Abstract
Calcific aortic valve disease (CAVD) can progress to symptomatic aortic stenosis in a subset of patients. The severity of aortic stenosis and the extent of valvular calcification can be evaluated readily by echocardiography, CT, and MRI using well-established imaging protocols. However, these techniques fail to address optimally other important aspects of CAVD, including the propensity for disease progression, risk of complications in asymptomatic patients, and the effect of therapeutic interventions on valvular biology. These gaps may be addressed by molecular imaging targeted at key biological processes such as inflammation, remodeling, and calcification that mediate the development and progression of CAVD. In this review, recent advances in valvular molecular imaging, including 18F-fluorodeoxyglucose (FDG) and 18F-sodium fluoride (NaF) PET, and matrix metalloproteinase-targeted SPECT imaging in the preclinical and clinical settings are presented and discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Calcific aortic valve disease (CAVD) is the most common cause of aortic stenosis (AS), which according to recent US statistics affects ~ 0.4% of the general population.1 The prevalence of AS increases with age with moderate to severe AS affecting nearly 3% of patients ≥ 75 years old.1 In addition, 17,000 deaths and 55,000 hospitalizations are annually attributable to aortic valve disease (not just AS). The Natural history of CAVD involves a long asymptomatic period during which the initial fibrotic thickening of aortic valve leaflets with limited calcification (aortic sclerosis) progresses to more extensive valvular calcification and ultimately, hemodynamically significant aortic stenosis. The resulting pressure overload promotes left ventricular hypertrophy, an adaptive response that underlies the typically long period of asymptomatic disease present despite gradual worsening of aortic stenosis. The rise in wall stress may lead to impairment of coronary perfusion and development of sub-endocardial ischemia, which in turn triggers cardiomyocyte apoptosis and myocardial fibrosis. It is postulated that eventually, ventricular decompensation triggers angina, heart failure, syncope, or sudden death. While symptomatic AS is indeed the hallmark of severe CAVD, not all patients with moderate AS progress to symptomatic disease. Even in patients with severe AS (peak systolic velocity ≥ 4 m/s by Doppler echocardiography), ~ 30% do not develop symptoms over a 5-year period.2 On the other hand, a non-negligible minority of such asymptomatic patients die of sudden cardiac death,2 highlighting the complex nature of the relation between aortic valve structure and physiology (including the severity of AS), ventricular response, and symptoms.
In addition to age, the number of leaflets is another major risk factor for the development of CAVD. Indeed, while bicuspid aortic valve (BAV) is found in ~ 1.4% of live births, BAV accounts for a large fraction of aortic valve surgeries.3,4,5 Reflecting the similarity between CAVD and atherosclerosis, male sex, smoking, hypertension, diabetes, hypercholesterolemia, and elevated Lp(a) are additional risk factors for the development of CAVD.4 Many of the factors that increase the risk of CAVD development are also risk factors for CAVD progression. In particular, disease progression is accelerated with the severity of stenosis, higher extent of calcification, older age, and presence of BAV.4 Moreover, aortic valve area and left ventricular hypertrophy are independent predictors of the development of symptoms.2 Importantly, the presence of CAVD, even in the absence of severe disease or symptoms, portends a higher risk of all-cause mortality.2,6,7 The predictors of all-cause mortality in aortic stenosis include age, chronic renal failure, inactivity, aortic valve velocity,2 left ventricle mass,8 and left ventricle wall stress.9
Currently, there is no known treatment to slow down the progression of CAVD, and surgical or transcatheter aortic valve replacement remains the only effective therapeutic options for advanced, symptomatic disease. Despite considerable overlap between the risk factors for CAVD and atherosclerosis and promising results of observational studies,10 randomized clinical trials of statins have failed to demonstrate benefit in slowing down the progression of aortic valve calcification or stenosis.11,12 Inadequate duration of statin therapy and late stage of the disease, as well as the pro-calcific effects of statins, may have contributed to this failure. In addition to statins, several other approaches are currently under investigation to prevent CAVD progression.13 Examples include Niacin [NCT02109614, to reduce Lp(a) levels], denosumab or alendronic acid (NCT02132026, to target bone metabolism), tadalafil (NCT01275339, to inhibit phosphodiesterase Type 5), and ataciguat (NCT02481258, to promote guanylate cyclase activity). Irrespective of the drug tested, considerable heterogeneity of CAVD progression makes it difficult to demonstrate therapeutic effectiveness in clinical trials. For instance in the Simvastatin and Ezetimibe in Aortic Stenosis (SEAS) trial, the mean (± SD) change in peak aortic-jet velocity during a median follow-up of 52.2 months was 0.62 ± 0.61 m·s−1 in the placebo group.12 It is reasonable to assume that by targeting therapeutic interventions to those patients who are at the highest risk for progression, it is easier to establish any potential therapeutic efficacy.
The initial diagnosis and evaluation of CAVD and AS is typically based on clinical findings and echocardiography. In addition, a subset of patients (e.g., those with inconclusive echocardiography results) may require invasive evaluation of the valve. However, classical non-invasive imaging techniques, echocardiography, CT, and MRI, which focus on aortic valve anatomy and physiology (e.g., calcification, area, flow rates and transvalvular gradient) and associated aortic and myocardial abnormalities (focal and diffuse myocardial fibrosis, global and basilar longitudinal strain) inadequately inform of the patient progression risk and prognosis. This risk is directly related to valvular (and myocardial) pathobiology, which can be appraised by molecular imaging. As such, in vivo assessment of valvular biology by molecular imaging can potentially help clinical decision making regarding the need for aortic valve replacement in patients with moderate AS who are to undergo coronary artery bypass grafting, and possibly in patients with severe asymptomatic AS, who might be at increased risk for sudden cardiac death. Selection of patients who might benefit from emerging medical therapies and assessment of the response to therapeutic interventions within a relatively short period of time are additional applications of such techniques for drug development. Last, but certainly not least, molecular imaging can help address important gaps in CAVD pathophysiology through in vivo assessment of aortic valve biology and serial imaging in the same subject.
Pathobiology
CAVD was once presumed to be a degenerative disease associated with passive calcium deposition in leaflets. Over the past two decades, advances in our understanding of aortic valve pathobiology have led to a paradigm shift of our view of the disease to an active process of valvular thickening and calcification starting with asymptomatic, hemodynamically insignificant aortic sclerosis that progresses to aortic stenosis.14
Aortic valve leaflets are covered by valvular endothelial cells (VECs) and are composed of three layers, namely the collagen-rich fibrosa on the aortic side, the proteoglycan-rich spongiosa, and the elastin-rich ventricularis.15 The valve-specific, fibroblast-like valvular interstitial cells (VICs) are interspersed within these layers and play a central role in fibrocalcific remodeling of the leaflets.16 Environmental factors, biomechanical perturbations, and genetic predisposition contribute to the leaflet remodeling process, which culminates in the development of hemodynamically significant aortic stenosis.
VEC activation induced by changes in shear stress or other stimuli, e.g., atherosclerotic risk factors, initiates the development of CAVD by promoting sub-endothelial lipid retention and oxidation, and recruitment of inflammatory cells to the leaflet.17,18 Inflammation, VIC transformation, angiogenesis, extracellular matrix remodeling, and calcification are pathologic hallmarks of CAVD. Infiltrated macrophages and T cells may trigger tissue remodeling by promoting oxidative stress, release of proteases, e.g., matrix metalloproteinases and cathepsins, and differentiation of quiescent VICs into myofibroblasts. This promotes fibrotic remodeling of the valve. In parallel, these cells may undergo osteogenic differentiation, resulting in the formation of calcific nodules, a process which starts at the base of the fibrosa layer.19,20,21,22 Ultimately, the thickening, fibrosis, and calcification of the leaflets lead to hemodynamically significant aortic stenosis in a subset of patients. Of note, while the immune-inflammatory (oxidative stress-driven) pathway underlies most cases of CAVD, an alternative hyperphosphatemia-driven pathway of calcification appears to dominate in patients with chronic kidney disease. These issues are discussed in detail elsewhere.14,23
Molecular Imaging
Preclinical Studies
The small size of aortic valve and motion are challenges to in vivo imaging. Accordingly, a number of studies have relied on ex vivo imaging modalities [near-infrared fluorescence (NIRF) imaging, and magnetic resonance imaging and spectroscopy (MRI/MRS)] to image various aspects of valvular pathobiology in rodent models of CAVD, including endothelial cell activation, proteolytic activity, phagocytic activity, osteogenic activity and angiogenesis.24,25,26 While valuable as complement to conventional molecular and valvular biology techniques, the inherent limitations of these techniques preclude their application in longitudinal studies and ultimately, clinical implementation. This is in sharp contrast with emerging nuclear imaging-based approaches, which can be readily applied to non-invasive imaging in humans.
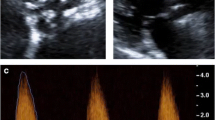
MMPs play a key role in extracellular matrix remodeling. Several members of MMP family, MMP-1, -9, and -12, are amongst the most highly up-regulated genes in CAVD.27 Inflammatory cells are major sources of MMP production and activity. Accordingly, MMP activation and inflammation are closely intertwined in CAVD. The feasibility of MMP-targeted imaging was recently shown in apolipoprotein E−/− mice fed a Western diet for up to 9 months to induce CAVD. In this model, a subset of animals develops hemodynamically significant aortic stenosis. In vivo microSPECT-CT imaging using RP805, a 99mTc-labeled tracer that binds to several activated MMPs, demonstrated specific in vivo aortic valve uptake of the tracer (Figure 1).28 Interestingly, the MMP signal peaked at 6 months of Western diet, while key features of CAVD (leaflet thickening, valvular calcification, and aortic stenosis) were most pronounced after 9 months. This suggests that MMPs are implicated in CAVD progression and MMP imaging may serve as a predictive tool in CAVD. A significant correlation between valvular macrophage staining (CD68) and MMP signal in vivo suggested that this technique may be used for detection of valvular inflammation and remodeling. Whether this technique can be used for imaging CAVD in the absence of atherosclerotic lesions (which co-exist in the aforementioned model) or tracking the response to therapeutic interventions remains to be determined.
In vivo matrix metalloproteinase-targeted imaging in apolipoprotein E−/− mouse fed on Western diet for up to 9 months to induce CAVD. Top panels Examples of transverse contrast-enhanced CT, 99mTc-RP805 (matrix metalloproteinase-targeted) SPECT, and fused images in a control, wild-type mouse, and an apolipoprotein E−/− mouse fed on Western diet for 6 months. Bottom panel Background-corrected quantification of aortic valve 99mTc-RP805 uptake in control animals and apolipoprotein E−/− mice fed on Western diet for up to 9 months. Arrows point to aortic valve area. cpv, counts per voxel. *P < .05, **P < .01, ***P < .0001, n = 7-13 in each group. This research was originally published in Journal of Nuclear Medicine (Jung et al.28 © by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
Clinical Studies
FDG PET
Despite some concern regarding its specificity, 18F-FDG PET has been extensively used for imaging vascular inflammation and has served as a non-invasive tool for early assessment of the effect of novel therapeutic interventions in clinical trials. Indeed, several studies have shown a close relationship between the 18F-FDG signal and macrophage content of atherosclerotic plaques.29,30,31,32,33 The potential role of 18F-FDG PET in aortic valve disease was first investigated in a retrospective analysis of whole body 18F-FDG PET/CT images acquired for oncological staging.34 In this study, FDG uptake [expressed as target-to-blood ratio (TBR)] was significantly higher in patients with aortic stenosis (n = 42) than in the matched control group (Figure 2). When categorized based on severity of stenosis and calcification, 18F-FDG uptake was significantly increased in patients with mild and moderate disease, but not in those with severe disease.

Adapted with permission from Elsevier and Copyright Clearance Center, Journal of the American College of Cardiology,34 © 2011
18F-FDG imaging of CAVD. Examples of CT images (A) and fused 18F-FDG-PET/CT images (B) of aortic valve in patients with different stages of CAVD. Arrows point to 18F-FDG uptake in aortic valve. AS, Aortic stenosis.
Another retrospective study evaluated the relation between aortic valve 18F-FDG signal in 111 patients without active cancer or aortic stenosis who underwent at least 2 18F-FDG PET/CT studies within a period of 1-5 years.35 When categorized as non-progressors or progressors based on aortic valve calcium score determined at baseline and follow-up time points, the 18F-FDG signal (expressed as SUVmax) in aortic valve was found to be higher in the progressor group compared to non-progressor group. A similar difference was seen in the subset of subjects without aortic valve calcification at baseline. Interestingly, in this study the 18F-FDG signal could independently predict subsequent calcification after adjusting for cardiovascular risk factors.
In a prospective study of 18F-FDG PET (and 18F-NaF) imaging involving 121 subjects (including 20 controls), the aortic valve 18F-FDG signal (maximum TBR) was significantly higher in patients with aortic stenosis than in controls and the uptake modestly increased with the severity of stenosis.36 A subset of the subjects of the original cohort (n = 30) was followed for 1 year, during which 12 subjects underwent aortic valve replacement (AVR) for symptomatic AS. In the remaining subjects without severe AS, there was no correlation between the baseline 18F-FDG signal and 1-year change in calcium score.37 In those subjects who underwent valve replacement, there was no correlation between the 18F-FDG signal in vivo and CD68 staining of the surgical specimens. A follow-up evaluation of the full cohort of the subjects (including the controls) at 2 years showed weak correlations between the baseline 18F-FDG signal and aortic calcification (CT) and stenosis (echocardiography). In addition, the baseline 18F-FDG signal was able to predict clinical outcomes (composite of cardiovascular death and aortic valve replacement) independently of age and sex.38
While the non-linear relation between 18F-FDG signal in aortic valve and the severity of aortic stenosis is intriguing, existing data raise many questions about the biological and clinical significance of the 18F-FDG signal in the valve. In addition, uptake in the myocardium is a practical barrier to broader implementation of aortic valve 18F-FDG PET imaging, indicating that alternative, more robust techniques are needed for evaluation of CAVD in humans.
NaF PET
18F-NaF is retained at the sites of calcification through binding to hydroxyapatite via an exchange mechanism with hydroxyl groups.39 However, the biological basis of 18F-NaF uptake in aortic valve is not well defined. Studies in human atherosclerotic lesions have linked the NaF signal in atherosclerosis to the sites of calcification. As such, in carotid endarterectomy specimens, NaF uptake ex vivo detected by autoradiography corresponded to the areas of calcification (Alizarin red staining), but not CD68 (macrophages), CD31 (endothelial cells) or alpha-smooth muscle actin (smooth muscle cells) expression.40 The higher 18F-NaF retention at the sites of microcalcification as opposed to larger masses of calcified material has been attributed to the relatively larger surface area of the former available for 18F-NaF binding.
The potential of 18F-NaF PET for imaging valvular biology in CAVD was brought up by retrospective analysis of aortic valve signal in 18F-NaF PET-CT studies of a small group of subjects (n = 5) with cancer or rheumatological disease and concomitant aortic stenosis (and control subjects without aortic valve calcification), which showed significantly higher 18F-NaF (both maximal SUVs and TBRs) in those with aortic stenosis.41 The aforementioned prospective study of 121 subjects with aortic sclerosis or stenosis of different severity or control subjects without apparent aortic valve disease who underwent both 18F-FDG and 18F-NaF PET/CT shortly followed this initial observation and demonstrated higher 18F-NaF signal in aortic valve of patients with CAVD than controls with the intensity of the signal increasing with the severity of the aortic stenosis.36 Of note, while 91% of patients with aortic stenosis had increased 18F-NaF uptake, only 35% showed increased 18F-FDG uptake. The poor correlation between aortic valve signal from these tracers indicated that they target different aspects of aortic valve biology. The 1-year follow-up study of a subset of the original cohort (n = 30, including 12 subjects who had undergone surgical AVR and 18 with asymptomatic disease) reported a statistically significant correlation between the baseline 18F-NaF signal and changes in calcium score. A similar correlation was found between the baseline and subsequent changes in calcium score.37 Histological analysis of the aortic valve from those subjects who underwent AVR during this 1-year period showed an association (albeit modest) between the baseline 18F-NaF signal and markers of calcification (alkaline phosphatase and osteocalcin), but not inflammation (CD68). Importantly, areas of osteocalcin expression in the leaflet extended beyond the area of Von Kossa staining (established calcium) and corresponded to the area with maximal ex vivo 18F-NaF uptake on autoradiography. The 2-year follow-up evaluation of this cohort reported the development of new sites of macrocalcification corresponding to the sites of baseline 18F-NaF signal, and a good correlation (r2 = 0.64) between the baseline 18F-NaF signal and subsequent changes in calcium score (Figure 3). However, the relationship with echocardiographic measures of hemodynamic progression was much weaker, highlighting the role of other factors (fibrosis, distribution of calcification) in aortic stenosis.38 Consistent with the observations at 1-year follow-up, 18F-NaF PET-CT was not an independent predictor of clinical outcomes at 2 years after correction for baseline CT-based calcium scores.

Adapted with permission from Elsevier and Copyright Clearance Center, Journal of the American College of Cardiology,38 © 2015
18F-NaF imaging and progression of valvular calcification in CAVD. Baseline CT images (left) and fused 18F-NaF-PET/CT images (middle), and 2-year follow-up CT images (right) in two patients with CAVD.
Perspective and Conclusions
The utility of 18F-NaF PET as a marker of disease activity in CAVD is currently under evaluation in several clinical trials [NCT02132026 (SALTIRE2), NCT02740088, NCT03095313]. While potentially useful as a tool to track the effect of therapeutic interventions on valvular calcification, 18F-NaF PET imaging probably misses the fibrotic component of the disease and additional molecular imaging tools are necessary to fully evaluate different aspects of this pathology. The size of aortic valve and its continuous motion are major hurdles to imaging quantitative assessment of valvular biology by molecular imaging. In the case of 18F-FDG, despite the emergence of several protocols aimed at suppressing myocardial uptake of the tracer, consistent, complete suppression remains an additional challenge. Recent advances in image acquisition and quantification methodology have improved the image quality and reproducibility of aortic valve 18F-NaF PET images, and more progress is expected to lead to more reliable data for clinical studies.42,43 While PET is a powerful tool for clinical imaging, the spatial resolution of microPET is a challenge to imaging in the mouse and microSPECT is more suitable in this setting. Preclinical studies of CAVD are also hampered by the paucity of relevant animal models, and in this regard, emergence of non-atherosclerotic models of the disease with leaflet calcification (similar to human disease) would be of great value. Furthermore, despite many similarities between valvular and vascular calcification, there are also key differences between the two processes that should not be overlooked.44 Nevertheless, a number of new tracers that are under evaluation for imaging atherosclerosis may be of value in CAVD.45 The limitations of 18F-FDG and 18F-NaF PET imaging highlight the need for such new approaches to detect key aspects of CAVD pathophysiology.
In conclusion, molecular imaging may address some of the existing gaps in CAVD management. Accordingly, there is a great need for non-invasive, highly specific molecular imaging tools with real potential for clinical translation. As only a subset of patients with aortic sclerosis progress to advanced CAVD, detection of early molecular and functional abnormalities in aortic valve disease that predict the future risk for disease progression can help identify subjects who would most benefit from novel therapeutic approaches to prevent aortic valve stenosis. In addition to its clinical relevance, the development of an imaging approach for tracking valvular biology in CAVD will help advance our understanding of pathophysiology and potentially lead to novel medical therapies for this prevalent disease.
References
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation 2017;135:e146-603.
Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, et al. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005;111:3290-5.
Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900.
Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, et al. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006.
Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: A systematic review. Heart 2017;103:1323-30.
Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 1999;341:142-7.
Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, et al. Mild and moderate aortic stenosis: natural history and risk stratification by echocardiography. Eur Heart J 2004;25:199-205.
Gerdts E, Rossebø AB, Pedersen TR, Cioffi G, Lønnebakken MT, Cramariuc D, et al. Relation of left ventricular mass to prognosis in initially asymptomatic mild to moderate aortic valve stenosis. Circ Cardiovasc Imaging 2015;8:e003644.
Ng ACT, Prihadi EA, Antoni ML, Bertini M, Ewe SH, Ajmone Marsan N, et al. Left ventricular global longitudinal strain is predictive of all-cause mortality independent of aortic stenosis severity and ejection fraction. Eur Heart J Cardiovasc Imaging 2017. https://doi.org/10.1093/ehjci/jex189.
Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol 2002;40:1723-30.
Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, Boon NA. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 2005;352:2389-97.
Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343-56.
Marquis-Gravel G, Redfors B, Leon MB, Généreux P. Medical treatment of aortic stenosis. Circulation 2016;134:1766.
Yutzey KE, Demer LL, Body SC, Huggins GS, Towler DA, Giachelli CM, et al. Calcific aortic valve disease: A consensus summary from the alliance of investigators on calcific aortic valve disease. Arterioscler Thromb Vasc Biol 2014;34:2387-93.
Hinton RB Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res 2006;98:1431-8.
Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Executive summary: Calcific aortic valve disease-2011 update. Circulation 2011;124:1783-91.
Butcher JT, Nerem RM. Valvular endothelial cells and the mechanoregulation of valvular pathology. Philos Trans R Soc Lond B 2007;362:1445-57.
Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol 2009;29:254-60.
New SE, Aikawa E. Molecular imaging insights into early inflammatory stages of arterial and aortic valve calcification. Circ Res 2011;108:1381-91.
Chester AH, Taylor PM. Molecular and functional characteristics of heart-valve interstitial cells. Philos Trans R Soc Lond B 2007;362:1437-43.
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: One function, multiple origins. Am J Pathol 2007;170:1807-16.
Mohler ER 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 2001;103:1522-8.
Aikawa E, Libby P. A rock and a hard place. Circulation 2017;135:1951.
Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 2007;115:377-86.
Waters EA, Chen J, Allen JS, Zhang H, Lanza GM, Wickline SA. Detection and quantification of angiogenesis in experimental valve disease with integrin-targeted nanoparticles and 19-fluorine MRI/MRS. J Cardiovasc Magn Reson 2008;10:43.
Aikawa E, Aikawa M, Libby P, Figueiredo JL, Rusanescu G, Iwamoto Y, et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation 2009;119:1785-94.
Bosse Y, Miqdad A, Fournier D, Pepin A, Pibarot P, Mathieu P. Refining molecular pathways leading to calcific aortic valve stenosis by studying gene expression profile of normal and calcified stenotic human aortic valves. Circ Cardiovasc Genet 2009;2:489-98.
Jung JJ, Razavian M, Challa AA, Nie L, Golestani R, Zhang J, et al. Multimodality and molecular imaging of matrix metalloproteinase activation in calcific aortic valve disease. J Nucl Med 2015;56:933-8.
Font MA, Fernandez A, Carvajal A, Gamez C, Badimon L, Slevin M. Imaging of early inflammation in low-to-moderate carotid stenosis by 18-FDG-PET. Front Biosci 2009;14:3352-60.
Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: A comparison with 18F-FDG PET/CT and histology. J Nucl Med 2009;50:959-65.
Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, et al. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol 2010;55:2527-35.
Tawakol A, Migrino RQ, Hoffmann U, Abbara S, Houser S, Gewirtz H, et al. Noninvasive in vivo measurement of vascular inflammation with F-18 fluorodeoxyglucose positron emission tomography. J Nucl Cardiol 2005;12:294-301.
Zhang Z, Machac J, Helft G, Worthley SG, Tang C, Zaman AG, Rodriguez OJ, Buchsbaum MS, Fuster V, Badimon JJ. Non-invasive imaging of atherosclerotic plaque macrophage in a rabbit model with F-18 FDG PET: A histopathological correlation. BMC Nucl Med 2006;6:3.
Marincheva-Savcheva G, Subramanian S, Qadir S, Figueroa A, Truong Q, Vijayakumar J, et al. Imaging of the aortic valve using fluorodeoxyglucose positron emission tomography increased valvular fluorodeoxyglucose uptake in aortic stenosis. J Am Coll Cardiol 2011;57:2507-15.
Abdelbaky A, Corsini E, Figueroa AL, Subramanian S, Fontanez S, Emami H, et al. Early aortic valve inflammation precedes calcification: A longitudinal FDG-PET/CT study. Atherosclerosis 2015;238:165-72.
Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76-86.
Dweck MR, Jenkins WS, Vesey AT, Pringle MA, Chin CW, Malley TS, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging 2014;7:371-8.
Jenkins WS, Vesey AT, Shah AS, Pawade TA, Chin CW, White AC, et al. Valvular (18)F-fluoride and (18)F-fluorodeoxyglucose uptake predict disease progression and clinical outcome in patients with aortic stenosis. J Am Coll Cardiol 2015;66:1200-1.
Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med 1972;2:31-7.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JLE, Dweck MR, et al. Identifying active vascular microcalcification by 18F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495.
Hyafil F, Messika-Zeitoun D, Burg S, Rouzet F, Benali K, Iung B, et al. Detection of 18fluoride sodium accumulation by positron emission tomography in calcified stenotic aortic valves. Am J Cardiol 2012;109:1194-6.
Pawade TA, Cartlidge TR, Jenkins WS, Adamson PD, Robson P, Lucatelli C, et al. Optimization and reproducibility of aortic valve 18F-fluoride positron emission tomography in patients with aortic stenosis. Circ Cardiovasc Imaging 2016. https://doi.org/10.1161/CIRCIMAGING.116.005131.
Doris MK, Rubeaux M, Pawade T, Otaki Y, Xie Y, Li D, et al. Motion-corrected imaging of the aortic valve with 18F-NaF PET/CT and PET/MR: A feasibility study. J Nucl Med. 2017. https://doi.org/10.2967/jnumed.117.194597.
Weiss RM, Miller JD, Heistad DD. Fibrocalcific aortic valve disease: Opportunity to understand disease mechanisms using mouse models. Circ Res 2013;113:209-22.
Tavakoli S, Vashist A, Sadeghi MM. Molecular imaging of plaque vulnerability. J Nucl Cardiol 2014;21:1112-1128; quiz 1129.
Disclosure
Jae-Joon Jung and Farid Jadbabaie have nothing to disclose. Mehran M. Sadeghi is a consultant for Bracco Research, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
This work was supported by grants from NIH (R01-HL114703, R01-HL138567), and Department of Veterans Affairs (I0-BX001750).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jung, JJ., Jadbabaie, F. & Sadeghi, M.M. Molecular imaging of calcific aortic valve disease. J. Nucl. Cardiol. 25, 1148–1155 (2018). https://doi.org/10.1007/s12350-017-1158-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-017-1158-7