Abstract
Introduction
Myocardial perfusion single photon emission computed tomography (MPS) is one of the most widely used diagnostic methods in patients with suspected ischemic heart disease (IHD). Recently, a novel technique based on cadmium-zinc-telluride (CZT) detectors, pinhole collimators, and a stationary gantry was introduced for MPS. The aim of this work was to investigate how patient positioning affects the reconstructed MPS images using this novel technique.
Materials and Methods
Eighteen patients referred for a clinical MPS due to suspected IHD were included in the study. All patients underwent MPS imaging on a GE Discovery NM 530c CZT camera. After image acquisition with the heart positioned in the center of the quality field of view (QFOV), the patients were re-imaged in different positions 5-20 mm off-center. The heart was still positioned within the limits of the QFOV during the off-center scans. The summed stress score and/or the summed rest score (SSS and/or SRS) for the acquisition performed in the center was compared to the same parameter for the acquisitions performed off-center.
Results
There was a statistically significant increase in SSS and/or SRS when imaging was performed with the heart 5-20 mm outside the center of the QFOV compared to optimal positioning (7.7 ± 1.3 vs 6.6 ± 1.3, P = .006). The SSS and/or SRS increased with ≥2 U in 35% (14/40) of the off-center examinations.
Conclusion
It is important to carefully position the patient’s heart within the center of the QFOV when performing MPS with the Discovery NM 530c CZT camera to avoid positioning-related image artifacts that could affect the diagnostic accuracy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease (IHD) is one of the most common causes of death in the western world1 and a major cause of heart failure.2 The pathophysiological basis of IHD is coronary artery disease (CAD) with coronary stenosis in one or more of the coronary arteries obstructing the myocardial blood flow at rest and/or stress. Presence of stress-induced myocardial ischemia due to significant coronary stenosis has been shown to be of fundamental clinical importance for patient prognosis3,4 and need for revascularization therapy.5 Current ESC/AHA (European Society of Cardiology/American Heart Association) guidelines6 strongly recommend having a stress examination performed in patients with suspected stable IHD before a decision for revascularization therapy is taken.
Myocardial perfusion single photon emission computed tomography (MPS) has been the most widely used method for assessment of stress-induced myocardial ischemia during the last decades. Traditionally, conventional MPS images have been acquired using a scintillation gamma camera with a parallel-hole collimator and a rotating gantry resulting in a large field of view in which the heart should be positioned. Recently, a novel gamma camera (Discovery NM 530c, GE Healthcare) based on focused multi-pinhole collimators combined with semi-conductor detector panels made of cadmium-zinc-telluride (CZT) and a stationary gantry was introduced. The CZT camera is associated with several benefits, such as improved spatial resolution, energy resolution, and count sensitivity compared to the conventional scintillation gamma cameras.7 The CZT technique has enabled reduction of administered activity8,9 and shorter scan times10 with preserved image quality and preserved diagnostic information11,12 compared to conventional MPS. The stationary pinhole collimators view the heart from different angles simultaneously. The volume that represents the intersection of the 19 views from each pinhole is named the quality field of view (QFOV) which is a set volume of a sphere with a diameter of 18 cm in which the heart needs to be positioned to be imaged correctly. Suboptimal positioning of the patient in relation to the QFOV could potentially affect the acquired image quality, but the importance of this is not known. Therefore, the aim of this study was to assess the impact of small alterations in patient positioning on the resulting reconstructed MPS images.
Methods
Study Population and Design
Eighteen patients referred for a clinical MPS with suspicion of stable IHD were included in the study. The patients were examined either by a 1-day stress-rest protocol, according to clinical routine, or by a rest examination only. The stress was accomplished either by a bicycle ergometer test or adenosine infusion. All patients were thoroughly positioned and imaged with the left ventricle in the center of QFOV. Thereafter, imaging was repeated in different positions according to Table 1. The investigated positions were as follows: moving the camera 5, 10, or 20 mm out from the patient, lowering the couch 20 mm or moving the couch 20 mm inwards in relation to the stationary gantry. The study was approved by the regional ethics committee and all patients gave their written informed consent to participate in the study.
Image Acquisition and Reconstruction
All patients were examined in a supine position with the arms over the head in a Discovery NM 530c CZT camera (GE Healthcare). Images were acquired 30-60 minutes after an intravenous injection of a perfusion tracer. The patients that were examined according to the 1-day stress-rest protocol received an intravenous injection of 2.5 MBq·kg−1 body weight of 99Tcm-tetrofosmin administered during stress. The activity administered at rest was calculated according to an in-house developed protocol taking time after stress injection (minimum 2 hours) into consideration to meet the American Society of Nuclear Cardiology (ASNC) recommended guidelines. The rest examination was only performed if the stress examination was interpreted as abnormal. The patients that did a rest examination only received an intravenous injection of 4 MBq·kg−1 bodyweight of 99Tcm-tetrofosmin. The initial acquisition was preceded by thorough positioning of the heart within the center of the QFOV utilizing the real-time persistence imaging tool. The positioning tool with automatic contouring to receive the same position of the left ventricle during the rest imaging session as for the stress imaging session was always applied. The acquisition time was 475 seconds for the stress and 285 seconds for the rest images. All acquisitions were ECG-gated and the cardiac cycle was divided into eight time frames. The images were reconstructed with an iterative reconstruction algorithm (stress: MLEM 40 iterations; Green OSL regularization α 0.51, β 0.3 and rest: MLEM 50 iterations; Green OSL regularization α 0.41, β 0.2). A Butterworth filter (frequency 0.37, order 7) was applied for post-processing of the reconstructed axial slices. Correct and similar positioning of the left ventricle within the rest and the stress imaging sessions was confirmed with the Scan QC tool (Figure 1) supplied by the vendor of the camera before image analysis was performed. The repeated image sessions to investigate the impact the effect of positioning were performed in either stress or rest as described above. The reconstructed images were reformatted to the standard cardiac axis format (short axis, vertical long axis, and horizontal long axis).
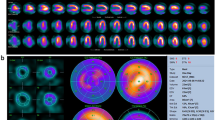
Quality control step (Scan QC) for positioning of the heart in the center of the quality field of view (QFOV). (A) The upper panel shows the raw data projections from the 19 detectors used to control for heart position. The dashed perpendicular lines indicate the center of the QFOV at origo. Below the 19 raw data projections, the reconstruction of the vertical long axis (VLA), horizontal long axis (HLA), and the short-axis projection (SHAX) is shown. On this reconstructed views the left ventricular myocardium is segmented. (B) This is the same case as in A after moving the camera 20 mm out from the patients. The segmentation from A is superimposed on the reconstructed projections from the mis-positioned patient. This clearly shows the misalignment, especially in the VLA and HLA projections
Image Analysis
The image analysis was performed using QGS/QPS (version 4.0, Cedars-Sinai Medical Center, Los Angeles, CA). Left ventricular end-diastolic and end-systolic volumes as well as the left ventricular ejection fraction were derived from QGS based on the ECG-gated data. Summed stress score or summed rest score, as appropriate, was derived from QPS for all examinations.
Basic Camera Performance
The reconstructed uniformity within the QFOV was investigated by placing a point source of 99Tcm at 19 positions uniformly distributed within the QFOV and normalize the reconstructed number of counts per second and unit activity (cps/MBq) to the center position. The center position reconstructed sensitivity, which was used for normalization, was the mean value of five measurements. The count rate performance of the CZT camera was investigated by placing point sources with activities of 0.5-800 MBq of 99Tcm in the center of the QFOV.
Statistical Analysis
Data is presented as the mean ± the standard error of the mean (SEM). A paired Student’s t test was employed to test for differences in left ventricular function and summed score between centered and off-centered patient examinations and to test for differences in SSS between gender. The correlation between difference in SSS center to off-center positioning and body mass index (BMI) was assessed using Pearson’s correlation coefficient. Statistical tests were performed using IBM SPSS Statistics v.20.0 (Chicago, IL, USA). A P value of <.05 was considered to indicate statistical significance.
Results
The 18 patients included (seven females) had a median age of 62.5 years (range 45-82 years). The patients had a median weight of 79 kg (range 46-110 kg) and a median BMI of 26 kg·m−2 (range 19-33 kg·m−2). Fifteen patients underwent the 1-day stress-rest protocol and three patients underwent a rest examination only. A total of 58 acquisitions were performed in the 18 patients. Forty of the acquisitions were performed 5-20 mm off-center according to Table 1. The effect of positioning was investigated at rest in 12 patients and after stress in six patients.
The summed score (SSS or SRS, as appropriate) increased when the left ventricle was positioned 5-20 mm off-center compared to when it was centered [6.6 ± 1.3 (centered) vs 7.7 ± 1.3 (off-centered), P = .006]. The summed score increased with ≥2 U in 35% (14/40) of the off-center images compared to the images acquired in the center of the QFOV. There was no significant difference between genders with regards to change in SSS when comparing center to off-center positioning (0.7 ± 2.1 vs 1.3 ± 3.0; P = .51). Furthermore, there was no significant correlation between change in SSS when comparing center to off-center positioning and BMI (r 2 = 0.04, P = .24).
Figure 2 shows an example where a loss of counts was introduced in different parts of the left ventricle when acquiring images in off-center patient positions. Figure 3 shows an example of a patient where a loss of counts appeared in the inferior left ventricular wall when the camera was moved 20 mm out from the patient resulting in a false perfusion defect that could be interpreted as stress-induced myocardial ischemia. Figure 4 shows an example where an inferolateral count reduction was seen in the image when the camera was moved 20 mm out from the patient. This patient also had a normal cardiac magnetic resonance examination with late gadolinium enhancement performed the same day proving that the count loss seen in off-center patient position at rest did not represent infarction.
Example of the effect of off-center positioning for a stress examination. The polar plots from the left to right show the count distribution at the center of the volume of interest, moving the camera out 20 mm from the patient, moving the couch down 20 mm and moving the couch in 20 mm. The short-axis images are displayed below each respective polar plot. For all off-center positions, a count loss in the inferior wall was seen (white arrows). When moving the couch down 20 mm, a slight count loss was also seen in the anterior wall (black arrow). When moving the couch 20 mm inwards, a count loss was observed in the basal anterolateral wall (dashed black arrow)
Example of a false stress-induced ischemia due to off-center positioning of the heart. The polar plot to the left and right show the count distribution with centered patient positioning at stress and rest, respectively. The short-axis images are displayed below each respective polar plot. The middle polar plot shows the count distribution when the camera has been moved out from the patient 20 mm, causing a significant loss of counts in the inferior left ventricle wall (arrow). If the stress examination shown in the middle would have been accepted and constituted the base for the clinical report, this patient would have been diagnosed with a stress-induced myocardial ischemia in the inferior wall
Example of a false rest perfusion defect introduced by off-center imaging at rest. The left polar plot shows the count distribution at rest with the heart positioned in the center of the quality field of view (QFOV). The right polar plot shows a reduction in counts in the inferolateral wall (arrow). The late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) images to the right (basal, mid, and apical short-axis images and a two-chamber long-axis image) reveal that the patient has no infarction, since there is no signs of hyperenhancement in the left ventricular myocardium due to infarction. White lines indicate the slice position of the three short-axis images. LA, Left atrium; LV, left ventricle; RV, right ventricle
The left ventricular end-diastolic volume and left ventricular end-systolic volume differed slightly when the images for the centered patient position was compared to the off-centered positions (108 ± 7 vs 105 ± 7 mL, P = .006 and 51 ± 5 vs 49 ± 5 mL, P = .006, respectively). Left ventricular ejection fraction did, however, not differ significantly (56% ± 2% vs 57% ± 2%, P = .07).
The basic camera performance measurements showed that the sensitivity is uniform within the camera sensitive volume. The sensitivity, shown as normalized counts per second per activity unit (normalized cps/MBq), in Table 2 was within ±6% (0.94-1.05). The count rate performance is linear up to approximately 800 MBq of 99Tcm put in a point source within the center of the QFOV (Figure 5).
Discussion
The findings in this study indicate that images acquired with the left ventricle positioned 5-20 mm off-center of the QFOV during MPS imaging with the Discovery NM 530c CZT gamma camera can induce regions of count loss in the reconstructed images. The artifacts can be interpreted as false perfusion defects and might affect the diagnosis and consequently the treatment of the patient. Thus, it is of outmost importance to always ensure optimal patient positioning.
The new camera technique with pinhole collimators and CZT-based detectors has an improved sensitivity and spatial resolution which gives a better image quality compared to the conventional gamma camera. The CZT camera allows shorter acquisition times10 and/or a decrease in the activity to administer to the patient9 which will reduce the radiation exposition to staff and to the patient. The stationary gantry is beneficial as it covers the entire myocardium simultaneously which enables, for example, dynamic acquisitions and thereby potentially assessment of absolute myocardial blood flow.13,14 The stationary gantry also introduces challenges as the QFOV has a fixed position in relation to the camera gantry. The fixed QFOV therefore constitute a specific challenge in obese patients where it might be difficult to obtain an acceptable position of the heart within the center of the QFOV due to the body habitus. Fiechter et al15 recently showed that in patients with BMI > 40 kg·m−2 the diagnostic image quality was unacceptable using a CZT camera, why these patients need to be scanned with conventional SPECT. They also showed that repositioning of the patients resulted in improved image quality with the CZT camera supporting the findings in the present study on the importance of patient positioning.
The reason why false perfusion defects, defined as ≥2 U for SSS and/or SRS, appeared in 35% (14/40) with suboptimal patient positioning is not fully known. Given that the count sensitivity within the QFOV was shown to be uniform in the present study, similar to previous reported data13 and that the count rate performance is linear in the range that applies to MPS, it is unlikely that these factors will cause loss of counts when moving the heart within the QFOV. Counts will be lost in parts of the myocardium if the heart is positioned so that parts of the myocardium end up outside the QFOV. However, moving the position of the heart 5-20 mm off-center will not place the heart outside the 18 cm diameter QFOV. The 19 focused pinhole collimators are stationary and all have unique views of the heart. Thus, the attenuation for each pinhole is unique and will change if the position of the heart and the surrounding tissues change in relation to each pinhole. The thorax is a complex region of the body when it comes to attenuation as it includes volumes of air, lung tissue, soft tissue, and bone. These tissues have densities varying from 0.001 g·cm−3 (air) and up to 1.8 g·cm−3 (cortical bone), causing significantly different amounts of attenuation seen in the projections from each pinhole collimator. Thus, a small change in positioning might cause large changes in attenuation from one position to another and this might be part of the explanation to why regions with count losses might appear in the image. If so, attenuation correction of the images could help.16 However, attenuation correction could also introduce defects because of difficulties in co-registration of the CT- and nuclear myocardial perfusion images.17,18
The introduction of count loss due to suboptimal patient positioning may affect patient management, especially if this is the case for the stress examination only as shown in Figure 3. Thus, a difference in patient position between the rest and the stress acquisition might result in a false positive stress-induced ischemia if the stress acquisition is performed off-center and the rest scan is centered in the QFOV. Inversely, an off-centered rest acquisition and a centered stress acquisition could potentially result in a false negative study with regards to stress-induced ischemia.
Limitations
The findings in this study should be interpreted in the light of the following limitations.
(1) The study population is limited and does not allow for separate analysis of gender differences, differences caused by different body habitus, how different off-center directions affect the counts and in what parts of the myocardium. However, a loss of counts was observed for all off-center directions tested (camera out, couch in and couch down). (2) Only a few patients were imaged 5 and 10 mm off-center. Still, some of the patients showed signs of a false count loss compared to the center position which indicates that even small deviations from the camera center could potentially have an impact on the interpretation of the examination. (3) The SSS and SRS values are not based on a CZT normal database using the QPS software. However, since the patient is its own control the magnitude of the differences in SSS or SRS due to differences in patient positions is still valid and does not change the conclusions.
New Knowledge Gained
When using a CZT camera with a stationary gantry based on multi-pinhole collimators such as the Discovery NM 530c, it is of great importance to ensure optimal patient position to avoid false positive myocardial perfusion SPECT studies. Thus, the quality assurance check for patient positioning as recommended by the vendor should be a part of the clinical routine and should be considered when writing clinical reports and interpreting research data acquired on this system.
Conclusions
It is important to carefully position the patient’s heart within the center of the quality field of view when performing myocardial perfusion scintigraphy with the Discovery NM 530c CZT camera. Suboptimal positioning can lead to regions of count loss within the reconstructed image and these artifacts can erroneously be interpreted as perfusion defects due to myocardial infarction or stress-induced ischemia. Thus, a standardized strategy that includes a quality control for optimal patient position is strongly recommended when acquiring MPS images using the Discovery NM 530c CZT camera.
References
Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 2006;367:1747-57.
Gheorghiade M, Bonow RO. Chronic heart failure in the United States: A manifestation of coronary artery disease. Circulation 1998;97:282-9.
Hachamovitch R, Berman DS, Shaw LJ, Kiat H, Cohen I, Cabico JA, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death: Differential stratification for risk of cardiac death and myocardial infarction. Circulation 1998;97:535-43.
Hachamovitch R, Hayes S, Friedman JD, Cohen I, Shaw LJ, Germano G, et al. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: What is the warranty period of a normal scan? J Am Coll Cardiol 2003;41:1329-40.
Hachamovitch R, Rozanski A, Hayes SW, Thomson LE, Germano G, et al. Predicting therapeutic benefit from myocardial revascularization procedures: Are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? J Nucl Cardiol 2006;13:768-78.
Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55.
Imbert L, Poussier S, Franken PR, Songy B, Verger A, Morel O, et al. Compared performance of high-sensitivity cameras dedicated to myocardial perfusion SPECT: A comprehensive analysis of phantom and human images. J Nucl Med 2012;53:1897-903.
Duvall WL, Croft LB, Godiwala T, Ginsberg E, George T, Henzlova MJ. Reduced isotope dose with rapid SPECT MPI imaging: Initial experience with a CZT SPECT camera. J Nucl Cardiol 2010;17:1009-14.
Oddstig J, Hedeer F, Jogi J, Carlsson M, Hindorf C, Engblom H. Reduced administered activity, reduced acquisition time, and preserved image quality for the new CZT camera. J Nucl Cardiol 2013;20:38-44.
Herzog BA, Buechel RR, Katz R, Brueckner M, Husmann L, Burger IA, et al. Nuclear myocardial perfusion imaging with a cadmium-zinc-telluride detector technique: Optimized protocol for scan time reduction. J Nucl Med 2010;51:46-51.
Esteves FP, Raggi P, Folks RD, Keidar Z, Askew JW, Rispler S, et al. Novel solid-state-detector dedicated cardiac camera for fast myocardial perfusion imaging: Multicenter comparison with standard dual detector cameras. J Nucl Cardiol 2009;16:927-34.
Buechel RR, Herzog BA, Husmann L, Burger IA, Pazhenkottil AP, Treyer V, et al. Ultrafast nuclear myocardial perfusion imaging on a new gamma camera with semiconductor detector technique: First clinical validation. Eur J Nucl Med Mol Imaging 2010;37:773-8.
Bocher M, Blevis IM, Tsukerman L, Shrem Y, Kovalski G, Volokh L. A fast cardiac gamma camera with dynamic SPECT capabilities: Design, system validation and future potential. Eur J Nucl Med Mol Imaging 2010;37:1887-902.
Ben-Haim S, Murthy VL, Breault C, Allie R, Sitek A, Roth N, et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: A feasibility study. J Nucl Med 2013;54:873-9.
Fiechter M, Gebhard C, Fuchs TA, Ghadri JR, Stehli J, Kazakauskaite E, et al. Cadmium-zinc-telluride myocardial perfusion imaging in obese patients. J Nucl Med 2012;53:1401-6.
Herzog BA, Buechel RR, Husmann L, Pazhenkottil AP, Burger IA, Wolfrum M, et al. Validation of CT attenuation correction for high-speed myocardial perfusion imaging using a novel cadmium-zinc-telluride detector technique. J Nucl Med 2010;51:1539-44.
Rajaram M, Tahari AK, Lee AH, Lodge MA, Tsui B, Nekolla S, et al. Cardiac PET/CT misregistration causes significant changes in estimated myocardial blood flow. J Nucl Med 2013;54:50-4.
Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: A definitive analysis of causes, consequences, and corrections. J Nucl Med 2007;48:1112-21.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hindorf, C., Oddstig, J., Hedeer, F. et al. Importance of correct patient positioning in myocardial perfusion SPECT when using a CZT camera. J. Nucl. Cardiol. 21, 695–702 (2014). https://doi.org/10.1007/s12350-014-9897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-014-9897-1