Abstract
Background
Prevalence and prognostic value of diastolic and systolic dyssynchrony in patients with coronary artery disease (CAD) + heart failure (HF) or CAD alone are not well understood.
Methods
We included patients with gated single-photon emission computed tomography (GSPECT) myocardial perfusion imaging (MPI) between 2003 and 2009. Patients had at least one major epicardial obstruction ≥ 50%. We assessed the association between dyssynchrony and outcomes, including all-cause and cardiovascular death.
Results
Of the 1294 patients, HF was present in 25%. Median follow-up was 6.7 years (IQR 4.9-9.3) years with 537 recorded deaths. Patients with CAD + HF had a higher incidence of dyssynchrony than patients with CAD alone (diastolic BW 28.8% for the HF + CAD vs 14.7% for the CAD alone). Patients with CAD + HF had a lower survival than CAD alone at 10 years (33%; 95% CI 27-40 vs 59; 95% CI 55-62, P < 0.0001). With one exception, HF was found to have no statistically significant interaction with dyssynchrony measures in unadjusted and adjusted survival models.
Conclusions
Patients with CAD + HF have a high prevalence of mechanical dyssynchrony as measured by GSPECT MPI, and a higher mortality than CAD alone. However, clinical outcomes associated with mechanical dyssynchrony did not differ in patients with and without HF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Left ventricular mechanical dyssynchrony (LVMD) indicates a difference in the timing of mechanical contraction or relaxation between different segments of the left ventricle (LV). It is distinct from electrical dyssynchrony, which is manifested by a prolonged QRS interval with or without left bundle branch block (LBBB), yet often associated with it.1 Systolic LVMD is well recognized as a risk factor for negative clinical outcomes in various patient populations2,3,4,5 and a valuable predictor of risk in patients with coronary artery disease (CAD)6 and heart failure with reduced ejection fraction (HFrEF).7,8,9,10 In patients with CAD, systolic LVMD had a stronger association with cardiovascular death compared to electrical dyssynchrony.6
Diastolic LVMD, which refers to the asynchrony in LV relaxation, was found to have only a weak relationship with systolic LVMD, suggesting that systolic and diastolic LVMD processes have different underlying pathogenic mechanisms, and therefore potentially different prognostic implications.11,12 While methods to study diastolic LVMD have only recently been outlined, the prognostic utility and clinical significance remain mostly unknown. Importantly, it appears that similarly to systolic LVMD, diastolic LVMD is common among patients with CAD1,13 and patients with heart failure (HF).11,14 Recent advances in gated single-photon emission computed tomography (GSPECT) myocardial perfusion imaging (MPI) imaging now also allow to measure diastolic LVMD.15,16 In patients with CAD, both diastolic and systolic LVMD measured by GSPECT MPI were associated with worse outcomes, independent of electrical dyssynchrony.16,17
In our current study, we aimed to examine (1) the prevalence of diastolic and systolic LVMD in the largest cohort of patients with CAD and HF; (2) test the prognostic value of diastolic and systolic LVMD using the novel GSPECT MPI-based technique in patients with CAD + HF vs CAD alone; and (3) newly explore potential differences in prognostic value of diastolic and systolic dyssynchrony between patients with HFrEF and heart failure with preserved ejection fraction (HFpEF).
Methods
Data Sources
Data were collected from two single-center, observational registries of patients undergoing cardiovascular procedures and cardiac imaging: Duke Nuclear Cardiology Databank and Duke Databank for Cardiovascular Disease (DDCD). Longitudinal records were created for patients who had at least one major epicardial CAD (≥ 50% stenosis). Clinical and demographic data were collected at the time of the procedure and are electronically linked across the above two registries.
Study Population
Our current analysis included patients who consecutively underwent GSPECT MPI between years 2003 and 2009 and had subsequent left heart catheterization to confirm CAD, which was defined as the ≥ 50% stenosis in at least one epicardial coronary artery. Heart failure status and symptom severity were defined by Duke clinical providers. Symptom severity was assessed with New York Heart Association (NYHA) classification during the most proximal clinic visit or at the time of the diagnostic study. If NYHA class was not assessed, then the patient was labeled as “NYHA – none” (Table 1). Ejection fraction was measured by GSPECT MPI, and HFrEF was defined as an EF of < 50% and HFpEF as ≥ 50%.18 Additional inclusion criteria were (1) presence of sinus rhythm at the time of imaging; and (2) availability of ECG results within 60 days before or after imaging. We also calculated LVMD for a control group of 103 patients. This control population consisted of patients who had no clinical history of CAD, had an EF of ≥ 50% on GSPECT MPI without any evidence of perfusion deficits, had a QRS duration ≤ 120 ms, and were in normal sinus rhythm. Notably, we have previously published an analysis using a portion of these patients in which we were able to demonstrate a high degree of reproducibility of phase analysis among controls.19
Stress Testing, GSPECT MPI, and Nuclear Dyssynchrony Measurement
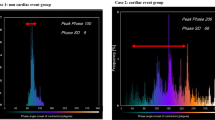
Patients capable of exercising underwent stress testing on a treadmill. The Bruce protocol was implemented unless the referring physician requested an alternative protocol. Patients who were unable to exercise underwent pharmacologic stress testing. Duke’s SPECT protocol, which we implemented in our current analysis, has been previously described.20,21 Multi-head detectors were used to obtain the images post stress. GSPECT images were obtained with Myoview (99mTc, 21-30 mCi; GE Healthcare). We then used a novel research upgrade of the commercially available software (Emory Cardiac Toolbox™ v4 with SyncTools™) to post-process raw stress SPECT data and obtain the dyssynchrony measurements of phase standard deviation (SD) and phase bandwidth (BW). The new methodology to measure diastolic dyssynchrony has been recently described.15,16 In brief, left ventricular diastolic dyssynchrony was measured via phase analysis. Regional maximal count detection was conducted for individual temporal frames in the short-axis GPECT images. Changes in regional counts are proportional to changes in regional myocardial wall thickening over the cardiac cycle and are the basis of the phase analysis. After the onset of mechanical contraction or relaxation, phase angles of the entire left ventricle were generated, and a phase distribution was created that provided data on the degree of systolic and diastolic dyssynchrony. The phase distribution was displayed in a polar map and histogram15 representing timing of mechanical contraction/relaxation. Systolic SD and systolic BW were calculated using phase histograms.
Follow-Up and Outcome Measures
The DDCD follow-up staff facilitated patient follow-up on an annual basis in order to determine vital status.22,23 Clinical follow-up was provided till the year 2015, thus providing information on long-term vital status on most patients. Deaths and their causes were adjudicated by two independent data abstractors and a faculty cardiologist using data from mailed questionnaires, telephone interviews with family members, death certificates, hospital discharge summaries, and/or matches from a search of the National Death Index with International Classification of Diseases, Ninth Revision, (ICD-9) coded cause of death. We used all-cause mortality and cardiovascular (CV) mortality as co-primary outcomes for our analysis.
Statistical Analysis
Distributions of baseline clinical characteristics are described using medians (25th and 75th percentiles) for continuous variables and percentages for ordinal and categorical variables. Testing for differences included Wilcoxon rank tests for continuous variables and Chi-Square or Exact tests for categorical variables. Means and SD are also provided for the continuous dyssynchrony measures. We provided histograms to graphically demonstrate the dyssynchrony distributions between patients with CAD + HF and those with CAD alone, and we assessed the normality of the dyssynchrony measures with Shapiro-Wilk testing. Estimates for time to all-cause death and CV death were obtained via the Kaplan-Meier method. For that purpose, we classified patients based on whether or not they have HF. Patients with HF were then sub-divided into 2 categories: EF ≥ 50% (HFpEF) and EF < 50% (HFrEF). The cumulative incidence function was used to estimate CV mortality, accounting for non-CV mortality as a competing risk. The cut-off for abnormal values was defined as two standard deviations above or below the mean of the respective dyssynchrony variable in the control population. We used Cox proportional hazards regression modeling to examine the associations between the two dyssynchrony parameters (phase BW and phase SD) with CV and all-cause death. Cubic polynomial spline testing was used to examine the linear relationships between continuous measures and outcomes, implementing transformations when necessary to satisfy the linearity assumption.24 Baseline clinical variables that were thought to be associated with prognosis were included for adjustment in multivariable Cox proportional hazards regression models. Those characteristics included age, gender, race, history of hypertension, HF, peripheral vascular disease, cerebrovascular disease, smoking, diabetes, prior myocardial infarction, chronic obstructive pulmonary disease, and chronic renal disease (defined as creatinine > 3.0, dialysis, or transplant). Multivariable models were examined to assess mechanical dyssynchrony measures (phase BW and SD) for patients with HF and those without HF using interaction terms. In addition to clinical characteristics, adjustment covariates included electrical dyssynchrony measured by QRS duration and presence of a LBBB and LVEF.
All tests were two-sided, and significance was determined by P < 0.05. Analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC). The Duke University Health System institutional review board approved the study and granted a waiver of consent.
Results
Baseline Characteristics
A total of 1294 patients were included in the analysis, of them 327 patients had a history of HF while 967 did not (Figure 1). Baseline characteristics of the study cohort were stratified based on HF status and are provided in Table 1. Across the population, the median age was 64 years (IQR 55-72), 69.9% were male, and 73.2% were Caucasian and 22.8% were African American. Patients with HF had a higher incidence of hypertension, peripheral artery disease, chronic obstructive pulmonary disease, renal disease, diabetes mellitus, and LBBB. Further, HF patients had longer QRS intervals (98 ms [IQR 88-120] vs 92 ms [IQR 84-102], P < 0.001), higher end-diastolic volumes (138 ml [IQR 97-190] vs 111 ml [IQR 88-139], P < 0.001), and lower LVEF (54% [IQR 38-67] vs 63% [SD 54-70], P < 0.001). There were 185 patients with HFpEF and 142 patients with HFrEF. There were 537 deaths in the study population, of which 298 were CV deaths. The median length of follow-up was 7.1 years [IQR of 5.0-9.3].
Mechanical Dyssynchrony Parameters
Prevalence (Figure 1) and distribution (Supplement Figure 1) of diastolic and systolic dyssynchrony parameters in patients with CAD and HF were compared to those with CAD alone. Also presented is the distribution of the mechanical dyssynchrony in the control population (Supplement Figure 1). Neither diastolic nor systolic dyssynchrony parameters had a normal distribution in the studied population. Patients with HF had a greater degree of dyssynchrony regardless of diastolic or systolic parameter (P < 0.001) and patients with CAD + HFrEF had greater degree of systolic and diastolic dyssynchrony than CAD + HFpEF. The cut-off for abnormal mechanical dyssynchrony (two SDs above mean) was: diastolic BW = 155.8, systolic BW = 134.0, diastolic SD = 51.3, and systolic SD = 46.9. The number of patients with any diastolic and any systolic BW above the upper limit of normal was 94 (28.8%) and 100 (30.6%) for the HF + CAD, respectively, vs 142 (14.7%) and 140 (14.5%) for the CAD alone group. The number of patients with any diastolic and any systolic SD above the upper limit of normal was 90 (27.5%) and 91 (27.8%) for the HF + CAD group, respectively, vs 130 (13.4%) and 131 (13.6%) for the CAD alone group. The majority of diastolic and systolic dyssynchrony was present in patients with HFrEF as opposed to HFpEF (Figure 2).
Unadjusted Outcomes
Kaplan-Meier event rates for all-cause mortality and CV mortality were stratified according to HF status and LVEF (HF with EF ≥ 50% vs HF with EF < 50%) (Figure 3). At 5 years, the Kaplan-Meier mortality estimate was 20.6% among patients without HF, 27.2% among patients with HF and EF ≥ 50%, and 51.4% among patients with HF and EF < 50% (overall P < 0.001). For CV death, 5-year event estimates for patients without HF were 10.8%, for those with HF and EF ≥ 50% were 16.3%, and for those with HF and EF < 50% were 38.2%. Unadjusted all-cause mortality and CV mortality rates indicate an increase in risk associated with HF, with HFrEF carrying a poorer prognosis than HFpEF.
Unadjusted and Adjusted Model Results
Adjusted Cox proportional hazard models results appear in Table 2 for both outcomes (death and CV death). Results from these prognostic main effects modeling are adjusted for clinical factors and QRS/LBBB, and suggest that both HF and dyssynchrony are independently associated with increased risk (Table 2). Hazard ratios for dyssynchrony measures provide the risk associated with a 10-unit increase. For example, an increase in diastolic BW or systolic BW by 10 was associated with increased mortality risk or negative clinical outcome beyond a history of HF (HR 1.034, 95% CI 1.022, 1.046, P < 0.0001) and (HR 1.037, 95% CI 1.025, 1.049, P < 0.0001), respectively.
Further, we examined unadjusted and two adjusted models (clinical characteristics + QRS/LBBB and Clinical characteristics + QRS/LBBB + LVEF) to determine whether the increased risk associated with dyssynchrony measures differ significantly for HF vs non-HF patients (Supplemental Table 1-unadjusted and Supplemental Table 2-adjusted). Graphical results from the interaction testing for HF and dyssynchrony appear in Figure 4. With the exception of systolic SD adjusted for clinical characteristics and QRS/LBBB (P = .0498) in the Cox survival model, there were no significant interactions between dyssynchrony parameters and HF. Following adjustment for clinical variables, QRS/LBBB and LVEF, systolic and diastolic dyssynchrony parameters no longer provide incremental predictive value for all-cause and CV death (Supplemental Table 2), without evidence for interaction between patient with and without a history of HF.
Discussion
There are several key findings in our analysis of patients with CAD and clinical HF. First, in this large prospective cohort of patients with CAD and HF, diastolic and systolic LVMD were detected in about 30% of the population, which was twice as common in patients with CAD alone. Second, while the presence of diastolic and systolic LVMD is associated with worse clinical outcomes, the presence of HF did not appear to modify or impact this association.
Prevalence and Significance of Mechanical Dyssynchrony in Heart Failure
LVMD can be measured with various modalities, including speckle tracking echocardiography, cardiac magnetic resonance, and nuclear imaging. GSPECT MPI has been widely used to diagnose epicardial CAD and to determine patient prognosis,25,26 but more recently, GSPECT MPI has been used to assess systolic and diastolic LVMD.27,28,29 Phase analysis as measured by GSPECT MPI was significantly correlated to results obtained from echocardiography (both tissue Doppler and speckle tracking) for the assessment of both systolic and diastolic LVMD.15,30 In our largest prospective cohort analysis of patients with CAD and HF, we found a high incidence of GSPECT MPI-based diastolic and systolic LVMD. A previous echocardiography-based analysis indicated comparably high prevalence of diastolic and systolic LVMD among patients with CAD and HF.1
Myocardial LVMD could represent an early indicator of LV dysfunction in CAD patients preceding any changes in regional wall motion or overt systolic and diastolic abnormalities. As an example, tissue Doppler-based analysis of systolic LVMD in CAD patients found systolic LVMD to be predictive of impaired exercise capacity independently of a history of previous myocardial infarction or regional wall abnormality.31 Preclinical work supports the notion that early stages of myocardial ischemia are sufficient to cause delayed subendocardial contraction, well before regional wall motion abnormalities first appear.32
The concept that systolic LVMD might be an indicator of early LV dysfunction appears to extend to diastolic LVMD, since the magnitude of regional diastolic asynchrony in CAD patients before and after percutaneous transluminal coronary angioplasty (PTCA) determined the degree of impaired global diastolic filling.33 Diastolic LVMD appears to be reversible after restoration of myocardial blood flow following successful PTCA. Reversibility was associated with an improvement in the rate and timing of global rapid diastolic filling without a measurable change in systolic function. Given presumably similar underlying pathomechanism, systolic and diastolic LVMD are commonly present at the same time. This is true for the whole population of CAD patients and patients with CAD and concomitant HF.
Conceivably, the culprit of systolic and diastolic LVMD does not necessarily have to be the result of epicardial vascular disease but could be driven by (regional) microvascular dysfunction. Microvascular dysfunction is now a well-recognized culprit of cardiac remodeling in HFpEF and HFrEF.34 Even in the absence of epicardial disease, microvascular ischemia which can result from local inflammation and vascular rarefaction has been demonstrated in HF.35,36,37 Furthermore, elevated filling pressures and abnormal transmural stress could be central to the pathophysiology of LVMD.38 Finally, the impact of ischemia on LV mechanical synchrony of contraction and relaxation frequently occurs without affecting the conduction system of the heart (QRS/bundle branch blocks), leading to the so-called electromechanical dissociation.1 This might be of particular importance in patients with HFrEF who are evaluated for cardiac resynchronization therapy (CRT). Some evidence now suggests that the lack of response to CRT in some patients meeting traditional (electrical) criteria may be due to the absence of LVMD.39,40
Increasing degrees of LVMD (systolic or diastolic) are associated with worse clinical outcomes in patients with CAD; however, despite the increased risk of death among patients with concomitant HF, the increased risk of death associated with dyssynchrony does not differ for HF vs non-HF patients. This raises the question whether our findings support the concept that underlying CAD with resultant macro- and microvascular dysfunction is the main mechanistic drivers of LVMD. Not surprisingly, both systolic and diastolic LVMD were associated with multi-vessel disease and higher class of angina, which suggests a significant impact of myocardial ischemia on the pathogenesis of mechanical dyssynchrony in CAD.16 While HFrEF-based analyses repeatedly indicate a close link between the degree of systolic dyssynchrony and worse outcomes in HFrEF, 2,3,41 similar findings for HFpEF are lacking.42 This means that while systolic LVMD appears to have predictive value and to serve as a therapeutic target in patients with HFrEF, its significance in HFpEF appears to be unclear. Notably, prevalence of diastolic and systolic dyssynchrony is lower in patients with HFpEF than HFrEF, indicating not only a diminished significance but also much lower prevalence of LVMD in CAD + HFpEF. It is important to acknowledge that the vast majority of HF patients had an LVEF > 35%, and neither systolic nor diastolic LVMD was independent predictors of adverse outcomes, when adjusted for LVEF. Whether the diastolic LVMD measures play a mechanistic or predictive role exclusively in HFrEF remains to be elucidated in future studies.
Limitations of the current analysis are several and should be acknowledged. First, only about a quarter of patients had HF and thus the performed analyses were underpowered to fully address the aims of the study. This is particularly true for the population with reduced LVEF, and thus results cannot be extrapolated to this population. Second, the significant interaction between LVMD and HF was only found for systolic SD and HF that occurs when LVEF is not used for adjustment. Given discrepancy with other interaction models, this relationship warrants additional scrutiny to validate this singular finding. Third, the registry is based at a single center. Replication at other sites is required to ascertain the generalizability of our findings. Fourth, the use of cardiovascular implantable electronic devices such as cardiac resynchronization therapy, implantable cardioverter-defibrillators, or pacemakers was not captured. The absence of these data may have led to unmeasured confounding. Residual confounding may exist and impact the validity of our results. Finally, we studied LVMD parameters at one time point only, and suspect that additional insights could be gained from serial measurements.
New Knowledge Gained
-
Patients with coronary artery disease and heart failure have a high prevalence of mechanical dyssynchrony as measured by GSPECT MPI
-
Clinical outcomes associated with mechanical dyssynchrony do not appear to differ in patients with and without clinical heart failure
Conclusions
This is the first study to evaluate the prognostic value of diastolic and systolic mechanical dyssynchrony on mortality in patients with CAD and HF. Patients with CAD and HF have a high prevalence of mechanical dyssynchrony as measured by GSPECT MPI, and a higher mortality than CAD alone. However, clinical outcomes associated with mechanical dyssynchrony do not appear to differ in patients with and without HF.
Abbreviations
- BW:
-
Phase bandwidth
- CAD:
-
Coronary artery disease
- GSPECT:
-
Gated single-photon emission computed tomography
- LV:
-
Left ventricular/ventricle
- MPI:
-
Myocardial perfusion imaging
- SD:
-
Standard deviation
References
Lee PW, Zhang Q, Yip GW, Wu L, Lam YY, Wu EB, et al. Left ventricular systolic and diastolic dyssynchrony in coronary artery disease with preserved ejection fraction. Clin Sci (Lond) 2009;116:521–9.
Goldberg AS, Alraies MC, Cerqueira MD, Jaber WA, Aljaroudi WA. Prognostic value of left ventricular mechanical dyssynchrony by phase analysis in patients with non-ischemic cardiomyopathy with ejection fraction 35-50% and QRS < 150 ms. J Nucl Cardiol 2014;21:57–66.
Aljaroudi WA, Hage FG, Hermann D, Doppalapudi H, Venkataraman R, Heo J, et al. Relation of left-ventricular dyssynchrony by phase analysis of gated SPECT images and cardiovascular events in patients with implantable cardiac defibrillators. J Nucl Cardiol 2010;17:398–404.
Aggarwal H, AlJaroudi WA, Mehta S, Mannon R, Heo J, Iskandrian AE, et al. The prognostic value of left ventricular mechanical dyssynchrony using gated myocardial perfusion imaging in patients with end-stage renal disease. J Nucl Cardiol 2014;21:739–46.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J Am Coll Cardiol 2014;63:2751–68.
Hess PL, Shaw LK, Fudim M, Iskandrian AE, Borges-Neto S. The prognostic value of mechanical left ventricular dyssynchrony defined by phase analysis from gated single-photon emission computed tomography myocardial perfusion imaging among patients with coronary heart disease. J Nucl Cardiol 2016;24:482–90.
Uebleis C, Hellweger S, Laubender RP, Becker A, Sohn HY, Lehner S, et al. Left ventricular dyssynchrony assessed by gated SPECT phase analysis is an independent predictor of death in patients with advanced coronary artery disease and reduced left ventricular function not undergoing cardiac resynchronization therapy. Eur J Nucl Med Mol Imaging 2012;39:1561–9.
Pazhenkottil AP, Buechel RR, Husmann L, Nkoulou RN, Wolfrum M, Ghadri JR, et al. Long-term prognostic value of left ventricular dyssynchrony assessment by phase analysis from myocardial perfusion imaging. Heart 2011;97:33–7.
Wang L, Yang MF, Cai M, Zhao SH, He ZX, Wang YT. Prognostic significance of left ventricular dyssynchrony by phase analysis of gated SPECT in medically treated patients with dilated cardiomyopathy. Clin Nucl Med 2013;38:510–5.
Zafrir N, Nevzorov R, Bental T, Strasberg B, Gutstein A, Mats I, et al. Prognostic value of left ventricular dyssynchrony by myocardial perfusion-gated SPECT in patients with normal and abnormal left ventricular functions. J Nucl Cardiol 2014;21:532–40.
Yu CM, Zhang Q, Yip GW, Lee PW, Kum LC, Lam YY, et al. Diastolic and systolic asynchrony in patients with diastolic heart failure: A common but ignored condition. J Am Coll Cardiol 2007;49:97–105.
Lee JH. Left ventricular diastolic dyssynchrony in post-myocardial infarction patients: Does it predict future left ventricular remodeling? J Cardiovasc Ultrasound 2016;24:193–4.
Gimelli A, Liga R, Giorgetti A, Favilli B, Pasanisi EM, Marzullo P. Determinants of left ventricular mechanical dyssynchrony in patients submitted to myocardial perfusion imaging: A cardiac CZT study. J Nucl Cardiol 2016;23:728–36.
Wang J, Kurrelmeyer KM, Torre-Amione G, Nagueh SF. Systolic and diastolic dyssynchrony in patients with diastolic heart failure and the effect of medical therapy. J Am Coll Cardiol 2007;49:88–96.
Boogers MJ, Chen J, Veltman CE, van Bommel RJ, Mooyaart EA, Al Younis I, et al. Left ventricular diastolic dyssynchrony assessed with phase analysis of gated myocardial perfusion SPECT: A comparison with tissue Doppler imaging. Eur J Nucl Med Mol Imaging 2011;38:2031–9.
Fudim M, Fathallah M, Shaw LK, Liu PR, James O, Samad Z, et al. The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary heart disease. JACC Cardiovascular Imaging 2018;12:1215–26.
AbouEzzeddine OF, Haines P, Stevens S, Nativi-Nicolau J, Felker GM, Borlaug BA, et al. Galectin-3 in heart failure with preserved ejection fraction. A RELAX trial substudy (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure). JACC Heart Fail 2015;3:245–52.
Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, et al. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol 2015;65:1668–82.
Trimble MA, Velazquez EJ, Adams GL, Honeycutt EF, Pagnanelli RA, Barnhart HX, et al. Repeatability and reproducibility of phase analysis of gated single-photon emission computed tomography myocardial perfusion imaging used to quantify cardiac dyssynchrony. Nucl Med Commun 2008;29:374–81.
Borges-Neto S, Tuttle RH, Shaw LK, Smith WT, Jain D, Coleman RE, et al. Outcome prediction in patients at high risk for coronary artery disease: Comparison between 99mTc tetrofosmin and 99mTc sestamibi. Radiology 2004;232:58–65.
Borges-Neto S, Pagnanelli RA, Shaw LK, Honeycutt E, Shwartz SC, Adams GL, et al. Clinical results of a novel wide beam reconstruction method for shortening scan time of Tc-99m cardiac SPECT perfusion studies. J Nucl Cardiol 2007;14:555–65.
Harris PJ, Harrell FE, Lee KL, Behar VS, Rosati RA. Survival in medically treated coronary artery disease. Circulation 1979;60:1259–69.
Mark DB, Nelson CL, Califf RM, Harrell FE, Lee KL, Jones RH, et al. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation 1994;89:2015–25.
Stone C, Koo C. Additive splines in statistics. In: Proc Stat Comp Sect Am Statist Assoc; 1985, p. 45-8.
Beller GA, Zaret BL. Contributions of nuclear cardiology to diagnosis and prognosis of patients with coronary artery disease. Circulation 2000;101:1465–78.
Beller GA. First annual Mario S. Verani, MD, Memorial lecture: Clinical value of myocardial perfusion imaging in coronary artery disease. J Nucl Cardiol 2003;10:529–42.
Chen J, Garcia EV, Folks RD, Cooke CD, Faber TL, Tauxe EL, et al. Onset of left ventricular mechanical contraction as determined by phase analysis of ECG-gated myocardial perfusion SPECT imaging: Development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol 2005;12:687–95.
Nakajima K, Okuda K, Matsuo S, Kiso K, Kinuya S, Garcia EV. Comparison of phase dyssynchrony analysis using gated myocardial perfusion imaging with four software programs: Based on the Japanese Society of Nuclear Medicine working group normal database. J Nucl Cardiol 2017;24:611–21.
Chen J, Henneman MM, Trimble MA, Bax JJ, Borges-Neto S, Iskandrian AE, et al. Assessment of left ventricular mechanical dyssynchrony by phase analysis of ECG-gated SPECT myocardial perfusion imaging. J Nucl Cardiol 2008;15:127–36.
Hsu TH, Huang WS, Chen CC, Hung GU, Chen TC, Kao CH, et al. Left ventricular systolic and diastolic dyssynchrony assessed by phase analysis of gated SPECT myocardial perfusion imaging: A comparison with speckle tracking echocardiography. Ann Nucl Med 2013;27:764–71.
Wang M, Yan GH, Yue WS, Siu CW, Yiu KH, Lee SW, et al. Left ventricular mechanical dyssynchrony impairs exercise capacity in patients with coronary artery disease with preserved left ventricular systolic function and a QRS duration ≤ 120 ms. Circ J 2012;76(3):682–8.
Wang J, Abraham TP, Korinek J, Urheim S, McMahon EM, Belohlavek M. Delayed onset of subendocardial diastolic thinning at rest identifies hypoperfused myocardium. Circulation 2005;111:2943–50.
Bonow RO, Vitale DF, Bacharach SL, Frederick TM, Kent KM, Green MV. Asynchronous left ventricular regional function and impaired global diastolic filling in patients with coronary artery disease: Reversal after coronary angioplasty. Circulation 1985;71:297–307.
Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: A multiorgan roadmap. Circulation 2016;134:73–90.
Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 2015;131:550–9.
Konerman MC, Greenberg JC, Kolias TJ, Corbett JR, Shah RV, Murthy VL, et al. Reduced myocardial flow reserve is associated with diastolic dysfunction and decreased left atrial strain in patients with normal ejection fraction and epicardial perfusion. J Card Fail 2017;24:90–100.
Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, et al. Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 2016;9:e002562.
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–95.
Emkanjoo Z, Esmaeilzadeh M, Mohammad Hadi N, Alizadeh A, Tayyebi M, Sadr-Ameli MA. Frequency of inter- and intraventricular dyssynchrony in patients with heart failure according to QRS width. Europace 2007;9:1171–6.
Perry R, De Pasquale CG, Chew DP, Aylward PE, Joseph MX. QRS duration alone misses cardiac dyssynchrony in a substantial proportion of patients with chronic heart failure. J Am Soc Echocardiogr 2006;19:1257–63.
Bader H, Garrigue S, Lafitte S, Reuter S, Jais P, Haissaguerre M, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol 2004;43(2):248–56.
Biering-Sørensen T, Shah SJ, Anand I, Sweitzer N, Claggett B, Liu L, et al. Prognostic importance of left ventricular mechanical dyssynchrony in heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;19:1043–52.
Disclosures
PLH received Career Development Award from the American Heart Association (19CDA34760126). MF received grant support from an American Heart Association Grant, 17MCPRP33460225 and NIH T32 Grant 5T32HL007101. JPP receives funding for clinical research from ARCA biopharma, Boston Scientific, Gilead, Janssen Pharmaceuticals, ResMed, Spectranetics, and St Jude Medical and serves as a consultant to Allergan, Amgen, GlaxoSmithKline, Johnson & Johnson, Medtronic, and Spectranetics. SB received research Grant support from GE Healthcare. All other authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
This analysis was supported by a Grant from GE Healthcare to S.B.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fudim, M., Fathallah, M., Shaw, L.K. et al. The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary artery disease and heart failure. J. Nucl. Cardiol. 27, 1622–1632 (2020). https://doi.org/10.1007/s12350-019-01843-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-019-01843-4