Abstract
Background
The prognostic value of normal stress myocardial perfusion single-photon emission computed tomography (MPS) in patients with diabetes has only been evaluated in single-center studies of relatively limited sample size. We performed a meta-analysis of published studies, including diabetic patients with known or suspected coronary artery disease (CAD), to assess the predictive value for adverse cardiac ischemic events of normal stress MPS.
Methods and Results
Studies published between January 1990 and December 2013 were identified by database search. We included studies using stress MPS to evaluate diabetic patients with known or suspected CAD and providing data on clinical outcomes of non-fatal myocardial infarction or cardiac death with a follow-up time ≥12 months. A total of 14 studies were finally included, recruiting 13,493 patients. The negative predictive value (NPV) for non-fatal myocardial infarction and cardiac death of normal MPS was 94.92% (95% confidence interval 93.67-96.05), during a weighted mean follow-up of 36.24 months, resulting in estimated event rate after a negative test equal to 5.08% (95% confidence interval 3.95-6.33). The corresponding annualized event rate after a negative test was 1.60% (95% confidence interval 1.21-2.04).
Conclusions
Stress MPS has a high NPV for adverse cardiac events in diabetic patients with known or suspected CAD leading to define a “relatively low-risk” patients category.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stress myocardial perfusion single-photon emission computed tomography (MPS) has taken a central role in risk stratify patients with suspected or known coronary artery disease (CAD). Accurate risk stratification has become increasingly important to optimize patient outcomes and contains rapidly escalating medical care costs.1 In particular, the prognostic value of negative stress MPS gives the possibility to identify low-risk patients, decreasing additional tests and interventions. However, after a normal stress MPS, diabetic patients are at higher risk for cardiac events than non-diabetic subjects also after balancing clinical characteristics and stress type by propensity score analysis.2 Despite cardiac risk assessment in diabetic patients is challenging, based on current data, routine screening is not generally recommended. Higher knowledge of prognostic value of normal MPS results in diabetic patients can be important considering that the reliance of temporal changes in risk during a long-term follow-up provides also a basis for identifying the timing of repeat testing.2,3
However, the prognostic value of a negative stress MPS in diabetic patients has only been evaluated in single-center studies of relatively limited sample size. Thus, we performed a meta-analysis of published studies, including diabetic patients with known or suspected CAD, to assess the predictive value for adverse cardiac ischemic events of normal stress MPS, defined as the absence of inducible perfusion defects.
Materials and Methods
Data Sources and Study Selection
An English literature search was performed using the PubMed, Cochrane, Web of Science, and Scopus database to identify articles published between January 1990 and December 2013. Studies search was restricted to data obtained in humans and adults and was conducted using the following keywords (alone and in different combinations): prognosis, outcome, follow-up, diabetes, diabetic patients, and myocardial perfusion imaging. The title and abstract of potentially relevant studies were screened for appropriateness before retrieval of the full article when relevant by two reviewers (V.C., R.G.) and disagreements were resolved by consensus. The full-published reports of the all abstracts selected by the reviewers were retrieved and the same reviewers independently performed a second-step selection based on the inclusion criteria; disagreements were resolved by consensus. In addition, one reviewer manually reviewed the bibliographies of retrieved articles for additional citations. A study was included if all of the following criteria were met: (1) the study had prospective or retrospective analysis of diabetic subjects referred for suspected or known CAD who underwent pharmacological or exercise stress MPS for searching inducible ischemia; (2) the study included a negative test defined in the absence of inducible perfusion defects during stress MPS; (3) the study provided the absolute number of diabetic patients with a negative test and data on clinical outcomes of non-fatal myocardial infarction and cardiac death; and (4) the follow-up time of the study was at least 12 months. In case of multiple studies reported from the same research group, potential cohort duplication was avoided by including the largest study only. Overall study quality was not used as a pre-specified inclusion criterion.4
Data Extraction and Quality Assessment
To evaluate eligibility for the meta-analysis, as previously described,5,6 each study was initially identified by considering journal, author, and year of publication. Additional extracted variables included study design (retrospective or prospective), imaging system, criterion used to classify a test as negative, type of stressor used, type of radiotracer used, the number of patients with and without disease, and modality of data assessment (qualitative, semiquantitative, or quantitative). Data were also collected on age and on prevalence of female sex, traditional cardiovascular risk factors besides diabetes (hypertension, dyslipidemia, family history of CAD, smoking), angina-like symptoms, and history of CAD (including previous myocardial infarction and previous myocardial revascularization). Mean or median follow-up time, number of events, or event rate based on negative tests, occurrence of non-fatal myocardial infarction, or cardiac death were recorded.
Quality assessment was performed using a previously described methodology7 and was on the basis of the presence of the following parameters: (1) complete follow-up in the majority of subjects (≥90% of the baseline cohort); (2) outcome data collected by investigators blinded to the test results; and (3) outcomes corroborated by hospital records and death certificates. Studies were defined as good quality if they fulfilled criterion 1 and ≥1 of the other 2 criteria, as fair quality if they fulfilled only the criterion 1, and as poor quality if they fulfilled none of the criteria.
Statistical Analysis
Demographical and clinical characteristics of all patients included in this meta-analysis were obtained as weighted averages of those reported in the single studies, the weights being the total sample size for each study. Statistical heterogeneity between studies was assessed using the Cochrane Q statistic (with a value of P < .1 reflecting significant heterogeneity) and I 2 statistic,8,9 which measures the percentage of total variability across studies not due to sampling error. Because of the large heterogeneity experienced, the pooled estimates of negative predictive value (NPV) for cardiac events and the pooled estimates of the event rate after negative tests (event rate after negative test = 1 − NPV) were computed with the 95% confidence interval (CI) using the random-effects model of DerSimonian and Laird.10
While fixed effects model assumes that the true effect size is the same in all study and that all of the variability between effect sizes is due to sampling error, the random-effects model assumes that the variability between effect sizes is due to sampling error (within study variance) plus variability in the effect size among the studies (between study variance). Accordingly, in a random-effects meta-analysis, the standard errors of the study-specific estimates are adjusted to incorporate a measure of the extent of between study variance.10 The influence of each study on the overall results of the meta-analysis is expressed as weight. The higher the percentage weight (and the bigger the box in the forest-plot depicting the meta-analysis results), the more influence the study has on the overall results. In the present meta-analysis, the weight of each study was calculated with the inverse variance method, as a function of the variance of that study (V i) and of the variance of the true effects of the compound between the different studies (T 2), using the formula: 1/(V i + T 2).11
To correct for over dispersion, the raw proportions (NPV and event rate after negative test) were initially converted using the Freeman-Tukey transformation and back transformed after quantitative data synthesis.12,13 For each study, the annualized event rate after negative test was obtained as average during the lengths of follow-up. In addition to the overall random-effects model, univariate meta-regression analyses were performed to assess the evidence of prevalence modifiers. To formally evaluate potential publication bias, we performed a test proposed by Egger et al.14 All analyses were performed with R statistics (version 2.15.0), using the additional packages META and METAFOR.
Results
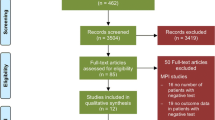
The initial database search identified 165 potentially eligible citations. The reviewers, after the evaluation of the titles and abstracts of these studies, discharged 131 citations because they were judged to be non-relevant or non-pertinent. To determine eligibility, each investigator reviewed the full text of the remaining 34 independently, and disagreements were resolved by consensus. After revision, 20 articles were excluded and the final analysis considered 14 studies2,15-27 including 13,493 patients. The complete literature search is presented in flow-chart form in Figure 1.
Demographical and clinical characteristics of patients included in the studies in the meta-analysis are detailed in Table 1. Data were obtained using exercise or pharmacologic stress test in ten studies and pharmacologic stress test in four studies (adenosine in three and dobutamine in one). The imaging agent was a Tc-99m-based tracer in 12 studies and thallium-201 in 2 studies. Of the 14 studies, 9 used as cut-off of normal myocardial perfusion a summed stress score (SSS) ≤3 (17-segment score model in 7 studies and 20-segment score model in 2 studies) and 2 a SSS ≤4 (17-segment score model in one study and 20-segment score model in another study). In the last three studies, myocardial perfusion was defined as normal when a perfusion defect involved <2 myocardial segments. Study sample sizes ranged from 87 to 4,628 patients enrolled, including a proportion of patients with known CAD ranging from 20% to 40%. The mean age ranged from 57 to 65 years, with the proportion of women ranging from 13% to 50%. The duration of follow-up ranged from 2.2 to 4.8 years. After negative test, the event rate for non-fatal myocardial infarction or cardiac death varied between 0.90% and 9.62% and the annualized event rate between 0.41% and 3.24%. After quality assessment, 11 studies were graded as good quality and 3 studies as fair quality.
Predictive Value of Stress MPS for Cardiac Death and Non-fatal Myocardial Infarction
Figure 2 shows the forest plot of NPV for non-fatal myocardial infarction and cardiac death for each study, as well as the pooled estimate yielded by the random-effect model. In the individual studies, NPV ranged from 90.38% to 99.10%. During a weighted mean follow-up of 36.24 months, the summary NPV was 94.92% (95% CI 93.67-96.05). The estimate of NPV was not significantly different (P = .14) between studies including both asymptomatic and symptomatic patients (94.19%, 95% CI 92.91-95.35) and studies including only asymptomatic patients (95.83%, 95% CI 92.80-98.09). The pooled event rate after negative test was 5.08% (95% CI 3.95-6.33) in the overall studies, without differences (P = .14) between studies including asymptomatic and symptomatic patients and those including only asymptomatic patients (Figure 3).
The corresponding pooled estimate of annualized event rate after negative test was 1.60% (95% CI 1.21-2.04). The estimate of annualized event rate was slightly lower (P = .06) for the subgroup of studies including only asymptomatic patients (1.20%, 95% CI 0.54-2.09) as compared to those including both asymptomatic and symptomatic patients (1.93%, 95% CI 1.48-2.45) (Figure 4). Noteworthy, the pooled estimate of annualized event rate was not significantly different (P = .41) between the four studies published before 2007 (1.95%, 95% CI 0.96-3.26) and those published after 2007 (1.48%, 95% CI 1.01-2.02).
A high level of heterogeneity was observed between studies for both NPV and event rate after negative test (I 2 = 78.4%, P < .0001) and for annualized event rate after negative test (I 2 = 84.6%, P < .0001). At meta-regression analysis, no significant association was detectable between NPV and the considered variables: age, sex, dyslipidemia, smoking, hypertension, family history of CAD, and prior myocardial infarction.
Publication Bias
For studies enrolled in this meta-analysis, no evidence of significant publication bias was found for NPV (bias 0.12, standard error 1.2, slope 2.66, P = .92) as well as for event rate after negative test (bias −0.12, standard error 1.2, slope 0.47, P = .92), and annualized event rate after negative test (bias −0.25, standard error 1.47, slope 0.27, P = .8).
Discussion
This systematic review and meta-analysis is the first addressing the prognostic value of negative stress MPS result in diabetic subjects with known or suspected CAD. Our results demonstrate that in this patient population a negative stress MPS predicts a relatively low risk of cardiovascular events over a mid-term follow up. One of the major strengths of stress MPS is the low subsequent cardiac event rate in patients with normal findings.3 Several studies reported that patients with negative stress MPS have an excellent outcome, as less than 1% of patients with a normal study will experience hard cardiac events, such as cardiac death or non-fatal myocardial infarction.2,3 However, the risk of hard event after a normal MPS is a function of the clinical and historical factors of the patients tested. In particular, diabetes is an important clinical variable in determining the risk of events after stress MPS. It has been demonstrated that after a normal stress MPS, diabetic patients are at higher risk (>1%) for cardiac events than non-diabetic subjects (<1%) also after balancing clinical characteristics and stress type by a propensity score analysis.2 Summary data according to diabetes status after a normal stress MPS might be very useful considering that the low risk associated with normal MPS is an important component of a testing strategy that can lower the overall cost and enhance the effectiveness of test.3
Data already published evaluating the stratification power of a normal MPS finding in diabetic patients have been performed in heterogeneous studies. Therefore, the prognostic value of a normal stress MPS in patients with diabetes has been extrapolated by an overall population with suspected or known CAD, and several potential confounding may have affect the results reported. Often, the prognostic power of a normal stress MPS has not been specifically evaluated in diabetic patients, but has been tested using diabetes as covariate in an overall patient population with suspected or known CAD.
From the present meta-analysis using restrict researching criteria (diabetic patients and normal MPS), it emerged that MPS has a high NPV leading to define a low-risk patients category. From published data it emerged that additional interventions can be avoided identifying a low-risk group of patients, defined by an annual event rate of <1%.28 However, in our meta-analysis the pooled annualized event rate with normal test is 1.60%, higher that in the general population (<1%), leading to define diabetic patients with negative stress MPS results as a “relatively low-risk” patients category. It should be considered that a significant heterogeneity in the studies selected in this meta-analysis was observed. In particular, the range of annualized event rate has been reassuring (<1%) only in four studies23,25-27 while in all the others studies it ranged from 1.47 to 3.24.2,15-22,24
It is still uncertain if all diabetic patients should be treated as CAD risk equivalents although many do not achieve that risk status29 or if they should be further stratify to better align intensity of therapy with levels of cardiovascular risk.30 The detection of occult CAD in order to initiate appropriate therapy at a time point when the disease process is more easily modifiable is the objective of non-invasively testing asymptomatic diabetic patients for risk stratification purposes. This strategy is expected to lead to declines in cardiac morbidity and mortality. Major society guidelines and position statements have made recommendations about testing for asymptomatic CAD in diabetic patients. The American College of Cardiology considers appropriate the use of radionuclide imaging for asymptomatic diabetic patients >40 years old and other coronary risk equivalents.31 The American College of Cardiology Foundation/American Heart Association guidelines give a weak class IIb (level of evidence C) recommendation to consider stress MPS for cardiovascular risk assessment in asymptomatic adults with diabetes.32 A position statement of the European Society of Cardiology states that asymptomatic diabetic patients without known CAD should be considered for coronary calcium imaging followed by MPS for those with significant coronary calcium, in order to identify subjects with a moderate to severe extent of ischemia which are likely to benefit from invasive evaluation and revascularization (class of recommendation IIa).33 The American Diabetes Association does not recommend screening for CAD in asymptomatic patients because it does not improve outcomes as long as cardiovascular risk factors are treated (level of evidence A).34 Important questions are still to be addressed, such as the potential of coronary revascularization to reduce cardiac events in asymptomatic diabetic patients; the yield of non-invasive testing in identifying reliably a considerable proportion of those patients likely to benefit from this type of intervention in a cost-effective manner; and who, when, and how to test.35,36 In the present meta-analysis, we found that the annualized event rate was not significantly different for studies including only asymptomatic patients compared to those including both asymptomatic and symptomatic patients.
Stress tests are most useful for patients with suspected CAD at intermediate pre-test probability of disease or in patients with known CAD at intermediate pre-test probability of ischemia, by moving them into a higher- or lower-risk group, thereby informing the choice of additional diagnostic tests, interventions, and medical management, which are costly and carry significant risks. Our study indicates that stress MPS in diabetic patients is useful in identifying such lower-risk subjects over a spectrum of pre-test probabilities. Even in study cohorts or subsets of patients with relatively high pre-test probability, such as those with a higher percentage of prior myocardial infarction, the event rate with normal stress MPS is relatively low. However, only identifying a low-risk group of patients, defined by an annual event rate of <1%, additional interventions can be avoided in most cases.37 In diabetic patients a normal MPS assumes an important significance also considering that helps to identify a warranty period in establishing the time at which repeat testing might be appropriate.20 Repeating testing remains a significant part in the evaluation of cost-effectiveness in the long-term follow-up of patients with suspected and known CAD.
Limitations
Variation in test performance among subjects at very high or very low risk of disease (spectrum bias) may affect the results of prognostic studies, also in assessment of NPV.37 Our systematic review includes studies of cohorts with supposedly varying pre-test risk of disease, as manifested by a broad range of percentage of subjects with major cardiovascular risk factor, history of CAD, prior myocardial infarction, history of previous coronary revascularization procedures or with symptoms (Table 1). The assessment of pre-test risk is relevant for evaluating the clinical impact of cardiovascular imaging,38 but the availability of incomplete clinical data in many of the included studies prevents the possibility to perform subgroups analyses and, consequently, to evaluate more carefully the NPV in relation to pre-test risk. In fact, meta-regression analysis was performed only considering the variables available in the majority of the studies. We could not address the effect of some confounding factors such as cardiac medications, glycemic control, microvascular dysfunction, and differences between exercise and vasodilator testing. Also the impact of ejection fraction could not be considered because it was not reported in the subset of patients with normal myocardial perfusion in most the studies included in the meta-analysis.
New Knowledge Gained
The current study underscores the high NPV of a normal stress MPS in diabetic patients with known or suspected CAD. However, the pooled annualized event rate in subjects with normal test is higher (1.60%) than in the general population (<1%). Thus, our findings suggest that diabetic patients with negative stress MPS results are in an intermediate-risk category.
References
Metz LD, Beattie M, Hom R, Redberg RF, Grady D, Fleischmann KE. The prognostic value of normal exercise myocardial perfusion imaging and exercise echocardiography: A meta-analysis. J Am Coll Cardiol 2007;49:227-37.
Acampa W, Petretta M, Cuocolo R, Daniele S, Cantoni V, Cuocolo A. Warranty period of normal stress myocardial perfusion imaging in diabetic patients: A propensity score analysis. J Nucl Cardiol 2014;21:50-6.
Hachamovitch R, Hayes S, Friedman JD, Cohen I, Shaw LJ, Germano G, et al. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: What is the warranty period of a normal scan? J Am Coll Cardiol 2003;41:1329-40.
Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427-37.
Gargiulo P, Petretta M, Bruzzese D, Cuocolo A, Prastaro M, D’Amore C, et al. Myocardial perfusion scintigraphy and echocardiography for detecting coronary artery disease in hypertensive patients: A meta-analysis. Eur J Nucl Med Mol Imaging 2011;38:2040-9.
Gargiulo P, Dellegrottaglie S, Bruzzese D, Savarese G, Scala O, Ruggiero D, et al. The prognostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: A meta-analysis. Circ Cardiovasc Imaging 2013;6:574-82.
Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323:42-6.
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88.
Cleophas TJ, Zwinderman AH. Meta-analysis. Circulation 2007;115:2870-5.
Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat 1950;21:607-11.
Miller JJ. The inverse of the Freeman-Tukey double arcsine transformation. Am Stat 1978;32:138.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34.
Ghatak A, Padala S, Katten DM, Polk DM, Heller GV. Risk stratification among diabetic patients undergoing stress myocardial perfusion imaging. J Nucl Cardiol 2013;20:529-38.
Valensi P, Pariès J, Brulport-Cerisier V, Torremocha F, Sachs RN, Vanzetto G, et al. Predictive value of silent myocardial ischemia for cardiac events in diabetic patients: Influence of age in a French multicenter study. Diabetes Care 2005;28:2722-7.
Acampa W, Petretta M, Evangelista L, Daniele S, Xhoxhi E, De Rimini ML, et al. Myocardial perfusion imaging and risk classification for coronary heart disease in diabetic patients. The IDIS study: A prospective, multicentre trial. Eur J Nucl Med Mol Imaging 2012;39:387-95.
Barmpouletos D, Stavens G, Ahlberg AW, Katten DM, O’Sullivan DM, Heller GV. Duration and type of therapy for diabetes: Impact on cardiac risk stratification with stress electrocardiographic-gated SPECT myocardial perfusion imaging. J Nucl Cardiol 2010;17:1041-9.
Giri S, Shaw LJ, Murthy DR, Travin MI, Miller DD, Hachamovitch R, et al. Impact of diabetes on the risk stratification using stress single-photon emission computed tomography myocardial perfusion imaging in patients with symptoms suggestive of coronary artery disease. Circulation 2002;105:32-40.
Hage FG, Wackers FJ, Bansal S, Chyun DA, Young LH, Inzucchi SE, et al. The heart rate response to adenosine: A simple predictor of adverse cardiac outcomes in asymptomatic patients with type 2 diabetes. Int J Cardiol 2013;167:2952-7.
Kasim M, Currie GM, Tjahjono M, Siswanto BB, Harimurti GM, Kiat H. Myocardial perfusion SPECT utility in predicting cardiovascular events among Indonesian diabetic patients. Open Cardiovasc Med J 2013;7:82-9.
Santos MT, Parker MW, Heller GV. Evaluating gender differences in prognosis following SPECT myocardial perfusion imaging among patients with diabetes and known or suspected coronary disease in the modern era. J Nucl Cardiol 2013;20:1021-9.
Matsuo S, Nakajima K, Yamasaki Y, Kashiwagi A, Nishimura T. Prognostic value of normal stress myocardial perfusion imaging and ventricular function in Japanese asymptomatic patients with type 2 diabetes: A study based on the J-ACCESS-2 database. Circ J 2010;74:1916-21.
Sultan A, Piot C, Mariano-Goulart D, Daures JP, Comte F, Renard E, et al. Myocardial perfusion imaging and cardiac events in a cohort of asymptomatic patients with diabetes living in southern France. Diabet Med 2006;23:410-8.
Pedone C, Schinkel AF, Elhendy A, van Domburg RT, Valkema R, Biagini E, et al. Incremental prognostic value of dobutamine-atropine stress 99mTc-tetrofosmin myocardial perfusion imaging for predicting outcome in diabetic patients with limited exercise capacity. Eur J Nucl Med Mol Imaging 2005;32:1057-63.
Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, DIAD Investigators, et al. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: The DIAD study: A randomized controlled trial. JAMA 2009;301:1547-55.
Wiersma JJ, Verberne HJ, ten Holt WL, Radder IM, Dijksman LM, van Eck-Smit BL, et al. Prognostic value of myocardial perfusion scintigraphy in type 2 diabetic patients with mild, stable angina pectoris. J Nucl Cardiol 2009;16:524-32.
Hachamovitch R, Shaw L, Berman DS. Methodological considerations in the assessment of noninvasive testing using outcomes research: Pitfalls and limitations. Prog Cardiovasc Dis 2000;43:215-30.
Diamond GA, Kaul S, Shah PK. Screen testing cardiovascular prevention in asymptomatic diabetic patients. J Am Coll Cardiol 2007;49:1915-7.
Miller TD, Shaw LJ. Risk stratification in diabetic patients: A continuing challenge. J Nucl Cardiol. 2009;16:486-9.
Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al.; American College of Cardiology Foundation Appropriate Use Criteria Task Force; American Society of Nuclear Cardiology; American College of Radiology; American Heart Association; American Society of Echocardiology; Society of Cardiovascular Computed Tomography; Society for Cardiovascular Magnetic Resonance; Society of Nuclear Medicine. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 appropriate use criteria for cardiac radionuclide imaging: A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol 2009;53:2201-29.
Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010;122:e584-e636.
Perrone-Filardi P, Achenbach S, Möhlenkamp S, Reiner Z, Sambuceti G, Schuijf JD, et al. Cardiac computed tomography and myocardial perfusion scintigraphy for risk stratification in asymptomatic individuals without known cardiovascular disease: A position statement of the Working Group on Nuclear Cardiology and Cardiac CT of the European Society of Cardiology. Eur Heart J 2011;32:1986-93.
American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care 2013;36:S11-66.
Di Carli MF, Hachamovitch R. Should we screen for occult coronary artery disease among asymptomatic patients with diabetes? J Am Coll Cardiol 2005;45:50-3.
Beller GA. Noninvasive screening for coronary atherosclerosis and silent ischemia in asymptomatic type 2 diabetic patients: Is it appropriate and cost-effective? J Am Coll Cardiol 2007;49:1918-23.
Mulherin SA, Miller WC. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Ann Intern Med 2002;137:598-602.
Schoenhagen P, Hachamovitch R. Evaluating the clinical impact of cardiovascular imaging: Is a risk-based stratification paradigm relevant? J Am Coll Cardiol 2013;61:185-6.
Disclosures
The authors have indicated that they have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Acampa, W., Cantoni, V., Green, R. et al. Prognostic value of normal stress myocardial perfusion imaging in diabetic patients: A meta-analysis. J. Nucl. Cardiol. 21, 893–902 (2014). https://doi.org/10.1007/s12350-014-9918-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-014-9918-0