Abstract
Purpose
To evaluate the effectiveness and safety of the dexamethasone intravitreal implant (DEX-I) in Non-Infectious Uveitis (NIU) in Chinese patients.
Methods
Ninety-one eyes of 77 patients (56 men, 21 women) receiving 130 implant injections for NIU were included. Treatment indication, uveitis diagnosis, best-corrected visual acuity (BCVA), central retinal thickness (CRT), vitreous haze score, intraocular pressure, phakic status, number of injections, time to reinjection, and systemic treatments were collected at baseline, 1 week, 1 month, 3 and 6 months after treatment.
Results
All patients were followed for at least 12 weeks and had a mean follow-up period of 5.1 months (range, 3–14 months) after the first implant. The main treatment indications were macular edema (ME), retinal vasculitis, retinal vasculitis with ME. Sixty-one eyes (67.03%) received only one injection, while 31 eyes (32.97%) received two or more. In eyes that received 2 injections, the mean time to the second injection was 3.83 months and in those that received 3 injections, the mean time to the third injection was 7.5 months. BCVA and CRT significantly improved at 1 week, 1 month, 3 months, and 6 months after treatment. When compared to baseline, the mean prednisone (or equivalent) dosage significantly decreased at 3- and 6-month follow-up evaluations after DEX implantation.14.29% of eyes developed a transient increase in intraocular pressure, and a cataract was removed from 1 phakic eye.
Conclusions
DEX implants, either alone or in combination with common adjunctive NIU treatments, is safe and effective in the treatment of NIU in Chinese patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uveitis is a common eye disease of variable etiology that causes blindness, with an incidence estimated at 17–52 cases per 100,000 persons per year and an annual prevalence of 69.0–114 per 100,000 persons [1,2,3,4]. Uveitis affects more patients of working age than age-related diseases (e.g., age-related macular degeneration, cataracts) and thus cause greater losses in productivity in the workforce and potentially more years of vision loss [5, 6]. There are currently 3 million to 4 million patients with uveitis in China, and 35% of them suffer from severe visual impairments [7]. According to the cause, uveitis can be divided into infectious and noninfectious uveitis. For noninfectious uveitis, the autoimmune response plays an important role in its pathogenesis. At present, the treatment of noninfectious uveitis is mainly systemically using of glucocorticoids and immunosuppressive agents, but some patients cannot be treated with these medicines because of peptic ulcers or abnormal liver and kidney functions [8, 9]. Intravitreal dexamethasone (DEX) implants (Ozurdex; Allergan, Inc., Irvine, CA) provide new treatment options for these patients [10, 11]. It has been reported in the literature that DEX implants can effectively control the active inflammation of noninfectious uveitis [12,13,14,15,16]. However, there are few Chinese data on the use of DEX implants. The objective of this study was to investigate the efficacy and safety of DEX implants in the treatment of noninfectious intermediate and posterior uveitis in Chinese patients.
Methods
A retrospective study was conducted on all patients with uveitis who were treated with DEX implants at our institution from December 2019 to April 2021. Institutional review board approval was obtained for the study.
Inclusion and exclusion criteria
All eyes treated with the DEX implants for uveitis as the main indicators were included. The criteria for DEX implant treatment were as follows: (1) Patients who were not candidates for systemic CSs and immunosuppressants due to systemic diseases such as gastric ulcers and cancer; (2) Patients who were unwilling to take systemic CSs because of concerns about side effects; and (3) Patients with chronic refractory noninfectious uveitis. Patients were excluded from the study if they had glaucoma or any active or suspected ocular or periocular infection, a history of macular edema from nonuveitic causes and those who were not followed up in time.
Data sources/measurements
Baseline data that were collected included age, sex, anatomical NIU classification (using the SUN criteria), etiology of uveitis, duration and number of occurrences/recurrences, and previous and current topical, local and systemic therapies. Following the comprehensive baseline clinical assessment, all patients were seen 1 week, 1 month, 3 months, and 6 months postimplantation. Data collected at each visit included BCVA, CRT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany), IOP measurements, and slit-lamp biomicroscopy of the anterior and posterior segments. Measurements of AC cells, flare and vitreous haze followed the SUN guidelines [17]. Visual acuity was measured on Snellen charts. For the purpose of statistical analysis, BCVA readings were converted to logMAR equivalents. The diagnosis of macular edema was made with the use of fundoscopy, fluorescein angiography (FA), and OCT.
The criteria for reinjection were the recurrence of uveitic macular edema or progression in inflammation with associated vision loss. The main outcome measures included changes in BCVA, CRT and the vitreous haze score.
Statistical methods
A paired t-test was used for statistical analysis, and one-way ANOVA was used for multiple comparisons.
Results
Study population
Ninety-one eyes from 77 patients (56 men, 21 women) receiving 130 implant injections for NIU were included in this study. The mean age of the patients was 49.97 ± 15.25 years (range, 16–79 years). All patients were followed for at least 12 weeks and had a mean follow-up period of 5.1 months (range, 3–14 months) after the first implant. The most frequent anatomic locations were panuveitis (70 eyes, 76.92%), followed by posterior uveitis (16 eyes, 17.58%) and intermediate uveitis (5 eyes, 5.49%), and the most frequent phenotypic diagnoses were idiopathic uveitis (47 eyes, 61.04%), followed by chronic recurrent Vogt–Koyanagi–Harada disease (9 eyes, 11.69%), suspected tuberculosis (5 eyes, 6.50%), birdshot chorioretinopathy (3 eyes, 3.90%), pediatric uveitis (2 eyes, 2.60%) and other diagnoses (11 eyes, 14.3%). The demographics and baseline characteristics of the cohort are listed below (Table 1). The main treatment indications were macular edema (ME) (37 eyes, 40.65%), retinal vasculitis (19 eyes, 20.88%), retinal vasculitis with ME (9 eyes, 9.90%), uveitic cataract surgery (8 eyes, 8.80%), retinochoroiditis (7 eyes, 7.70%), vitritis (6 eyes, 6.60%) and other causes (1 eye, 1.10%).
Efficacy
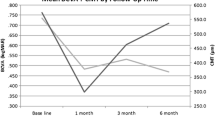
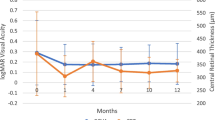
BCVA increased by a mean of 0.245 logarithm at Week 1 (P < 0.001), 0.437 logarithm at Month 1 (P < 0.001), 0.380 logarithm at Month 3 (P < 0.001), and 0.316 logarithm at Month 6 (P < 0.001) (Fig. 1). CRT decreased from 649.076 to 340.923 μm at Week 1 (P < 0.001), 287.913 μm at Month 1 (P < 0.001), 357.2 μm at Month 3 (P < 0.05), and 387.071 μm at Month 6 (P < 0.05) (Fig. 2). The proportion of eyes with a vitreous haze score improvement (2-step decrease or change from + 0.5 to 0) at 1 week was 53.12%, at 1 month was 81.48%, at 3 months was 66.67%, and at 6 months was 63.64%. Fourteen patients had bilateral DEX implantations, with the second eye receiving an implant 7 days after the first eye. In 4 patients, after implantation in the first eye, there was also a response on the fellow eye in bilateral noninfectious uveitis, with a reduction in CRT and improvement in BCVA. In 31 eyes that received two or more DEX implants, the clinical response was similar to that after the first implantation for both BCVA and CRT.
Duration of effect
The overall average number of injections per eye was 1.43 (range, 1–4). Twenty-two eyes in the study group received a second DEX implant injection within an average of 3.83 months (median 3.5 months), with the shortest duration to retreatment being 2 months. Seven eyes received a third DEX implant injection within an average of 7.5 months (median 7.25 months), and one eye received a fourth DEX implant injection within 9 months. In the subgroup analysis of patients receiving systemic treatment, the average time to the second implantation was 15.5 weeks.
Systemic steroid sparing effect
A total of 31 patients (40.26%) received some form of systemic therapy before undergoing DEX implant injection: 6.5% of the patients received steroids alone, 33.77% received steroids combined with immunosuppressant therapy and 3.9% were treated using steroids combined with biologics therapy. This percentage decreased to 29.87% at 3 months and 20.78% at 6 months. At baseline, the mean prednisone (or equivalent) dosage was 16.71 ± 10.25 mg/day. When compared to baseline, the mean prednisone (or equivalent) dosage significantly decreased at the 3-month (11.81 ± 9.81 mg/day) and 6-month (12.2 ± 11.45 mg/day) follow-up evaluations after DEX implantation (both P < 0.05).
Safety
A total of 14.29% of eyes developed a transient increase in intraocular pressure (IOP > 21 mmHg). 3.3% (3/91) of the patients’ eyes had an IOP > 31 mmHg. Three eyes with increased IOP were observed at the second injection. IOP elevation was successfully managed with topical treatment alone. A cataract was removed from 1 phakic eye after the second DEX implantation. There were no reported cases of endophthalmitis or retinal detachment.
Discussion
In this study, we retrospectively analyzed the safety and effectiveness of DEX implants in a group of Chinese patients with uveitis. The results of our study demonstrate that DEX implants were able to successfully control inflammation and improve BCVA and CRT. CRT was significantly reduced from baseline by 1 week after injection and improved further during the 6-month follow-up, and the improvement in visual acuity was consistent with that of CRT. At the same time, our results suggested that DEX implant treatment was safe. In this group of patients, only a few patients had transient intraocular pressure increases, and only 1 patient required surgery for cataracts. Our results are basically consistent with the previously reported results [18].
HURON (cHronic Uveitis evaluation of the intRavitreal dexamethasONe implant) was the first study to assess the effects of a single dexamethasone implant in eyes with noninfectious uveitis. The results demonstrated improved BCVA, reduced CRT, and less vitreitis with a relatively good safety profile and no systemic effects. The authors suggested that a single DEX implant significantly improved intraocular inflammation and visual acuity that persisted for 6 months [12]. However, because of the study design, several issues were not explored, such as repeat implantations, long-term effects, and safety among patients, including steroid responders. In our study, the mean reinjection interval was 3.83 months between the first and second DEX implant injections. However, the interval was extended to 7 months between the second and third injections and extended to 9 months between the third and fourth injections. This may be related to severe uveitis inflammation in the initial stage of the injection. Inflammation could not be controlled approximately 3 months after the first injection, and reinjection was required. However, after the second and third injections, the inflammation was partly controlled, and only a small drug concentration was needed to prevent recurrence, so the injection interval was gradually extended. Each treatment with DEX implants also produced similar significant mean reductions in CRT and the control of inflammation. The anatomical improvements in central retinal thickness in individual patients were not always associated with improvements in BCVA, perhaps because of irreversible tissue damage caused by a long duration of inflammation and edema before the patients underwent DEX implant treatment.
The present study demonstrated that the use of DEX implants allows an important reduction in systemic medication including conventional immunosuppressive/biologic agents in patients treated for chronic noninfectious uveitis. In our patients, the dosage of prednisone (or equivalent) was significantly tapered from the first week after implantation. The dosage of systemic CSs was significantly lower than at baseline, and nearly 20% of the patients could stop systemic medication at the end of the study period. In addition, given that systemic treatment may take several weeks to months to achieve effects, the DEX implant may be effectively used as a bridging therapy in patients with severe active inflammation.
Similar to previous reports, in our study, improvement of contralateral vitreitis and macular edema was observed in 4 patients [19]. The mechanism of this phenomenon is currently unclear. It has been suggested that corticosteroid molecules may escape into the systemic circulation and then reach the contralateral eye [20]. However, further research is needed to clarify the underlying mechanism.
Regarding the side effects of DEX implants, our research findings are basically consistent with other reports. The most common side-effect is increased intraocular pressure and cataracts. The increase in intraocular pressure is temporary and can be controlled with drugs. In our study, only one patient developed cataract aggravation and required surgical treatment. Due to the relatively short follow-up time of some patients in this study, we need to collect more data to further explore the safety of this implant.
The main limitation of our study is its retrospective nature. Owing to its retrospective nature, the evaluation could not be performed at specific time points. Moreover, it is likely that patients were selected for treatment with DEX implants due to a perception by investigators that they were good candidates for such treatment. The strengths of this study include the wide variation in patient demographics and clinical features in Chinese patients.
In summary, the results of this study demonstrate that the clinical use of DEX implants, either alone or in combination with common adjunctive NIU treatments, is safe and effective in the treatment of NIU in Chinese patients. Decreases in inflammation and improvements in visual acuity continued to be seen after each subsequent DEX implant injection, and no new safety concerns developed after the use of multiple implants.
References
Gritz DC, Wong IG (2004) Incidence and prevalence of uveitis in Northern California; the Northern California epidemiology of uveitis study. Ophthalmology 111(3):491–500. https://doi.org/10.1016/j.ophtha.2003.06.014 (discussion 500)
Suhler EB, Lloyd MJ, Choi D, Rosenbaum JT, Austin DF (2008) Incidence and prevalence of uveitis in veterans affairs medical centers of the Pacific Northwest. Am J Ophthalmol 146(6):890-896 e898. https://doi.org/10.1016/j.ajo.2008.09.014
Acharya NR, Tham VM, Esterberg E, Borkar DS, Parker JV, Vinoya AC, Uchida A (2013) Incidence and prevalence of uveitis: results from the pacific ocular inflammation study. JAMA Ophthalmol 131(11):1405–1412. https://doi.org/10.1001/jamaophthalmol.2013.4237
Miserocchi E, Fogliato G, Modorati G, Bandello F (2013) Review on the worldwide epidemiology of uveitis. Eur J Ophthalmol 23(5):705–717. https://doi.org/10.5301/ejo.5000278
Durrani OM, Tehrani NN, Marr JE, Moradi P, Stavrou P, Murray PI (2004) Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol 88(9):1159–1162. https://doi.org/10.1136/bjo.2003.037226
Thorne JE, Skup M, Tundia N, Macaulay D, Revol C, Chao J, Joshi A, Dick AD (2016) Direct and indirect resource use, healthcare costs and work force absence in patients with non-infectious intermediate, posterior or panuveitis. Acta Ophthalmol 94(5):e331-339. https://doi.org/10.1111/aos.12987
Wakefield D, Chang JH (2005) Epidemiology of uveitis. Int Ophthalmol Clin 45(2):1–13. https://doi.org/10.1097/01.iio.0000155938.83083.94
Deschenes J, Murray PI, Rao NA, Nussenblatt RB, International Uveitis Study G (2008) International uveitis study group (IUSG): clinical classification of uveitis. Ocul Immunol Inflamm 16(1):1–2. https://doi.org/10.1080/09273940801899822
Tomkins-Netzer O, Taylor SR, Lightman S (2012) Corticosteroid-sparing agents: new treatment options. Dev Ophthalmol 51:47–56. https://doi.org/10.1159/000336186
Sella R, Oray M, Friling R, Umar L, Tugal-Tutkun I, Kramer M (2015) Dexamethasone intravitreal implant (Ozurdex(R)) for pediatric uveitis. Graefes Arch Clin Exp Ophthalmol 253(10):1777–1782. https://doi.org/10.1007/s00417-015-3124-x
Lightman S, Belfort R Jr, Naik RK, Lowder C, Foster CS, Rentz AM, Cui H, Whitcup SM, Kowalski JW, Revicki DA (2013) Vision-related functioning outcomes of dexamethasone intravitreal implant in noninfectious intermediate or posterior uveitis. Invest Ophthalmol Vis Sci 54(7):4864–4870. https://doi.org/10.1167/iovs.12-10981
Lowder C, Belfort R Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, Li XY, Cui H, Whitcup SM, Ozurdex HSG (2011) Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol 129(5):545–553. https://doi.org/10.1001/archophthalmol.2010.339
Moisseiev E, Goldstein M, Waisbourd M, Barak A, Loewenstein A (2013) Long-term evaluation of patients treated with dexamethasone intravitreal implant for macular edema due to retinal vein occlusion. Eye (Lond) 27(1):65–71. https://doi.org/10.1038/eye.2012.226
Fabiani C, Vitale A, Lopalco G, Iannone F, Frediani B, Cantarini L (2016) Different roles of TNF inhibitors in acute anterior uveitis associated with ankylosing spondylitis: state of the art. Clin Rheumatol 35(11):2631–2638. https://doi.org/10.1007/s10067-016-3426-3
Fardeau C, Champion E, Massamba N, LeHoang P (2016) Uveitic macular edema. Eye (Lond) 30(10):1277–1292. https://doi.org/10.1038/eye.2016.115
Fabiani C, Alio JL (2015) Local (topical and intraocular) therapy for ocular Adamantiades-Behcet’s disease. Curr Opin Ophthalmol 26(6):546–552. https://doi.org/10.1097/ICU.0000000000000210
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G (2005) Standardization of uveitis nomenclature for reporting clinical data Results of the first international workshop. Am J Ophthalmol 140(3):509–516. https://doi.org/10.1016/j.ajo.2005.03.057
Nobre-Cardoso J, Champion E, Darugar A, Fel A, Lehoang P, Bodaghi B (2017) Treatment of non-infectious uveitic macular edema with the intravitreal dexamethasone implant. Ocul Immunol Inflamm 25(4):447–454. https://doi.org/10.3109/09273948.2015.1132738
Habot-Wilner Z, Sorkin N, Goldenberg D, Goldstein M (2015) Bilateral effect of unilateral dexamethasone intravitreal implant in a case of noninfectious uveitic macular edema and vitritis. Retin Cases Brief Rep 9(2):151–153. https://doi.org/10.1097/ICB.0000000000000122
Sharma A, Sheth J, Madhusudan RJ, Sundaramoorthy SK (2013) Effect of intravitreal dexamethasone implant on the contralateral eye: a case report. Retin Cases Brief Rep 7(3):217–219. https://doi.org/10.1097/ICB.0b013e31828993a1
Funding
This study was funded by the National Natural Science Foundation of China (81300752), the Jilin Province Science and Technology Development Plan Project (20200201333JC) and the Jilin Province Health Special Project (2020SCZT058).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethics Committee of the Second Hospital of Jilin University and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zeng, S., Yang, L., Bai, F. et al. Intravitreal dexamethasone implant for noninfectious uveitis in Chinese patients. Int Ophthalmol 42, 2063–2069 (2022). https://doi.org/10.1007/s10792-021-02204-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-02204-2