from Astellas Pharma Inc., Bayer Pharmaceutical Co., Ltd., Daiichi-Sankyo Pharma Inc., and MSD K. K. Dr. Murohara received lecture fees from Bayer Yakuhin., Ltd., Daiichi-Sankyo Co., Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd. Dr. Yoshida, Takagi, Morishima, Tanaka, Morita, Kanzaki, Nagai, Watanabe, Furui, Shibata, Yoshioka, Yamauchi, Komeyama, Sugiyama, and Tsuboi have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
This work was not supported by any funding. Department of Cardiology, Nagoya University Graduate School of Medicine received research Grant from Astellas Pharma Inc., Daiichi-Sankyo Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Kowa Co., Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co., Ltd., Pfizer Japan Inc., and Teijin Pharma Ltd.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoshida, R., Takagi, K., Ishii, H. et al. Myocardial salvage after ST-segment-elevation myocardial infarction: comparison between prasugrel and clopidogrel in the presence or absence of high-residual platelet reactivity. J. Nucl. Cardiol. 28, 1422–1434 (2021). https://doi.org/10.1007/s12350-019-01852-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01852-3