Abstract
Purpose
To assess the efficacy and safety of intravitreal dexamethasone implant injection in the management of sarcoidosis-related uveitis.
Methods
A retrospective analysis was performed of the efficacy and safety of intravitreal dexamethasone implant injection for indications such as intractable vitritis, vasculitis, or cystoid macular edema.
Results
This study comprised 20 patients with sarcoidosis-related uveitis. A single injection was performed in 13 eyes (65%) and 35% required more than 2 injections during the follow-up period [median 16.5 months (range 6–32)]. The best-corrected visual acuity showed significant improvement at 1 month (P = 0.004) and 3 months (P = 0.001), but there was no significance at 6 months after implant injection (P = 0.186). One month after treatment, the central macular thickness decreased to 278.95 ± 52.20 μm (P = 0.023). It further decreased to 274.70 ± 55.88 μm at 3 months (P = 0.027), but there was no significance at 6 months (280.65 ± 64.48 μm, P = 0.074).The anterior chamber cell grade (P = 0.003) and vitreous haze (P = 0.001) were significantly decreased for up to 6 months after a single implant injection. The most common ocular complication was worsening of cataracts during the first 6 months.
Conclusion
Intravitreal dexamethasone implant injection is efficacious in reducing anterior chamber inflammation, vitreous haze, and cystoid macular edema in patients with sarcoidosis-related uveitis. Considering that sarcoidosis shows a chronic course of disease in a significant proportion of cases, intravitreal dexamethasone implant injection is a possible option to relieve intraocular inflammation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sarcoidosis is a multisystemic chronic inflammatory disorder of unknown origin, characterized by non-caseating granuloma formation in the involved organs [1,2,3]. Ocular involvement in sarcoidosis ranges from 30 to 60% of cases, and uveitis is the initial presentation of sarcoidosis in 11–30% of cases [4,5,6,7].
Sarcoidosis-related uveitis tends to cause either acute self-limiting anterior uveitis or insidious chronic intraocular inflammation with ocular complications and frequent relapses [4]. Ocular sarcoidosis can cause sight-threatening and recurrent uveitis in both eyes. Therefore, it is important to make an accurate diagnosis and adopt an appropriate treatment strategy for these patients. A stepwise approach is recommended, and corticosteroids are the cornerstone of treatment [8]. The use of immunosuppressants and biologic agents has been increasing in refractory cases or those requiring prolonged treatment with intolerable adverse effects in recent years [9,10,11].
The intravitreal dexamethasone implant (Ozurdex, Allergan, Inc., Irvine, CA, USA) is a biodegradable, sustained-release, dexamethasone-containing implant that has been FDA approved for intermediate and posterior noninfectious uveitis [8]. The efficacy of intravitreal dexamethasone implants in sarcoidosis-related uveitis has been reported in small case series [12, 13]. The efficacy was shown by improvement in visual acuity and vitreous haze and by a decrease in retinal thickness. In a multicenter study Zarranz-Ventura et al. evaluated the use of the implant in sarcoidosis-related uveitis in 82 eyes with noninfectious uveitis [13]. This study demonstrated improvement in vitreous haze and macular edema, but it included only six patients with ocular sarcoidosis. There are limited data regarding the efficacy and safety of the intravitreal dexamethasone implant in ocular sarcoidosis with the optimal length of follow-up. Also, the re-implantation rate or indications in ocular sarcoidosis are not well known.
In this study, we described the efficacy and safety of the intravitreal dexamethasone implant in treatment of ocular sarcoidosis in our cohort.
Methods
Study Population
We retrospectively reviewed the medical records of patients with ocular sarcoidosis (20 eyes). Patients who had received their first injection with an intravitreal dexamethasone implant were included. If both eyes were eligible for the study, the eye that was injected earlier was included in the study. All participants were recruited between November 2016 and September 2018 at Seoul St. Mary’s Hospital in Korea. This study adhered to the tenets of the Declaration of Helsinki, and all protocols were approved by the Institutional Review Board of The Catholic University of Korea. The requirement for informed patient consent was waived because of the retrospective nature of the study.
Exclusion criteria were as follows: any active intraocular disease or suspicion of infection; any history of significant intraocular pressure (IOP) elevation in response to corticosteroid treatment; presence of concomitant retinal diseases including glaucoma, diabetic retinopathy, age-related macular degeneration, or retinal vein occlusion; uveitis unresponsive to prior corticosteroid treatment; high myopia with refractive error over ± 6 diopters (as spherical equivalent); or any uncontrolled systemic disease. We also excluded patients whose uveitis was unlikely to be related to sarcoidosis and those with uveitis with atypical features.
Ophthalmic and Systemic Evaluations
Demographic information and comprehensive medical and ophthalmologic history were recorded at the initial visit. All subjects underwent ocular examinations, including slit-lamp biomicroscopy, dilated fundus examination, best-corrected visual acuity (BCVA) evaluation [logarithm of the minimum angle of resolution scale (logMAR)], and non-contact pneumatic tonometry. Classification and grading of uveitis were performed in accordance with the SUN criteria [14]. Anterior chamber cells were graded from 0 to 4 using a semiquantitative scoring system. Vitreous haze was measured using a standardized photographic scale ranging from 0 to 4 with 0 = no inflammation; + 0.5 = trace inflammation (slight blurring of the optic disc margins and/or loss of the nerve fiber layer reflex); + 1 = mild blurring of the retinal vessels and optic nerve; + 1.5 = optic nerve head and posterior retinal view obscuration > + 1 but < +2; + 2 = moderate blurring of the optic nerve head; + 3 = marked blurring of the optic nerve head; + 4 = optic nerve head not visible [15].
Thorough laboratory tests were performed for the uveitis work-up including complete blood count, kidney and liver function tests, erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies, rheumatoid factor, HLA-B27 and HLA-B51 determination, interferon gamma-releasing assay, angiotensin-converting enzyme, syphilis rapid plasma regain test, chest radiographs, and sacroiliac joint radiographs. In cases of intraocular inflammation that provoked a suspicion of viral etiology, anterior chamber paracentesis and aqueous analysis for certain viruses (varicella-zoster, cytomegalovirus, and herpes simplex I and II) were performed. Patients were referred to the pulmonologist in our center, and a thorough evaluation for systemic sarcoidosis was performed. Sarcoidosis-related uveitis was diagnosed in accordance with the diagnostic criteria of the International Workshop on Ocular Sarcoidosis (IWOS) [3]. Various clinical signs, laboratory investigations, and biopsy results were used to define four diagnostic categories of sarcoidosis-related uveitis: definite, presumed, probable, and possible ocular sarcoidosis.
The intravitreal dexamethasone implant was injected through the pars plana into the vitreous cavity. All injection procedures were done by an experienced vitreoretinal surgeon (M.K.) in the operating room.
Optical Coherence Tomography Image Acquisition
OCT imaging was performed with a swept source (SS)-OCT device (DRI Triton, Topcon, Tokyo, Japan). A six-line radial pattern scan (1024 A-scans) centered on the fovea was obtained from each eye. Retinal thickness was obtained with the automatic built-in software associated with the SS-OCT device. Thickness maps were created in accordance with the conventional ETDRS grid with nine independent sectors, using central macular thickness. If there was any suspicion of segmentation error, segmentation lines were manually corrected by a vitreoretinal specialist.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows version 23.0 (SPSS Inc., Chicago, IL, USA). An exploratory analysis was conducted for all variables. Categorical data are expressed as absolute numbers and continuous data as mean ± SD (95% confidence interval). The paired t test and Wilcoxon signed-rank test were used to compare pre- and post-treatment BCVA, central macular thickness, anterior chamber cell grade, and vitreous haze grade. P values < 0.05 were considered statistically significant.
Results
Demographic and Clinical Characteristics
A total of 20 patients were included in this study. Table 1 summarizes their demographic and clinical characteristics. The mean age of the patients was 61.3 ± 10.1 years, and 16 of the 20 patients (80.0%) were female. The mean refractive error was − 0.34 ± 1.80 diopters. Thirteen of the 20 patients (65.0%) showed bilateral involvement of uveitis. Among these, four patients (30.8%) received an intravitreal dexamethasone implant in the fellow eye. The most common anatomical location of uveitis was posterior uveitis (45.0%), followed by panuveitis (40.0%) and intermediate uveitis (15.0%). Of the 20 eyes, 15 were phakic and 5 were pseudophakic.
Sixty percent of the participants showed recurrent episodes of uveitis separated by inactive periods without any treatment over 3 months. Of the remaining patients, 35.0% showed a chronic disease course, and 5.0% showed an acute episode of uveitis. Of the 20 patients in the study, 12 (60.0%) were confirmed as having systemic sarcoidosis. Of the 20 patients, the mean serum ACE level was 66.53 ± 32.73. The most common form of systemic manifestation was pulmonary sarcoidosis, followed by multiorgan and cardiac sarcoidosis.
Efficacy Analysis
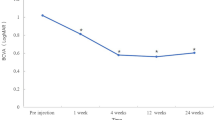
Table 2 shows BCVA, central macular thickness, intraocular inflammation grade, and intraocular pressure according to follow-up time after intravitreal dexamethasone implant injection. The mean BCVA at baseline was 0.44 ± 0.22 (logMAR). After intravitreal dexamethasone implant injection, the mean BCVA was 0.26 ± 0.20 at 1 month, 0.23 ± 0.15 at 3 months, and 0.31 ± 0.19 at 6 months after injection. The mean BCVA showed significant improvement at 1 month (P = 0.004) and 3 months (P = 0.001) after treatment, but there was no significance at 6 months after treatment (P = 0.186).
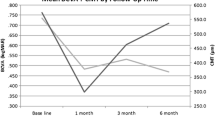
The mean central macular thickness at baseline was 308.90 ± 59.26 μm. After intravitreal dexamethasone implant injection, the mean central macular thickness decreased to 278.95 ± 52.20 μm (P = 0.023) at 1 month. The central macular thickness further decreased to 274.70 ± 55.88 μm at 3 months and 280.65 ± 64.48 μm at 6 months after treatment (P = 0.027 and P = 0.074, respectively). Figure 1 depicts improvement in BCVA and a decrease of central macular thickness after a single intravitreal dexamethasone implant injection. Anterior chamber cell grade (P = 0.003) and vitreous haze grade (P = 0.001) were significantly decreased after intravitreal dexamethasone implant injection (Fig. 2).
Among the study population, a single injection was performed in 13 eyes (65%). Two eyes (10%) required two injections, two eyes (10%) required three injections, and three eyes (15%) required four injections during the follow-up period [median 16.5 months (range 6–32)]. The probability of a second injection at 6 months was 15%, increasing to 25% at 12 months and 35% at 24 months, with a median time to second injection of 7 months.
At the time of the first implant injection, 70% (14 of 20 patients) were on systemic medications. Among these, six patients (12%) were on a combination regimen of corticosteroid and single immunosuppressant, five patients (10%) were on a single immunosuppressant, two patients (10%) were on a corticosteroid only, and one patient (5%) was on dual immunosuppressants: cyclosporine and mycophenolate mofetil. Three months after implant injection, the proportion of patients using systemic medications was reduced to 40%. Six patients discontinued oral corticosteroids, and two patients were able to achieve dose reduction of immunosuppressant.
Indications of Intravitreal Dexamethasone Implant
The most common indication for intravitreal dexamethasone implant was intractable vitritis (10 of 20 patients). Six patients used the implant to improve cystoid macular edema, and four patients used the implant to control vasculitis. Systemic corticosteroids and immunosuppressants were not tolerated in two patients because of adverse effects. Among the seven eyes that required repeated injections, the indications for re-treatment were vitritis (4 eyes) and recurrent cystoid macular edema (3 eyes).
Safety Analysis
The most common ocular complication was worsening of cataracts during the first 6 months (3 cases of the 15 phakic eyes, 20%). IOP elevation was observed in two cases, and aggravation of the epiretinal membrane was observed in two cases. The mean IOP was 13.00 ± 2.90 at baseline. After treatment, the mean IOP was 13.25 ± 1.97 mmHg at 1 month, 11.50 ± 1.88 mmHg at 3 months, and 12.25 ± 2.10 mmHg at 6 months. The mean IOP showed no difference after a single intravitreal dexamethasone implant injection (P = 0.050), but two patients showed IOP elevation over 21 mmHg at 1 month after treatment.
Discussion
In the current study, we investigated the efficacy and safety of the intravitreal dexamethasone implant in patients with sarcoid uveitis. We found that, after implant injection, anterior chamber inflammation and vitreous haze improved within 1 month, and they remained improved at 6 months after treatment. Best-corrected visual acuity and central macular thickness also improved within 1 month, but the improvement lasted only 3 months after treatment. Thirty-five percent of patients required more than two injections during follow-up.
Corticosteroids have become the cornerstone of treatment in non-infectious uveitis. However, treatment with systemic corticosteroids is often accompanied by adverse effects such as hyperglycemia, hypertension, fluid retention, gastrointestinal ulceration, osteoporosis, higher susceptibility to infections, and psychologic problems [16, 17]. In this aspect, localized corticosteroid treatment can achieve adequate intraocular concentration without systemic adverse effects. In our study population, the proportion of patients using systemic medications was reduced from 70 to 40% at 3 months after implant injection.
The use of intravitreal dexamethasone implants has been shown in many studies for treatment of retinal vein occlusion, diabetic macular edema, and noninfectious uveitis [13, 18,19,20,21,22,23]. The Ozurdex HURON trial was the first clinical trial to evaluate the efficacy of intravitreal dexamethasone implant in treatment of noninfectious intermediate or posterior uveitis [15]. After that, there were reports on the efficacy of the intravitreal dexamethasone implant in sarcoidosis-related uveitis. Zarranz-Ventura et al. reported that the dexamethasone implant significantly improved visual acuity, vitreous haze, and macular edema, but the study included only six patients with ocular sarcoidosis [13]. Myung et al. reported one case in which a single dexamethasone implant was effective in control of papillitis and retinal vasculitis until 6 months after injection [12]. To the best of our knowledge, ours is the largest case series of ocular sarcoidosis treated with intravitreal dexamethasone implant.
However, despite these favorable results, clinicians should keep in mind the potential for ocular adverse events such as worsening of cataract or IOP elevation. Malcles et al. in the SAFODEX study [24] reported that 28.5% of patients developed ocular hypertension after intravitreal dexamethasone implant. However, in this report, the IOP elevation was transient in most cases and was well controlled with topical eye drops in 97% of cases. The proportion of eyes with IOP elevation in our study was smaller than in the SAFODEX study [24]. This could be explained by our exclusion of patients with any history of significant IOP elevation in response to corticosteroid treatment. The risk factors associated with IOP elevation after intravitreal corticosteroid injection were young age, male sex, preexisting glaucoma, higher baseline IOP, uveitis, higher steroid dosage, and fluocinolone implant [25].
The current study had some limitations. Due to the nature of the retrospective design, this study may have an intrinsic drawback with respect to bias; the treatment strategy or follow-up schedule was not well stratified. Furthermore, the indications for starting the intravitreal dexamethasone implant treatment and re-treatment were not pre-defined. Second, we analyzed a relatively small number of patients who experienced their first intravitreal injection. Thus, our cohort may not be representative of all patients with sarcoidosis-related uveitis in a real-world clinical setting. Third, the total follow-up period was relatively short. Despite these limitations, the strengths of this study include a relatively large study population with characterization of the ocular sarcoidosis phenotype according to IWOS [3] criteria. To confirm the efficacy and assess the disease course, a prospective, large-scale, longitudinal study should be conducted. These limitations should be addressed in future studies.
In conclusion, this study suggests that treatment with an intravitreal dexamethasone implant for sarcoidosis-related uveitis results in improved visual acuity and a decreased intraocular inflammation grade with a low risk of systemic adverse effects. Considering that a significant proportion of patients with sarcoidosis have a chronic course of disease, intravitreal dexamethasone implant injection is one possible option to relieve intraocular inflammation. However, due to potential ocular adverse effects including ocular hypertension, all patients receiving the implant should be warned and their IOP monitored following implantation. Future large-scale studies are necessary to confirm the results of the current study.
References
Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–34. https://doi.org/10.1056/nejm199704243361706.
Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee. 1999. Am J Respir Crit Care Med 160: 736–755. https://doi.org/10.1164/ajrccm.160.2.ats4-99.
Herbort CP, Rao NA, Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm. 2009;17:160–9. https://doi.org/10.1080/09273940902818861.
Jamilloux Y, Kodjikian L, Broussolle C, Seve P. Sarcoidosis and uveitis. Autoimmun Rev. 2014;13:840–9. https://doi.org/10.1016/j.autrev.2014.04.001.
Ma SP, Rogers SL, Hall AJ, Hodgson L, Brennan J, Stawell RJ, Lim LL. Sarcoidosis-related uveitis: clinical presentation, disease course, and rates of systemic disease progression after uveitis diagnosis. Am J Ophthalmol. 2019;198:30–6. https://doi.org/10.1016/j.ajo.2018.09.013.
Rothova A, Alberts C, Glasius E, Kijlstra A, Buitenhuis HJ, Breebaart AC. Risk factors for ocular sarcoidosis. Doc Ophthalmol. 1989;72:287–96.
Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H Jr, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, McLennan G, Moller DR, Newman LS, Rabin DL, Rose C, Rybicki B, Weinberger SE, Terrin ML, Knatterud GL, Cherniak R. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–9. https://doi.org/10.1164/ajrccm.164.10.2104046.
Matsou A, Tsaousis KT. Management of chronic ocular sarcoidosis: challenges and solutions. Clin Ophthalmol. 2018;12:519–32. https://doi.org/10.2147/opth.s128949.
Riancho-Zarrabeitia L, Calvo-Rio V, Blanco R, Mesquida M, Adan AM, Herreras JM, Aparicio A, Peiteado-Lopez D, Cordero-Coma M, Garcia Serrano JL, Ortego-Centeno N, Maiz O, Blanco A, Sanchez-Burson J, Gonzalez-Suarez S, Fonollosa A, Santos-Gomez M, Gonzalez-Vela C, Loricera J, Pina T, Gonzalez-Gay MA. Anti-TNF-alpha therapy in refractory uveitis associated with sarcoidosis: multicenter study of 17 patients. Semin Arthritis Rheum. 2015;45:361–8. https://doi.org/10.1016/j.semarthrit.2015.05.010.
Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–65. https://doi.org/10.1056/NEJMra071714.
Cottin V. Update on bioagent therapy in sarcoidosis. F1000 medicine reports, 2, 13. 2010. https://doi.org/10.3410/m2-13. Accessed 8 Aug 2019.
Myung JS, Aaker GD, Kiss S. Treatment of noninfectious posterior uveitis with dexamethasone intravitreal implant. Clin Ophthalmol. 2010;4:1423–6. https://doi.org/10.2147/opth.s15696.
Zarranz-Ventura J, Carreno E, Johnston RL, Mohammed Q, Ross AH, Barker C, Fonollosa A, Artaraz J, Pelegrin L, Adan A, Lee RW, Dick AD, Sallam A. Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, and reinjection frequency. Am J Ophthalmol. 2014;158:1136–1145.e1135. https://doi.org/10.1016/j.ajo.2014.09.003.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16.
Lowder C, Belfort R Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, Li X-Y, Cui H, Whitcup SM, Group OHS. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–53. https://doi.org/10.1001/archophthalmol.2010.339.
Stanbury RM, Graham EM. Systemic corticosteroid therapy–side effects and their management. Br J Ophthalmol. 1998;82:704–8.
Buchman AL. Side effects of corticosteroid therapy. J Clin Gastroenterol. 2001;33:289–94.
Tomkins-Netzer O, Taylor SR, Bar A, Lula A, Yaganti S, Talat L, Lightman S. Treatment with repeat dexamethasone implants results in long-term disease control in eyes with noninfectious uveitis. Ophthalmology. 2014;121:1649–54. https://doi.org/10.1016/j.ophtha.2014.02.003.
Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jacques ML, Jiao J, Li XY, Whitcup SM. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146.e1133. https://doi.org/10.1016/j.ophtha.2010.03.032.
Haller JA, Bandello F, Belfort R Jr, Blumenkranz MS, Gillies M, Heier J, Loewenstein A, Yoon YH, Jiao J, Li XY, Whitcup SM, Li J. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–60. https://doi.org/10.1016/j.ophtha.2011.05.014.
Moisseiev E, Goldstein M, Waisbourd M, Barak A, Loewenstein A. Long-term evaluation of patients treated with dexamethasone intravitreal implant for macular edema due to retinal vein occlusion. Eye (Lond). 2013;27:65–71. https://doi.org/10.1038/eye.2012.226.
Calvo P, Ferreras A, Al Adel F, Dangboon W, Brent MH. Effect of an intravitreal dexamethasone implant on diabetic macular edema after cataract surgery. Retina. 2018;38:490–6. https://doi.org/10.1097/iae.0000000000001552.
Malcles A, Dot C, Voirin N, Agard E, Vie AL, Bellocq D, Denis P, Kodjikian L. Real-life study in diabetic macular edema treated with dexamethasone implant: the Reldex Study. Retina. 2017;37:753–60. https://doi.org/10.1097/iae.0000000000001234.
Malcles A, Dot C, Voirin N, Vie AL, Agard E, Bellocq D, Denis P, Kodjikian L, Safety of Intravitreal Dexamethasone Implant (Ozurdex): The SAFODEX study. Incidence and risk factors of ocular hypertension. Retina. 2017;37:1352–9. https://doi.org/10.1097/iae.0000000000001369.
Kiddee W, Trope GE, Sheng L, Beltran-Agullo L, Smith M, Strungaru MH, Baath J, Buys YM. Intraocular pressure monitoring post intravitreal steroids: a systematic review. Surv Ophthalmol. 2013;58:291–310. https://doi.org/10.1016/j.survophthal.2012.08.003.
Acknowledgements
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2016R1A6A1A03010528). Article processing charges were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Mirinae Kim, Seong Ah Kim, Wookyung Park, Rae Young Kim, and Young-Hoon Park have nothing to declare.
Compliance with Ethics Guidelines
This study adhered to the tenets of the Declaration of Helsinki, and all protocols were approved by the Institutional Review Board of The Catholic University of Korea. The requirement for informed patient consent was waived because of the retrospective nature of the study.
Data Availability
The datasets analyzed for the current study are available from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8101067.
Rights and permissions
About this article
Cite this article
Kim, M., Kim, S.A., Park, W. et al. Intravitreal Dexamethasone Implant for Treatment of Sarcoidosis-Related Uveitis. Adv Ther 36, 2137–2146 (2019). https://doi.org/10.1007/s12325-019-00989-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00989-4