Abstract
Background
The aim of this study was to evaluate the prognostic values of sympathetic nerve system using 123I-MIBG myocardial scintigraphy and using Holter electrocardiogram (ECG) in patients with heart failure with preserved ejection fraction (HFpEF).
Methods and results
Among 403 consecutive patients with stable HF who underwent 123I-MIBG myocardial scintigraphy and Holter ECG, we identified 133 patients (64 ± 16 years) who had preserved ejection fraction (≥ 50%) by echocardiography. Multivariate Cox model was used to assess if washout rate (WR) by 123I-MIBG scintigraphy and very low frequency power (VLFP) by Holter ECG was associated with major adverse cardiovascular events (MACE). During a mean follow-up of 5.4 ± 4.1 years, 39 MACE occurred. The lower nighttime VLFP (HR 3.29, 95% CI 1.56 to 6.92) and higher WR (HR 4.01, 95% CI 1.63 to 9.88) were the significant prognostic factors for MACE. As compared to high nighttime VLFP and low WR group, MACE risk was significantly the highest in the low nighttime VLFP and high WR group (HR 40.832; 95% CI 5.378 to 310.012, P < 0.001).
Conclusion
This study demonstrated that the nighttime VLFP adding to WR could be a potential prognostic value among patients with HFpEF.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) contributed to one in every nine deaths in 2009, and half of those who develop HF die within 5 years of diagnosis, regardless of having reduced or preserved left ventricular ejection fraction (LVEF).1 Despite the high prevalence of HF with preserved LVEF (HFpEF), its prognosis has not improved during the past 2 decades.2 The most common cause of HFpEF is diastolic HF, and no pharmacological treatment has been proven to improve survival and shown to be effective in large clinical trials of patients with HFpEF.3 In chronic HF, abnormalities in cardiac autonomic control, characterized by sympathetic overactivity and parasympathetic withdrawal, potentially contribute to the progression of the disease and are associated with an unfavorable prognosis.4 Therefore, assessing cardiac autonomic status is clinically important in the management of patients with chronic HF.
123I-meta-iodobenzylguanidine (123I-MIBG) myocardial scintigraphy imaging plays an important role in the assessment of the progression of HF among patients with chronic HF.5123I-MIBG scintigraphy is often used before and after treatments such as β-blockers.6 It also has prognostic value for future cardiac events in patients with HFpEF.7 On the other hand, heart rate variability (HRV) analysis by using Holter electrocardiogram (ECG) was proposed as a non-invasive tool for the assessment of cardiac autonomic regulation8 and has been shown to predict the clinical outcome in patients with chronic HF.9 Although prior investigators have demonstrated the relationship and compared the prognostic value between the parameters of 123I-MIBG scintigraphy and HRV analysis in patients with chronic HF,10 their prognostic value among patients with HFpEF remains unclear. The aim of this study was to evaluate the prognostic value of 123I-MIBG scintigraphy compared with that of HRV in patients with HFpEF.
Methods
Patient Population
Among 403 consecutive patients who were admitted to our hospital because of congestive HF and underwent 123I- MIBG scintigraphy and Holter ECG for clinical indications within 30 days between July 2004 and December 2016, we finally enrolled 133 patients with preserved EF (> 50%) on echocardiography retrospectively (Figure 1). 123I-MIBG scintigraphy and Holter ECG were performed because of suspected myocardial ischemia, cardiomyopathy, or evaluation of sympathetic activity, therapeutic effect of drugs when the condition of HF was decided to be stable by chief physician after treatment during hospitalization. The institutional review board approved this retrospective study and the requirement to obtain informed consent was waived (M17089).
Echocardiographic Imaging
Echocardiographic images were obtained from the parasternal window for the evaluation of left ventricular function (Vivid E9device; GE Vingmed, Horten, Norway). LVEF was calculated using the Teichholz formula.11 A LVEF > 50% was defined as a preserved EF.12
123I-MIBG Scintigraphy
Patients were injected with 123I-MIBG (111MBq) while resting. Five-minute anterior planar imaging was carried out at 15 and 240 minutes after 123I-MIBG injection. All images were acquired using a 256 × 256 matrix and a double head gamma camera (Infinia GP3; GE Healthcare, Little Chalfont, UK) equipped with low-energy general-purpose collimators. The planar 123I-MIBG images were analyzed using a region-of-interest technique to obtain semi-quantitative parameters for tracer distribution by using software (smartMIBG ver.3.1.0.0; FUJIFILM RI Pharma Co., Ltd, Tokyo, Japan). The 123I-MIBG count densities of the heart (H) and the mediastinum (M) were calculated from 15- and 240-minute images. The heart-to-mediastinum (H/M) ratios of 123I-MIBG uptake at 15 minutes (early H/M) and at 240 minutes (delayed H/M) were calculated. The washout rate (WR) from the myocardium was calculated as [(H/M) at 15 minutes − (H/M) at 240 min] × 100/(H/M) at 15 minutes (%).5 The 123I-MIBG data were interpreted by the nuclear medicine specialist. Neither the patient information nor the other specialists’ results were accessible to any of the specialists.
Holter ECG
Twenty-four-hour Holter ECG recordings (FM120, FM160, FM180, FM180S, FM200, FM960; Fukuda Denshi Co, Ltd.,Tokyo, Japan) were performed in the study population. Recordings with atrial fibrillation, more than 15% noise or ectopic beats during 24 hours and those with < 20 hours of analyzable data were excluded from the analysis. Independent from clinical characteristics and 123I-MIBG scintigraphy data, variables of HRV were analyzed using the HRV system (SCM-8000; MemCalc/Chiram3, GMS Co.,Ltd, Tokyo, Japan), according to the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology.8 The time domain analysis of HRV included the standard deviation of all normal to normal intervals (SDNN). Spectral analysis was performed using the maximum entropy method.13 Power spectra were quantified using the area within the following frequency band: very low frequency power (VLFP: 0.003 to 0.04 Hz), low-frequency power (LFP: 0.04 to 0.15 Hz), and high-frequency power (HFP: 0.15 to 0.4 Hz). The LF/HF ratio was also calculated by dividing LFP by HFP. The Holter ECGs’ data were interpreted by the cardiologist. Neither the patient information nor the other cardiologists’ results were accessible to any of the cardiologists.
Assessment of Clinical Outcome
The endpoint was defined as the occurrence of major adverse cardiac events (MACE) including cardiac deaths (deaths caused by HF, acute myocardial infarction (AMI), lethal ventricular arrhythmias, or other definitive cardiac disorders), cardiovascular events (AMI or unstable angina), severe HF requiring hospitalization, or stroke. For the diagnosis of AMI, unstable angina, and stroke standard laboratory, ECG or examination criteria were used. HF exacerbation was defined as dyspnea, accompanied by pulmonary edema or congestion on chest X-ray requiring hospitalization. Only the first event was counted, even if patients experienced several cardiac events during follow-up. The event data were retrospectively gathered from the patients’ medical records, including in-hospital and out-of-hospital medical records.
Statistical Analysis
Data are expressed as average ± standard deviation of continuous variables. Continuous variables from patients with and without events were compared using the Mann-Whitney U test, and categorical data were analyzed using the chi-square test. Then, age, sex, and factors with a significance level of P value < 0.05 were included in a univariate Cox regression model. Thereafter, variables that were significant probable values were included in multivariate Cox regression models to determine whether the future occurrence of MACE was associated with the parameters of echocardiographic imaging, 123I-MIBG scintigraphy, or HRV. To evaluate the clinical importance of nighttime VLFP and WR, all patients were divided into 2 groups based on their nighttime VLFP values and WR values. Each of the cut-off value was determined using the area under the curve (AUC) from a receiver operating characteristic (ROC) analysis based on MACE occurrences. The proportion of event-free patients was estimated using the Kaplan-Meier method and compared between each of the high and low nighttime VLFP and WR groups by using the log-rank test. A P value < 0.05 was considered statistically significant. All statistical analyses were performed using StatMate IV software version 4.01 (Advanced Technology for Medicine and Science, Tokyo, Japan). The results of this study were not available to the treating physicians. Neither the patient information nor the other investigators’ results were accessible to any of the investigators in this study.
Results
Patient characteristics are presented in Table 1. Arrhythmia-induced HF and dilated cardiomyopathy were common, each with a prevalence of 12% (n = 16). Other patients had hypertensive heart disease (n = 15), ischemic cardiomyopathy (n = 13), tachycardia induced cardiomyopathy (n = 13), valvular heart disease (n = 8), hypertrophic cardiomyopathy (n = 6), and unknown etiology (n = 46).
Overall, 39 patients (29%) experienced MACE during 5.4 ± 4.1 years of median follow-up. Cardiac deaths occurred in 16 patients (AMI in 4, deterioration of HF in 12), non-fatal AMI in 1, severe HF requiring hospitalization in 21, and stroke in 1. Table 1 shows that age, New York Heart Association (NYHA), BNP, left atrial dimension (LAD), left ventricular end-diastolic volume index (LVEDVI), left ventricular mass index (LVMI), delayed H/M, and WR were significantly higher, and body mass index (BMI), all types of SDNN, VLFP, and LF/HF were significantly lower in patients with MACE. In the univariate analysis, NYHA, BNP, LAD, LVEDVI, LVMI, delayed H/M, WR, total SDNN, total VLFP, daytime VLFP, daytime LF/HF, nighttime SDNN, nighttime VLFP, and nighttime LF/HF were determined to be the significant factors for MACE (Table 2). The values of hazard ratio in Tables 2 and 3, BMI, all types of SDNN, VLFP, and LF/HF were inverted to allow better conceptualization of risk. WR, total VLFP, daytime VLFP, daytime LF/HF, nighttime VLFP, and nighttime LF/HF were determined to be the significant prognostic factors of MACE in each model in multivariate analysis after adjusting for NYHA and BNP (Table 3, models 1–5). Among the variables of HRV, nighttime VLFP was determined to be the most significant prognostic factor of MACE in multivariate analysis after adjusting for NYHA, BNP, and WR (Table 3, model 6). Therefore, we investigated the clinical importance of nighttime VLFP and WR, all patients were divided into 2 groups based on their nighttime VLFP values and WR values. From the ROC analysis, 74 patients were assigned to the higher nighttime VLFP group, whereas the remaining 59 were in the lower group; 74 patients were assigned to the low WR group, whereas the remaining 59 were assigned to the higher WR group. The AUC of the ROC in predicting MACE was 0.72 for nighttime VLFP and 0.82 for WR. The cut-off values for lower nighttime VLFP and higher WR were 825 ms2 and 42%, respectively. Of the 39 incidents of events, 29 cases occurred in the lower nighttime VLFP group. The proportion of patients who experienced MACE was significantly higher in the lower nighttime VLFP group than in the higher nighttime VLFP group (Figure 2). Of the 39 incidents of events, 31 cases occurred in the higher WR group. The proportion of patients who experienced MACE was significantly higher in the higher WR group than in the lower WR group (Figure 3). Kaplan-Meier curves for MACE in the combined groups of nighttime VLFP and WR are shown in Figure 4. The proportion of patients who experienced MACE was significantly higher in the higher WR and lower nighttime VLFP group than in the lower WR and higher nighttime VLFP group (61% vs 2%, P < 0.001). In the multivariate Cox proportional model, as compared to high nighttime VLFP and low WR group, MACE risk was significantly the highest in the low nighttime VLFP and high WR group, followed by the high nighttime VLFP and high WR group, and low nighttime VLFP and low WR group after adjusting for NYHA and BNP (Table 3, model 7).
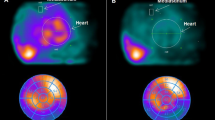
Case Presentation
Figure 5 shows a typical patient in the lower nighttime VLFP and higher WR group. The patient was a 66-year-old man with HF due to valvular heart disease (clinical scenario: 2, NYHA: class III, Nohria-Stevenson classification: wet and cold). He had a history of hypertension and a smoking habit. He underwent echocardiography and his LVEF was 59%. The score of VLFP was 303.8 and that of WR was 55.5. In this case, the patient had cardiac death due to deterioration of HF 240 days after Holter ECG.
Discussion
In the present study of patients with HFpEF, our findings demonstrated that HRV was associated with increase in MACE and 123I-MIBG scintigraphic findings, and the evaluation of nighttime VLFP added to WR had predictive value for the identification of future MACE among patients with HFpEF.
Prognostic Value of 123I-MIBG Scintigraphy in HFpEF
Given the high-risk nature of HFpEF, prediction of future cardiac risk by using non-invasive imaging modalities is essential. Several prior investigations demonstrated the prognostic utility of 123I-MIBG scintigraphy7 or echocardiography14 among patients with HFpEF. Katoh et al. reported that 123I-MIBG WR was an independent predictor of cardiac events in patients with HFpEF.7 Several underlying mechanisms of this finding could be considered. First, the marked sympathetic activation in patients with hypertension depended on an impairment of the arterial baroreflex. The serum norepinephrine level was similar in patients with diastolic HF and systolic HF, and was markedly increased as compared with that in normal subjects.15 Second, it was suggested that the renin-angiotensin system (RAS) is associated with cardiac sympathetic activation. The activation of RAS was associated with norepinephrine release from cardiac sympathetic nerve endings in HF.16 Therefore, 123I-MIBG scintigraphic findings directly reflect cardiac sympathetic nerve activity and ongoing myocardial damage in patients with HFpEF.
Prognostic Value of HRV add to 123I-MIBG Scintigraphy in HFpEF
In the current study, on multivariate analysis, nighttime VLFP showed a significant association with cardiac events. HRV, which depends on postsynaptic signal transduction, reflects the end-organ response of the sinus node. In conditions characterized by marked, persistent sympathetic condition, which is often observed in chronic HF, the sinus node may diminish its responsiveness to neural input.17 Bigger et al. reported that VLFP after myocardial infarction was strongly associated with a poor prognosis.18 Yamada et al. reported that VLFP showed a significant association with cardiac events in chronic HF.17 VLFP may be influenced by a number of factors other than autonomic balance such as thermoregulation, RAS, or chemoreceptors.19,20 Other reports showed that breathing disorders increase the spectral power to the VLFP range in patients with chronic HF.21 Therefore, VLFP, especially nighttime VLFP reveals sympathetic nerve system abnormality during nighttime that is associated with cardiac events. In previous reports about other indicators of sympathetic nerve system disorders, breathing abnormalities in HF indicate a poor prognosis22 and ambulatory blood pressure variability is a risk for organ damage and cardiovascular events.23 These theories do not contradict our results.
In the present study, lower nighttime VLFP and higher WR were associated with poor prognosis for cardiac events. It is speculated that WR evaluation added to nighttime VLFP enables early risk stratification of patients with HFpEF.
Study Limitations
This study has several limitations. The number of patients was relatively small, which limited the statistical reliability. However, our results have clearly demonstrated that a higher WR and lower nighttime VLFP was significantly associated with MACE. We did not have the Biplane Simpson data of all patients in this study. Therefore, EF calculated from Treicholz method may be evaluated inaccurately. We also did not have data on parameters of echocardiography such as E/e’ that may be important for determining diastolic function in the current study. The parameter of LAD may be inappropriate for diastolic parameter, because the patients with valvular disease were included in this study. Since other diastolic parameters, LVEDVI and LVMI were calculated without using the data of LAD, we thought LVEDVI and LVMI can be substituted LAD for diastolic parameters. However, LVEDVI and LVMI measured using echocardiography were included in the analysis and did not predict MACE better than WR and nighttime VLFP. A further limitation was that this study retrospectively analyzed 123I-MIBG scintigraphy data, Holter ECG data, and the outcomes from patients with HFpEF. Therefore, the timing of examinations differed among patients according to the severity of each patient’s HF, and the outcomes reviewed of medical records might have been incomplete. Future prospective studies of large populations are needed to confirm the prognostic value of WR and nighttime VLFP in patients with HFpEF.
New Knowledge Gained
HFpEF patients with low nighttime VLFP has a poor prognosis. As compared to high nighttime VLFP and low WR group, MACE risk was significantly the highest in the low nighttime VLFP and high WR group, followed by the high nighttime VLFP and high WR group, and low nighttime VLFP and low WR group.
Conclusion
In this study, nighttime VLFP demonstrated a high prognostic value for MACE in patients with HFpEF. The evaluation of nighttime VLFP added to WR could have predictive value for the identification of future MACE among patients with HFpEF.
Abbreviations
- HFpEF:
-
Heart failure with preserved left ventricular ejection fraction
- HRV:
-
Heart rate variability
- SDNN:
-
Standard deviation of all normal to normal intervals
- VLFP:
-
Very low frequency power
- LFP:
-
Low-frequency power
- HFP:
-
High-frequency power
- WR:
-
Washout rate
- AUC:
-
Area under the curve
- ROC:
-
Receiver operating characteristic
- MACE:
-
Major adverse cardiac events
References
Levy WC, Anand IS. Heart failure risk prediction models: What have we learned? JACC Heart Fail. 2014;2:437–9.
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92.
Packer M. The neurohumoral hypothesis: A theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–54.
Agostini D, Carrio I, Verberne HJ. How to use myocardial 123I-MIBG scintigraphy in chronic heart failure. Eur J Nucl Med Mol Imaging. 2009;36:555–9.
Fujimoto S, Inoue A, Hisatake S, Yamashina S, Yamashina H, Nakano H, et al. Usefulness of meta-[123I]iodobenzylguanidine myocardial scintigraphy for predicting cardiac events in patients with dilated cardiomyopathy who receive long-term beta blocker treatment. Nucl Med Commun. 2005;26:97–102.
Katoh S, Shishido T, Kutsuzawa D, Arimoto T, Netsu S, Funayama A, et al. Iodine-123-metaiodobenzylguanidine imaging can predict future cardiac events in heart failure patients with preserved ejection fraction. Ann Nucl Med. 2010;24:679–86.
Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standard of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65.
Bonaduce D, Petretta M, Marciano F, Vicario ML, Apicella C, Rao MA, et al. Independent and incremental prognostic value of heart rate variability in patients with chronic heart failure. Am Heart J. 1999;138:273–84.
Kurata C, Shouda S, Mikami T, Uehara A, Ishikawa K, Tawarahara K, et al. Metaiodobenzylguanidine and heart rate variability in heart failure. Jpn Circ J. 1998;62:770–2.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Emande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.
Jessup M, Marwick TH, Ponikowski P, Voors AA, Yancy CW. 2016 ESC and ACC/AHA/HFSA heart failure guideline update—What is new and why is it important? Nat Rev Cardiol. 2016;13:623–8.
Ohtomo N, Terachi S, Tanaka Y, Tokiwano K, Kaneko N. New method of time series analysis and its application to Wolf’s sunspot number data. Jpn J Appl Phys. 1994;33:2821–31.
Donal E, Lund LH, Oqer E, Hage C, Persson H, Reynaud A, et al. New echocardiographic predictors of clinical outcome in patients presenting with heart failure and a preserved left ventricular ejection fraction: A subanalysis of the Ka (Karolinska) Ren (Rennes) Study. Eur J Heart Fail. 2015;17:680–8.
Kizman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50.
Levi R, Silver RB, Mackins CJ, Seyedi N, Koyama M. Activation of a renin-angiotensin system in ischemic cardiac sympathetic nerve endings and its association with norepinephrine release. Int Immunopharmacol. 2002;2:1965–73.
Yamada T, Shimonagata T, Fukunami M, Kumagai K, Ogita H, Hirata A, et al. Comparison of the prognostic value of cardiac iodine-123 metaiodobenzylguanidine imaging and heart rate variability in patients with chronic heart failure: a prospective study. J Am Coll Cardiol. 2003;41:231–8.
Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart rate variability and mortality after myocardial infarction. Circulation. 1992;85:164–71.
Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92.
Parti G, Saul JP, Di Rieuzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation: A critical appraisal. Hypertension. 1995;25:1276–86.
Morata A, Sleight P, Pinna GD, Maestri R, Prpa A, La Rovere MT, et al. Abnormal awake respiratory patters are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circulation. 1997;96:246–52.
Ushijima R, Joho S, Akabane T, Oda Y, Inoue H. Differing effects of adaptive servoventilation and continuous positive airway pressure on muscle sympathetic nerve activity in patients with heart failure. Circ J. 2014;78:1387–95.
Kario K. Morning surge and variability in blood pressure: a new therapeutic target? Hypertension. 2005;45:485–6.
Acknowledgments
We are grateful to the radiology technologists Mr. Tadashi Kokubo, Mr. Kazuhiro Tachiki, Mr. Nobutomo Ishii, and Mr. Takushi Ookubo for their technical assistance in the administration of myocardial 123I-MIBG scintigraphy, and to the clinical physiology technologist Mr. Naoji Masuya for his technical assistance in Holter ECG.
Disclosures
Takanori Ikeda has received Grant support through his institution from Daiichi Sankyo, Bristol-Myers Squibb, Boehringer Ingelheim; and honoraria for lectures from Bayer Healthcare, Daiichi Sankyo, Bristol-Myers Squibb, Pfizer, Tanabe-Mitsubishi, and Ono Pharmaceutical. Regarding this study, all authors declare that there is no potential conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hashimoto, H., Nakanishi, R., Mizumura, S. et al. Prognostic values of 123I-MIBG myocardial scintigraphy and heart rate variability in patients with heart failure with preserved ejection fraction. J. Nucl. Cardiol. 27, 833–842 (2020). https://doi.org/10.1007/s12350-018-01494-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-018-01494-x