Abstract
Background
Regadenoson is a selective A2A receptor agonist that is used for vasodilator stress myocardial perfusion imaging (MPI). Since the drug is partially metabolized by the liver, its safety in patients with end-stage liver disease (ESLD) needs to be determined.
Methods and Results
We studied 168 consecutive patients with ESLD who had regadenoson stress gated single photon emission computed tomography MPI between January 2008 and March 2010 before planned orthotopic liver transplantation and compared the hemodynamic responses and safety profile to 168 control patients. There were 72 women (43%) in ESLD versus 87 (52%) in the control group (P = .1). The patients with ESLD were younger (58 ± 7 vs 62 ± 12 years, P = .0002), but more likely to be Caucasians (P = .002). The MPI images were normal in 161 patients (96%) in each group. The left ventricular ejection fraction was 72 ± 10% in ESLD and 66 ± 11% in the control patients (P = .0001). The heart rate increase in response to regadenoson was lower in patients with ESLD than in the control group (16 ± 11 vs 23 ± 16 bpm, P = .0001), but the changes in systolic and diastolic blood pressures were similar (−9 ± 12 vs −11 ± 14 mmHg and −6 ± 8 vs −7 ± 10 mmHg, respectively, P = NS). There were no deaths or medication-related adverse events that required hospitalization in either group within 30 days of the study.
Conclusion
This is the first study to document the tolerability and safety profile of regadenoson in patients with ESLD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myocardial perfusion imaging (MPI) is widely used for the detection of coronary artery disease (CAD), risk stratification, and pre-operative evaluation. Approximately half of the studies are performed with a vasodilator stress agent.1 This is particularly true in patients with end-stage liver disease (ESLD) undergoing liver transplant evaluation as these patients have limited exercise capacity. Orthotopic liver transplantation is the last hope of treatment in patients with ESLD. The number of these procedures continues to increase and the results continue to improve.2 The procedure is costly and the resources are limited and hence pre-operative screening to identify high-risk patients is often done.3 Regadenoson, unlike adenosine, is given as a fixed dose. Another relevant pharmacokinetic property of regadenoson is that it is not metabolized and predominately excreted by the kidney (55-60%), while the remaining 40% is excreted by the liver through the bile, also unchanged.4 There are no safety data of the drug in patients with ESLD. The purpose of this study is to examine the hemodynamic changes, safety, and tolerability of regadenoson in patients with ESLD.
Methods and Materials
Patient Selection
We identified from our database, 168 consecutive patients with ESLD who had regadenoson stress gated MPI for clinical indications from 2008 to 2010. The MPI was done before anticipated orthotopic liver transplantation. Since the majority of the patients had normal perfusion images,3,5 we selected a control group of patients with no liver disease (N = 168), from the same time period, and with comparable MPI results. The patients’ records were reviewed for demographics, co-morbidities, medications at the time of the study, electrocardiographic findings, changes in heart rate (HR) and blood pressure (BP), serious side effects based on self-reporting, and death or procedure-related hospitalization within 30 days.
SPECT Imaging
Gated single photon emission computed tomography (SPECT) MPI was obtained after stress (0.4 mg intravenous bolus administration of regadenoson) using technetium-99m sestamibi according to ASNC guidelines.6 The SPECT images were acquired 1 hour after tracer injection with an elliptical 180° orbit of 32 projections, using a dual-head detector gamma camera with a 15% window centered on the 140 keV gamma peak. The gating was done with 8-16 frames per RR cycle and image interpretation was done without attenuation or scatter correction. The left ventricular (LV) ejection fraction (EF), end-diastolic and end-systolic volumes, and LV mass were measured from the rest gated images (as this study was usually done with a larger dose of 30-45 vs 10-15 mCi for the stress study), based on the method described by Germano et al7 and as previously described.8 The presence of a perfusion defect (reversible, fixed, or a combination) was quantitatively determined using a well-validated program (MDSPECT, Ann Arbor, MI, USA). The perfusion defect size was expressed as % of LV myocardium as total (scar and ischemia), fixed (scar), or reversible (ischemia).9-11
Statistical Analysis
The HR and Bp were monitored for 5 minutes, unless side effects warranted more prolonged monitoring. The changes in HR, systolic, and diastolic BP were expressed as absolute (peak-resting) and percent changes (peak-resting/resting × 100), and maximum/minimum values were used. Data were presented as percentages for categorical variables, and as means ± standard deviation for continuous variables. The patients with ESLD and control cohort were compared using Student’s t test or Chi-square test as appropriate. All P values were two-tailed. A P value <.05 was considered statistically significant with 95% confidence interval. The protocol was approved by our Institutional Review Board.
Results
The baseline characteristics of the patients are listed in Table 1. The ESLD group was younger than the control group and included more Caucasians, and fewer patients with hypertension or peripheral vascular disease. The most common causes fro liver transplant were: hepatitis C (40%), non-alcoholic steatohepatitis (18%), alcohol (12%), crypotogenic (11%), primary biliary cirrhosis (6%), and alpha-1 antitrypsin deficiency (4%). Both the groups had a similar proportion of patients with diabetes mellitus, known CAD (abnormal coronary angiogram, prior myocardial infarction, or prior coronary revascularization) and smoking.
The results of gated-SPECT MPI are listed in Table 2. Patients with ESLD had similar LV volumes, lower LV mass, and higher LVEF than patients with no liver disease. The percentages of patients with abnormal perfusion pattern and the perfusion defect size were similar in the two groups.
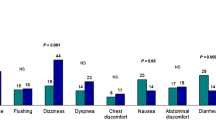
Regadenoson was associated with a less increase in HR in patients with ESLD but comparable decrease in systolic and diastolic BP than the control group despite the ESLD group having lower baseline BP (Table 3; Figure 1). The percentage of patients with severe decrease in systolic BP (>−30 mmHg) was low and not statistically different in both the groups (Table 3). Regadenoson was associated with small increase in systolic BP in 14% and 13% of the patients with mean increase of 7 ± 5 vs 8 ± 5 mmHg in the ESLD and control groups, respectively (P = NS). The ST changes consistent with ischemia were unusual in either group. Very few patients had symptomatic hypotension in either group (P = NS).
Hemodynamic changes associated with regadenoson. Panel A represent the absolute changes with the standard deviations, while panel B represent the % changes with standard deviations. *P < .001. ADVANCE MPI (adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: phase III trial), bmp, beats per minute; DBP, diastolic blood pressure; ESLD, end-stage liver disease; SBP, systolic blood pressure
There were very few reported symptoms in both the groups, and no serious events leadings to hospitalization or death within 30 days of the study (P = NS for all variables) (Table 3). Aminophylline was used in 2 patients (one in each group) because of nausea and vomiting.
Discussion
This is the first study that documents the safety of regadenoson in patients with ESLD. Further, the changes in BP were comparable to those in the control group but the HR response was less. Blunted HR response to adenosine and regadenoson has been described by our group in patients with diabetes, metabolic syndrome, and end-stage renal disease.12,13
Most patients undergoing stress MPI for liver transplant evaluation have normal myocardial perfusion, which led some to question the benefit of such studies.5,14 Also, the many competing factors that affect eligibility for transplantation may further limit the value of the screening MPI.14 Yet, the prevalence of CAD in patients with ESLD varies from 3% to 16%,15,16 and has been shown to be associated with increased peri-operative mortality.17,18 The current guidelines recommend screening in patients with one or more risk factor for CAD.2 A negative SPECT MPI3,19 study or dobutamine stress echocardiogram19 are strongly predictive of very low cardiovascular events in the peri-operative period. At our institution, stress MPI is performed as part of the liver transplant work-up. The image quality is excellent, especially because of decreased liver uptake, less background noise, and subsequently higher target-to-background ratio20 (Figure 2). Most patients with ESLD also have ascites and splenomegaly clearly visible on the rotating images as was initially reported earlier by our group20 (Figure 2).
Raw cine image and SPECT MPI of patients with liver cirrhosis. Raw cine image showing lack of tracer uptake in the cirrhotic liver (arrow, panel A), and an enlarged spleen (arrow, panel B) with ascites (arrowhead, panel C) of another patient with liver cirrhosis. Panel D is a SPECT myocardial perfusion images in short, vertical, and horizontal long-axis projections showing normal perfusion, with an excellent image quality and target to background contrast ratio. The 3-dimentional images (panel E) show normal left ventricular size and function and the time activity curve (panel F) shows normal ejection fraction
There were few side effects reported in both the groups, consistent with our clinical experience and as previously published in patients with end-stage renal disease.21 The discrepancy between symptoms reported in clinical daily practice21 and in pivotal phase III trials22,23 is not uncommon, and was previously seen with adenosine.1
Regadenoson is a selective A2A receptor agonist that is administered intravenously as a fixed bolus of 0.4 mg with no weight-adjustment.4 The weight-independent dosing is important, especially in patients with ESLD who have ascites (66% of cases3), as the extra fluid overestimates the real weight, and often leads to larger doses of adenosine or dipyridamole with potentially more side effects. On the other hand, unlike adenosine, regadenoson is liver cleared (40% of elimination route) with renal clearance accounting for the remainder.24 Regadenoson binds to the A2A receptor on the coronary arterial bed and promotes peak hyperemia within 30 seconds of the bolus injection, with a mean duration of action of 2.3 minutes.25 It remains in the circulation at low concentrations that are clinically irrelevant, even in patients with moderate chronic kidney disease.26 Although we did not measure the serum concentration of regadenoson or its clearance in patients with ESLD, the study clearly shows similar hemodynamic responses and tolerability as in patients with normal liver function. We made similar observations in patients with end-stage renal disease.21
The hemodynamic changes were comparable or even less dramatic to those in the control group and to the entire cohort of patients who were enrolled in the adenosine versus regadenoson comparative evaluation in MPI, phase III trial (ADVANCE MPI),22,23 P = NS (Figure 1). There were no serious side effects such as bronchospasm, high degree atrio-ventricular block, severe ischemia, and there were no long-term complications up to 30 days of the study.
According to unpublished data (data on file of CV Therapeutics, Paolo Alto, CA, USA), regadenoson non-renal clearance remains constant and greatly exceeds renal clearance in subjects with severe renal impairment. Accordingly, one might postulate the reverse happening in patients with ESLD, with a significant increase in renal clearance. However, this hypothesis needs to be verified.
The patients included in this study may not require screening according to ACC/AHA appropriateness criteria27; however, the main purpose of the study is to report on the safety of regadenoson in such patients. While other imaging modalities have been used for risk assessment, the thrust of this study is on safety rather than the outcome. It is quite possible that the penetration of the criteria to non-cardiology community is lagging but that could very well be time-related. Furthermore, the retrospective nature of the study and the fact it is from a single tertiary center are obvious limitations. Also, we had no measures of liver function studies after administration of regadenoson; however, by selection criteria, all patients had advanced liver disease. While the sample size was large enough to support the conclusions, future studies in large sample size should include some measures of randomization, broader spectrum of patients, and measurement of liver function studies before and after testing.
In conclusion, regadenonson administration during stress testing is safe in patients with ESLD.
References
Iskandrian AE, Garcia EV. Nuclear cardiac imaging: Principles and applications. In: Zoghbi GJ, Iskandrian AE, editors. Pharmacological stress testing. 4th ed. New York, NY: Oxford University Press; 2008. p. 293-315.
Murray KF, Carithers RL Jr. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 2005;41:1407-32.
Zoghbi GJ, Patel AD, Ershadi RE, Heo J, Bynon JS, Iskandrian AE. Usefulness of preoperative stress perfusion imaging in predicting prognosis after liver transplantation. Am J Cardiol 2003;92:1066-71.
Al Jaroudi W, Iskandrian AE. Regadenoson: A new myocardial stress agent. J Am Coll Cardiol 2009;54:1123-30.
Kryzhanovski VA, Beller GA. Usefulness of preoperative noninvasive radionuclide testing for detecting coronary artery disease in candidates for liver transplantation. Am J Cardiol 1997;79:986-8.
Hansen CL, Goldstein RA, Akinboboye OO, Berman DS, Botvinick EH, Churchwell KB, et al. Myocardial perfusion and function: Single photon emission computed tomography. J Nucl Cardiol 2007;14:e39-60.
Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995;36:2138-47.
Aljaroudi WA, Hage FG, Hermann D, Doppalapudi H, Venkataraman R, Heo J, et al. Relation of left-ventricular dyssynchrony by phase analysis of gated SPECT images and cardiovascular events in patients with implantable cardiac defibrillators. J Nucl Cardiol 2010;17:398-404.
Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: The Michigan method for quantitative nuclear cardiology. J Nucl Cardiol 2007;14:455-65.
Iskandrian AE, Garcia EV, Faber T, Mahmarian JJ. Automated assessment of serial SPECT myocardial perfusion images. J Nucl Cardiol 2009;16:6-9.
Hage FG, Venkataraman R, Aljaroudi W, Bravo PE, McLarry J, Faulkner M, et al. The impact of viability assessment using myocardial perfusion imaging on patient management and outcome. J Nucl Cardiol 2010;17:378-89.
Hage FG, Heo J, Franks B, Belardinelli L, Blackburn B, Wang W, et al. Differences in heart rate response to adenosine and regadenoson in patients with and without diabetes mellitus. Am Heart J 2009;157:771-6.
Hage FG, Perry G, Heo J, Iskandrian AE. Blunting of the heart rate response to adenosine and regadenoson in relation to hyperglycemia and the metabolic syndrome. Am J Cardiol 2010;105:839-43.
Bradley SM, Soine LA, Caldwell JH, Goldberg SL. Screening stress myocardial perfusion imaging and eligibility for liver transplantation. Am J Cardiol 2010;105:1010-3.
Carey WD, Dumot JA, Pimentel RR, Barnes DS, Hobbs RE, Henderson JM, et al. The prevalence of coronary artery disease in liver transplant candidates over age 50. Transplantation 1995;59:859-64.
Plotkin JS, Johnson LB, Rustgi V, Kuo PC. Coronary artery disease and liver transplantation: The state of the art. Liver Transpl 2000;4:S53-6.
Plotkin JS, Scott VL, Pinna A, Dobsch BP, De Wolf AM, Kang Y. Morbidity and mortality in patients with coronary artery disease undergoing orthotopic liver transplantation. Liver Transpl Surg 1996;2:426-30.
Safadi A, Homsi M, Maskoun W, Lane KA, Singh I, Sawada SG, et al. Perioperative risk predictors of cardiac outcomes in patients undergoing liver transplantation surgery. Circulation 2009;120:1189-94.
Donovan CL, Marcovitz PA, Punch JD, Bach DS, Brown KA, Lucey MR, et al. Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation 1996;61:1180-8.
Tallaj JA, Kovar D, Iskandrian AE. The use of technetium-99m sestamibi in a patient with liver cirrhosis. J Nucl Cardiol 2000;7:722-3.
Aljaroudi W, Hermann D, Hage F, Heo J, Iskandrian AE. Safety of regadenoson in patients with end-stage renal disease. Am J Cardiol 2010;105:133-5.
Iskandrian AE, Bateman TM, Belardinelli L, Blackburn B, Cerqueira MD, Hendel RC, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: Results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol 2007;14:645-58.
Cerqueira MD, Nguyen P, Staehr P, Underwood SR, Iskandrian AE. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc Imaging 2008;1:307-16.
Gordi T, Frohna P, Sun HL, Wolff A, Belardinelli L, Lieu H. A population pharmacokinetic/pharmacodynamic analysis of regadenoson, an adenosine A2A-receptor agonist, in healthy male volunteers. Clin Pharmacokinet 2006;45:1201-12.
Lieu HD, Shryock JC, von Mering GO, Gordi T, Blackburn B, Olmsted AW, et al. Regadenoson, a selective A2A adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J Nucl Cardiol 2007;14:514-20.
Gordi T, Blackburn B, Lieu H. Regadenoson pharmacokinetics and tolerability in subjects with impaired renal function. J Clin Pharmacol 2007;47:825-33.
Hendel RC, Berman DS, Di Carli MF, Heidenreich PA, Henkin RE, Pellikka PA, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol 2009;53:2201-29.
Disclosures
Dr Ami E. Iskandrian is an investigator and a consultant to Gilead Sciences, Inc. The other authors have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
AlJaroudi, W., Iqbal, F., Koneru, J. et al. Safety of regadenoson in patients with end-stage liver disease. J. Nucl. Cardiol. 18, 90–95 (2011). https://doi.org/10.1007/s12350-010-9288-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-010-9288-1