Abstract
The differential diagnosis of vertigo or dizziness as a result of cerebellar disorders can be difficult as many patients with a cerebellar pathology do not present with the full spectrum of cerebellar signs. The main goal of this study was to describe the typical clinical features of these patients with vertigo or dizziness of a cerebellar origin. We reviewed the medical records of 5400 patients with vertigo and dizziness from our tertiary outpatient clinic for vertigo and balance disorders. In 459 the diagnosis of “cerebellar vertigo or dizziness” was made; 90 patients were excluded from further analysis due to evident structural changes in MRI. Of the remaining 369 patients (67.0 ± 15.1, 54% female, symptom duration until diagnosis 5.5 ± 6.9 years), 81% suffered from persistent vertigo or dizziness, 31% from attacks of vertigo and dizziness and 21% from both. Neuro-ophthalmologically, 95% had saccadic smooth pursuit, 80% gaze-holding deficits, 64% a pathological fixation suppression of the VOR, 24% central fixation nystagmus (in 64% of these cases downbeat nystagmus (DBN)), 23% rebound nystagmus, and an ocular misalignment in 84% in near view and 50% in distance view. Eleven percent had isolated mild to moderate cerebellar ocular motor disturbances without any other typical cerebellar signs. The most common diagnoses were sporadic adult-onset degenerative ataxia in 26%; idiopathic DBN syndrome in 20%; cerebellar ataxia, neuropathy, and vestibular areflexia syndrome in 10%; episodic ataxia type 2 in 7%; and multiple system atrophy cerebellar type in 6%. In posturography, a typical cerebellar 3-Hz sway was found in 16%. The diagnostic key to patients with cerebellar vertigo or dizziness is a careful examination of eye movements since practically all of them have cerebellar ocular disturbances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vertigo and dizziness as a multisensory syndrome of various etiologies and pathogeneses is one of the most common clinical chief complaints in medicine with an annual prevalence of around 11% [1] and a lifetime prevalence of around 20–30% [2]. This syndrome can also be caused by lesions of the vestibulo-cerebellar, vestibulo-spinal, or cerebellar ocular motor systems [2,3,4]. The cerebellum is known to be involved in sensorimotor operations, cognitive tasks, and affective processes [5, 6]. Thus, the consequences of cerebellar vertigo and dizziness can be very complex. Cerebellar vertigo and dizziness can be categorized into (1) acute onset of cerebellar vertigo and/or dizziness (e.g., in cerebellar strokes), (2) recurrent attacks of cerebellar vertigo and/or dizziness (e.g., in episodic ataxias), and (3) persisting symptoms [6].
In those patients with clear cerebellar signs and symptoms, such as patients with spinocerebellar ataxia (SCA) or downbeat nystagmus (DBN) syndrome, diagnosis is easy [7]. However, in patients with only subtle cerebellar signs, such as isolated cerebellar ocular motor disturbances or isolated gait ataxia, diagnosis can be challenging and a careful examination is indispensable. Clinical experience, however, shows that cerebellar vertigo or dizziness can often be overlooked because the ocular motor system is not, or not properly, examined. Typical cerebellar ocular motor disturbances are gaze-holding deficits, downbeat, rebound, central positional, periodic alternating nystagmus, hypermetria/hypometria of saccades, saccadic smooth pursuit, skew deviation (ocular misalignment), or impairment of the vestibulo-ocular reflex (VOR) [5]. Topographic anatomically cerebellar ocular motor disturbances can be assigned to specific areas of the cerebellum such as the flocculus and paraflocculus, the oculomotor vermis (lobules VI, VII, crus I–II of ansiform lobule), the nodulus and uvula, the caudal part of the fastigial nucleus, the lateral part of the interpositus nucleus, and the caudal part of the dentate nucleus [5, 7]. There are three principal cerebellar ocular motor syndromes: (1) the syndrome of the flocculus and paraflocculus, (2) the syndrome of the nodulus and ventral uvula, and (3) the syndrome of the dorsal vermis and the underlying caudal fastigial nuclei (fastigial ocular motor region) [7]. Besides characteristic ocular motor disturbances, ataxia of stance and gait is another clinical main symptom of cerebellar disorders with a typical postural 3-Hz sway being pathognomonic for cerebellar disease, especially described in patients with anterior lobe atrophy [8]. Other clinical features are, for example, a vestibular hyperreactivity [9, 10].
Based on this knowledge the major aim of this study was as follows: (1) a detailed description of the types of cerebellar vertigo and dizziness, (2) a detailed workup of cerebellar ocular motor disturbances in patients with cerebellar vertigo and dizziness in general and more specifically an analysis of whether distinct cerebellar ocular motor disturbances are a sensitive clinical marker for an underlying etiology of the cerebellar disease, and (3) a description of the relative frequency of other characteristic cerebellar signs such as postural 3-Hz sway. Moreover, the co-occurrence of different cerebellar symptoms, ocular motor deficits, and other clinical entities was evaluated.
Methods
Standardized History
We retrospectively analyzed the files of 5400 consecutive patients from 2011 to 2015 who presented with vertigo and dizziness in our outpatient clinic of the German Center for Vertigo and Balance Disorders (DSGZ) at the University Hospital Munich. We selected those patients with an underlying cerebellar syndrome according to clinical standards as well diagnostic criteria for sporadic adult-onset degenerative ataxia (SAOA) [11], multiple system atrophy cerebellar type (MSA-C) [12], and cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS) [13]. Patients with cerebellar disease due to acquired focal lesions (i.e., because of infarction, tumor, or demyelinization) were excluded from the further analysis because of the different pathogenesis in these diseases. A detailed standardized history was taken for the patients. The following parameters were assessed:
-
1.
Vertigo and dizziness as well as unsteadiness of gait and stance with onset, duration, time course, frequency, and associated symptoms, such as visual and ocular motor symptoms including oscillopsia, double vision, blurred vision, associated otological symptoms (including tinnitus, hearing loss, fullness of ear), associated cerebellar symptoms (ataxia, dysarthria), and other symptoms such as headache, phonophobia, photophobia, nausea, and vomiting.
-
2.
Past medical history with exposure to toxins, ethanol, lithium, amiodarone, antiepileptic medications, cardiovascular risk factors, other diseases, and family history.
Neurological, Neuro-Otological, and Neuro-Ophthalmological Examination
All patients underwent a standardized neurological, neuro-otological, and neuro-ophthalmological examination at the DSGZ as described previously (for example, see [14]). The neuro-ophthalmological examination included the clinical head-impulse test (cHIT), evaluation of ocular alignment when looking at a near target and into the distance as well as during lateral gaze in the distance (i.e., orthophoria/esophoria/exophoria, vertical deviation, congenital strabismus), spontaneous nystagmus with Frenzel’s goggles in primary position and during gaze deviations, gaze-evoked nystagmus, smooth pursuit, saccades, optokinetic nystagmus, visual fixation suppression of the VOR, rebound nystagmus, head-shaking nystagmus, and determination of the subjective visual vertical (SVV). Deviations above 2.5° were set as pathological. Further neuro-otological and neuro-ophthalmological diagnostic examinations included videooculography (VOG) with bithermal caloric irrigation, two-dimensional VOG (EyeSeeCam®) to assess ocular motor function, and the video-assisted head-impulse test (vHIT) as described elsewhere (for further information, see [15, 16]). Bilateral vestibulopathy in caloric examination was defined as the sum of the maximal peak velocities of the slow phase caloric-induced nystagmus for stimulation with warm and cold water on each side < 6°/s [17]. As there is no standard definition of caloric hyperactivity, we operationally defined caloric hyperactivity as responses showing > 70°/s.
Posturography
Posturographic measurements were carried out in the upright position using the standard protocol with 10 different conditions with increasing difficulty [18]. The total body sway during 30 s of the posturographic measurement provided the sway path values (m/min). Furthermore, root mean square (RMS) (mm) and frequency spectrum between 2.4 and 3.5 Hz as well as between 5 and 8 Hz (FFT) of the z axis (kgf/Hz) of the measurements were obtained. The conditions in posturography relevant for cerebellar patients are condition 1 (standing on firm ground with eyes open), condition 2 (standing on firm ground with closed eyes), condition 5 (standing on foam ground with eyes open), and condition 6 (standing on foam ground with closed eyes).
Additional Diagnostic Procedures
Polyneuropathy (PNP) was diagnosed either clinically (reduced or absent ankle reflexes, distal hypaesthesia for sensation, and/or a reduced sense of vibration of less than 4/8 in both feeds) and confirmed by nerve conduction studies and electromyography in selected patients. Cerebellar function was evaluated in detail by ataxia rating scales, namely, the Scale for the Assessment and Rating of Ataxia (SARA) [19]. Selected patients received specific genetic examinations, lumbar puncture including autoimmune and infectiological examinations, and laboratory examinations.
Ethical Standards
The study was approved by the ethics committee of the Ludwig-Maximilians University Munich and was performed in accordance with the ethical standards according to the 1964 Declaration of Helsinki and its later amendments.
Statistics
Posturography data were collected and evaluated in MATLAB (MathWorks Inc., Natick, MA); other data were evaluated using Excel (Microsoft, Redmond, WA) spreadsheet software. Statistical analysis was performed using R version 3.3 [20]. Figures were designed with GraphPad Prism (v5; GraphPad Software Inc., La Jolla, CA, USA). We report mean and standard deviation for continuous variables and frequencies for categorical data. We tested for difference between the groups using t test for continuous data and chi-squared test for categorical data. To express the correlation we used nonparametric testing with Spearman R. Differences were considered significant if p < 0.05. As this study is exploratory, we did not adjust for multiple testing.
Results
Cerebellar Vertigo and Dizziness—Frequency and Underlying Etiology
Of a total of 5400 consecutive patients between 2011 and 2015, 459 patients (mean age at diagnosis: 65.8 ± 15.6 years, 53% female) were diagnosed with a cerebellar disease. Patients with acquired focal lesions due to infarction, tumor, or demyelinization (n = 90, 61.1 ± 16.8 years, 50% female) were not included in the further more detailed analysis. In total, 369 patients (67.0 ± 15.1 years, 54% female) with underlying cerebellar disease and no evidence of focal lesions were analyzed.
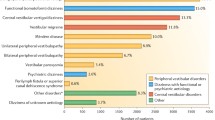
The most common clinical diagnoses among those patients were as follows: SAOA (26%, 66.3 ± 14.2 years, 55% female), DBN syndrome (20%, 73.5 ± 10.7 years, 59% female), isolated mild to moderate cerebellar ocular motor disturbances (11%, 71.6 ± 13.5 years, 63% female), patients with CANVAS (10%, 76.4 ± 7.2, 53% female), patients with MSA-C (6%, 74.7 ± 6.8 years, 46% female) followed by patients with spinocerebellar ataxia (SCA) (8%, 55.3 ± 17.1 years, 60.7% female), and episodic ataxia type 2 (EA2) (7%, 46.3 ± 14.9 years, 16% female). The remaining 13% of patients (61.2 ± 13.7 years, 55% female) were diagnosed with either toxic, autoimmune, or paraneoplastic cerebellar disease (for further details, see Fig. 1).
Etiology of different cerebellar disease in 459 patients in the German Center for Vertigo and Balance Disorders in Munich between 2011 and 2015. MSA-C multiple system atrophy of cerebellar type; CANVAS cerebellar ataxia, neuropathy, vestibular areflexia syndrome; DBN downbeat nystagmus; SAOA sporadic adult-onset degenerative ataxia. Absolute numbers are given
All diagnoses were classified into three groups depending on the etiology: (1) patients with degenerative forms of ataxia (n = 267, 67.0 ± 15.1 years, 57% female) including SAOA, DBN syndrome, CANVAS, pure mild to moderate cerebellar ocular motor disturbances, MSA-C, and other degenerative diseases; (2) patients with hereditary forms of ataxia (n = 50, 50.5 ± 16.9 years, 36% female) including SCA of different types and EA2; and (3) patients with acquired non-degenerative forms of ataxia (n = 52, 71.0 ± 12.4 years, 52% female) including paraneoplastic, immunologic/autoimmune, alcohol, and toxic induced ataxia. Mean time until diagnosis was 5.5 ± 6.9 years; the delay was longest in patients with hereditary diseases (10.4 ± 9.9 years) compared to degenerative (5.0 ± 6.1 years) and acquired non-degenerative forms of cerebellar ataxia (3.1 ± 5.1 years) (p < 0.001). For further details, see Table 1.
Family history was positive in 11% (n = 39) of patients, commonly in hereditary forms of ataxia (52% positive family history, p < 0.0001). Age of manifestation was younger in patients with hereditary forms of ataxia (40.1 ± 20.7 years) compared to patients with degenerative (66.0 ± 13.5 years) and acquired forms of ataxia (58.9 ± 15.6 years) (p < 0.0001). Fifty-three percent (n = 197) of patients reported a subjective progression of their symptoms over time (for further details, see supplemental Table 1).
Cerebellar Vertigo and Dizziness—Symptoms
Eighty percent (n = 295) of patients suffered from persistent vertigo and dizziness, with postural imbalance in 77% (n = 227), light-headedness in 16% (n = 50), and rotatory vertigo in 2% (n = 6). Persistent vertigo and dizziness in cerebellar patients was motion-dependent in n = 227 patients. Triggers were reported in n = 207 (56%) of patients: darkness (n = 105), ground dependency (n = 84), situations (n = 27), emotions (n = 35), and alcohol (n = 19).
Attacks of vertigo and dizziness were reported in 28% (n = 104) of patients (rotatory vertigo n = 55, postural imbalance in n = 48, light-headedness in n = 1). The typical durations of attacks (seconds: n = 46, minutes n = 25, hours n = 25, days n = 7) and frequency of attacks (several times daily n = 27, daily n = 31, weekly n = 15, monthly n = 4) showed a broad spectrum. Seventy-two percent of patients described triggers, most commonly positional changes in 83% of these patients. Eighteen percent of patients had persistent vertigo and persistent attacks (n = 67).
Other reported symptoms of cerebellar patients were vegetative symptoms (n = 63) like nausea (n = 33), vomiting (n = 16), urinary incontinence (n = 20), erectile dysfunction (n = 3), recurrent attacks of sweating (n = 2), and anxiety (n = 71). Typical “cerebellar” symptoms like gait disturbances (n = 268), falls (n = 149), fine motor disturbances (n = 72), or dysarthria (n = 83) were reported in 73%, 40%, 20%, and 22% of the patients, respectively. Approximately 20% of the patients had ocular symptoms such as oscillopsia (n = 81, 22%), double vision (n = 67, 18%), or blurred vision (n = 63, 17%). For further details, see Supplemental Table 2.
Oculomotor Findings
Saccadic smooth pursuit was the most common, but unspecific finding in patients with cerebellar disorders (n = 312, 85% saccadic smooth pursuit in all directions; n = 27, 7% saccadic smooth pursuit only in vertical direction; n = 12, 3% saccadic smooth pursuit only in horizontal directions) (p < 0.001).
Overall, saccadic smooth pursuit occurred in 95% of patients with degenerative cerebellar disorders and in 88% of patients with hereditary and 90% of patients with acquired non-degenerative forms of cerebellar disorders. Central fixation nystagmus occurred in 24% (n = 88) of cases (degenerative n = 68, 25%; hereditary n = 8, 16%; acquired non-degenerative n = 12, 23%; p = 0.006) with DBN the most common in 64% (n = 50), causing oscillopsia as well as vertigo and dizziness. Gaze-holding function deficits were documented in 80% of the patients (n = 297). Horizontal gaze-evoked nystagmus was more common in degenerative forms (n = 147, 55%) than in patients with hereditary (n = 21, 42%) or acquired non-degenerative cerebellar diseases (n = 17, 33%) (p = 0.006). Rebound nystagmus as a typical cerebellar ocular dysfunction was found in n = 86 (23%) of patients, whereas 57% (n = 218) patients had head-shaking nystagmus (in horizontal direction n = 81, 22%; in vertical direction n = 143, 39%). Disturbances of vertical saccades (n = 125) (slowed n = 51/40%, hypometric n = 46/37%, hypermetric n = 45/36%) and of horizontal saccades in (n = 126) (slowed n = 46, 37%; hypometric n = 40, 32%; hypermetric n = 52, 41%) were each found in 34%.
Forty-nine percent of the patients clinically had a bilateral pathological head-impulse test, more frequently in patients with degenerative (54%) and hereditary (44%) than acquired non-degenerative (27%) forms of ataxia (p = 0.0021), whereas a unilateral pathological head-impulse test was found in 8% of the patients with no significant differences in the different forms of ataxia.
Conjugate eye movement abnormalities were observed in 29% (n = 106), while misalignments in distant view were evident in 50% (n = 183) and in near view in 84% (n = 309) of the patients. Impaired fixation suppression was documented in 64% of all patients (n = 187 in all directions, n = 17 in horizontal direction, and n = 34 in vertical direction). The clinical head-impulse test was pathologic in 56% of patients (n = 180 pathologies both sides, n = 27 one side). For further details, see Fig. 2 and Table 2.
Frequencies of various findings in the neuro-orthoptic/oculomotor examination (a) and other clinical findings (b) in selected cerebellar diseases. Higher frequencies are of more intense color. SAOA sporadic adult-onset degenerative ataxia; DBN downbeat nystagmus; CANVAS cerebellar ataxia, neuropathy, vestibular areflexia syndrome; MSA-C multiple system atrophy of cerebellar type; SCA spinocerebellar ataxia; VOR vestibular ocular reflex
Additional Symptoms and Findings
The most common findings in the neurological examination of the cerebellar patients were problems with coordination and fine motor skills with dysmetria in the finger-to-finger test (46%, n = 134), in the finger-to-nose test (41%, n = 108), in the heel-to-shin test (35%, n = 77), dysdiadochokinesia (34%, n = 125), intention tremor (12%, n = 43), and fine motor disturbances (15%, n = 53). Furthermore, patients suffered from dysarthria (20%, n = 58) as well as postural instability (17%, n = 55). In positioning maneuvers, 15% of patients (n = 57) showed a central positional or positioning nystagmus while 4% (n = 15) showed peripheral nystagmus implying the occurrence of benign paroxysmal positional vertigo (BPPV) coincidentally. Mean SARA was 14.1 ± 6.6 in all patients; patients with acquired non-degenerative forms of cerebellar disease seemed to be more affected (28.8 ± 4.6) than patients with degenerative (13.2 ± 5.8) and hereditary cerebellar disease (13.2 ± 5.8, p < 0.001). A total of 105 patients (28%) had limb ataxia. Twenty-eight percent (n = 102) had polyneuropathy with no differences between the groups (degenerative vs. hereditary vs. acquired non-degenerative: 29% vs. 22% vs. 27%; p = 0.606).
Diagnostic Procedures/Other Findings
Of all cerebellar patients, 35% (n = 115) had caloric irrigation pathologies with 16% of all patients (n = 53) showing a bilateral vestibulopathy, 8% of patients (n = 27) unilateral vestibulopathy, and another 8% of all patients (n = 25) caloric hyperactivity. Caloric hyperactivity was most common in patients with paraneoplastic etiologies of cerebellar ataxia (29%), DBN syndrome (13%), and episodic ataxia (9%, p = 0.0234). Square wave jerks (documented by videooculography) were found in n = 103 (n = 36%) of patients. An experienced neuroradiologist detected cerebellar atrophy in 27% of patients (n = 78) (the etiology of these was degenerative in 29%, hereditary in 32%, and acquired non-degenerative in 12%; p = 0.07). Posturography was obtained in 238 patients; in 8% of patients, posturography in artificial-network-based posturographic analysis was rated normal (n = 19). A typical cerebellar 3-Hz sway was evident in 13% (n = 37) of patients, whereas 16% (n = 37) had a typical pattern of cerebellar ataxia but without the 3-Hz sway. Another common diagnosis in artificial-network-based posturographic analysis was a vestibular deficit in 18% (n = 43) as well as phobic postural vertigo (PPV) in 32% (n = 76) (see Table 3).
In terms of posturography, the patients were able to perform 8.4 ± 1.8 conditions, patients with hereditary forms of ataxia only 7.9 ± 2.1 (p = 0.040) (see supplement Fig. 1). For detailed information on results in posturography, see supplemental Table 3. For a graphic illustration of the frequencies of certain oculomotor findings as well as other clinical findings, see Fig. 2.
Correlations of Selected Oculomotor Deficits and Other Specific Findings Associated With Cerebellar Disease
Ocular Alignment
“Eso-Pathologies” in Distant Gaze
More than one third of patients (36.9%) had eso-pathologies (esophoria and esotropia); these patients had a positive family history in 16% compared to 7% of patients without eso-pathologies (p = 0.022). On neurological examination, eso-pathologies in distant gaze straight ahead were only associated with gait ataxia (p = 0.018, 46% vs. 33%).
Eso-pathologies showed a strong association with motility pathologies (p < 0.0001), especially with abduction deficits (p = 0.0446). Furthermore, eso-pathologies were associated with gaze-evoked nystagmus (87% vs. 77%, p = 0.0286), rebound nystagmus (30% vs. 19%, p = 0.0246), saccade pathologies in the vertical direction (42% vs. 29%, p = 0.017), impaired fixation suppression (p = 0.001), and optokinetic nystagmus pathologies (p = 0.037).
Clinically, the presence of a pathological head impulse test was associated with eso-pathologies in distant gaze (p = 0.033) as well as the finding of cerebellar atrophy in imaging (p = 0.008).
Eso-pathologies were most often found in SAOA (26%), followed by idiopathic DBN syndrome (17%) and CANVAS, cases with isolated cerebellar oculomotor disturbances and hereditary cerebellar ataxia (each 10%).
“Exo-Pathologies” in Distant Gaze
Five percent of patients (n = 21) had exo-pathologies (in distant gaze straight ahead). Clinically, gait disturbances were associated with this finding (95% compared to 71%, p = 0.032). In terms of oculomotor dysfunction, the finding of a strabismus sursoadductorius was associated with exo-pathologies (p = 0.013).
Vertical Deviation in Distant Gaze
Twelve percent (n = 45) of patients had vertical deviation in distant gaze. Thirty-one percent of these patients showed a strabismus deorsoadductorius (p < 0.001). Ninety-six percent of these patients had gaze-evoked nystagmus (p = 0.012) compared to 78% of patients without vertical deviation. Sixty-one percent had hypometric vertical saccades (p = 0.041).
Central Positioning Nystagmus
Central positioning nystagmus was found in 15% (n = 57). These patients more often had attacks of vertigo (n = 26/45%) compared to n = 78 out of 312 (25%) (p < 0.001). Triggers for the attacks were changes of head position relative to gravity (77 to 48%, p = 0.011). Moreover, these patients more often had nausea (92 to 43%, p = 0.007) as a typical vegetative symptom. Gaze-evoked nystagmus was more evident in these patients (91% vs. 79%, p = 0.041); this was true for spontaneous nystagmus (18% vs. 7%, p = 0.020) as well. Central positioning nystagmus was more frequent in degenerative etiologies (88% compared to 70%, p = 0.008). Eighteen percent of patients with central positional nystagmus had a spontaneous nystagmus (compared to 7%, p = 0.023).
Square Wave Jerks
Square wave jerks were found in 28% of cases (n = 103). In patients with square wave jerks, primary position nystagmus (35% compared to 20%, p = 0.007) was a common sign, in 50% of cases a downbeat nystagmus (n = 18). Furthermore, square wave jerks were associated with hypometric vertical saccades (p = 0.041). Square wave jerk was most often found in patients with idiopathic downbeat nystagmus (25%), followed by SAOA (19%), EA2 (10%), and CANVAS (10%).
Impaired Fixation Suppression
Of all cerebellar patients, 64% (n = 238) showed an impaired fixation suppression. Patients with an impaired fixation suppression more frequently showed dysarthria (27% compared to 27%, p = 0.009). Furthermore, DBN in convergence was found in n = 81 patients (34%) compared to only n = 25 patients (19%) without impaired fixation suppression (p = 0.005). Of the patients, 136 (57%) had pathologic ocular alignment in distance (p < 0.001) compared to n = 47 (36%) without impaired fixation suppression. All in all, of these patients n = 14 patients had exo-deficits (exophoria n = 13, exotropia n = 1), n = 104 patients had eso-deficits (exophoria in n = 69, exotropia in n = 35), and n = 33 (24%) of patients showed pathological vertical deviation. In contrast, ocular alignment pathology in near gaze straight ahead was not associated with impaired visual fixation (pathologic in n = 200 patients/84% compared to n = 109/83%; p = 0.953). Taken together, 161 patients had exo-pathologies (n = 155 exophoria, n = 6 exotropia), n = 29 had eso-pathologies (n = 12 esophoria, n = 17 esotropia), and n = 18 had pathological vertical deviation. Saccadic smooth pursuit showed a high correlation with impaired fixation suppression (n = 214/90% compared to n = 98, 75%; p < 0.001).
The finding of rebound nystagmus was associated with impaired fixation suppression (n = 69, 29% compared to n = 17, 13%; p < 0.001) as was the finding of head-shaking nystagmus (n = 151, 63% compared to n = 60, 46%; p = 0.002), especially in the horizontal direction (n = 63, 26% compared to n = 18, 14%; p = 0.007), whereas the finding of head-shaking nystagmus in the vertical direction did not show an association (n = 99, 42% compared to n = 44, 34%; p = 0.162).
Slowed vertical saccades (p = 0.001; n = 27, 31% compared to n = 24, 63%) and horizontal saccades (n = 25, 28% compared to n = 21, 55%; p = 0.008) were less often seen in patients with impaired fixation suppression.
Patients with impaired fixation suppression more often showed a cerebellar atrophy (p = 0.037, n = 61, 31% compared to n = 17; 19%), but had significant fewer caloric irrigation pathologies (p = 0.011, 30% impaired to 44%).
Impaired fixation suppression was not associated with vertical hypometric saccades (p = 0.962), horizontal hypometric (p = 1), or horizontal hypermetric (p = 0.2095) but with hypermetric vertical saccades (p = 0.040).
Correlation of 3-Hz Sway in Posturography With Clinical and Oculomotor Findings
Thirty-seven patients (66.1 ± 15.2 years) out of 238 patients (16%) had a typical 3-Hz sway in posturography. Patients with 3-Hz sway in posturography more often had gait disturbances (95% compared to 69% in patients without 3-Hz sway, p = 0.003) fine motor disturbances (49% compared to 17%, p < 0.001) and coordination disturbances in clinical examination (46% compared to 20%, p = 0.001) with a dysmetric finger-nose test (p = 0.021) and heel-to-shin test (p = 0.010). Dysdiadochokinesia was more frequent (p = 0.015) and the Romberg standing test pathologic in 84% of patients (compared to 62%, p = 0.045). On neurological examination, gait disturbances were evident (p = 0.012) with postural instability (p = 0.023). On neuro-ophthalmological examination, the finding of a 3-Hz sway was associated with vertical saccades pathologies (p = 0.0031) as well as horizontal saccades pathologies (p = 0.019). The finding of a 3-Hz sway could not help in diagnosing a specific disease as 3-Hz sway was found in SAOA (35% of patients) as well as in hereditary cerebellar ataxia (10%), MSA-C (6%), idiopathic DBN syndrome (5%), and alcohol-induced cerebellar ataxia (3%).
Hypersensitivity in Caloric Irrigation
Of all patients, n = 25 showed a caloric hyperactivity with no differences regarding sex (female 44% compared to 55%). Age (67.5 ± 13.5 years) in patients with caloric hyperactivity was the same as in patients without (67.8 ± 14.8 years) (p = 0.925). Patients with caloric hyperactivity had significant less gait/stance ataxia (p = 0.029).
The finding of caloric hyperactivity was associated with a vertical deviation in distant view (p = 0.031) and near view (p = 0.017); there were no other significant associations between caloric hyperactivity and oculomotor disturbances. Caloric hyperactivity was found most often (36%) in idiopathic DBN.
Discussion
The major findings of the study are as follows:
First, this study shows that cerebellar dysfunction is a relevant and frequent cause of vertigo and dizziness which may lead to a major and permanent limitation of daily life and severe functional impairment in affected patients. All of our patients showed typical cerebellar oculomotor disturbances, which highlights the necessity of a thorough examination of the ocular system in patients with vertigo and dizziness. Particularly, the detection of distinct cerebellar disease such as DBN syndrome and EA2 is of clinical relevance, since these syndromes can be pharmacologically treated, for instance, with aminopyridines [21,22,23,24,25,26] or others with acetyl-DL-leucine [27].
Second, 81% of the patients suffered from persistent dizziness, 31% from vertigo and dizziness attacks, and 21% from both. Classical “cerebellar symptoms,” i.e., gait disturbances, fine motor disturbances, or dysarthria, were reported in 73%, 20%, and 22% of patients, respectively, and were therefore not that common. Ocular symptoms like double vision, blurred vision, and oscillopsia were each found in about 20% of cases. Usually, attacks of vertigo are described in patients with focal cerebellar lesions, i.e., caused by an infarction in the territory of the cerebellum. In chronic cerebellar disease, vertigo and dizziness has been described as part of selected cerebellar diseases like DBN syndrome, EA2, SCA6, or CANVAS. Onset of hereditary or degenerative forms of cerebellar ataxia is difficult to specify due to the insidious and progressive course of the disease. In a systemic study on disease onset in SCA, known for varying affection of different parts of the nervous system and a broad variability of symptoms, gait ataxia was the initial complaint in only two thirds of patents, whereas 16% of these patients reported other symptoms like diplopia or episodic vertigo. Another study cohort of SCA patients showed that 20% had non-gait ataxia onset without specifying these symptoms, especially in relation to vertigo or dizziness. Therefore, a standardized description of vertigo- and dizziness-related symptoms in cerebellar patients had not been carried out so far.
Third, the study provides very detailed and quantitative information on the relative frequency of various oculomotor disorders in cerebellar vertigo and dizziness: saccadic smooth pursuit in 87%, gaze-holding deficits in 80%, head-shaking nystagmus in 57%, ocular misalignments in distant view in 50% and in near view in 84%, impairment of vertical and horizontal saccades (slowed, hypometric, hypermetric saccades) each in 34%, central fixation nystagmus in 24%, rebound nystagmus in 23%, and impaired fixation suppression of the VOR in 64%. The association of cerebellar dysfunction with eso-pathologies described in a previous study on 400 patients, in whom 199 had a known or newly diagnosed cerebellar dysfunction [14], could also be confirmed in our study with a different cohort of a similar size. As a consequence one should consider cerebellar disorders in patients with esophoria or esotropia who complain of double vision.
The most common “cerebellar nystagmus” forms are as follows: 80% of patients had gaze-evoked nystagmus and 57% of patients had head-shaking nystagmus; of these patients, 65% had perverted head-shaking nystagmus in the vertical direction. In general, the mechanism for perverted head-shaking nystagmus is thought to be either a directional error in the input circuitry or a directional error in the central velocity storage mechanisms that perseverated vestibular input [2, 7]. One quarter of the patients had rebound nystagmus and/or central fixation nystagmus with DBN being the most common. Different topo-anatomical studies were able to localize the dysfunctional cerebellar structure [5]. Therefore, with these studies, a topo-anatomical localization of other cerebellar oculomotor disturbances can be made. Impaired fixation suppression as a very common finding in cerebellar patients was statistically significantly associated with DBN, pathologies in ocular alignment in distant view, especially eso-pathologies, saccadic smooth pursuit, and rebound nystagmus. These findings point to a possible pathophysiological involvement of the cerebellar flocculus, while the association with head-shaking nystagmus suggests involvement of the nodulus and uvula. Central positional or positioning nystagmus may be due to a lesion of the nodulus-uvula or oculomotor vermis region [6], but the exact location of the lesions causing central positional or positioning nystagmus is not well understood. However, in our study cohort, the association of central positional or positioning nystagmus with gaze-evoked nystagmus might demonstrate a pathophysiological involvement of the flocculus and paraflocculus as well. Eighteen percent of patients with central positional or positioning nystagmus also had spontaneous nystagmus. As central positional or positioning nystagmus has to be differentiated from benign paroxysmal positional vertigo (BPPV), in which the nystagmus correlated with the semicircular canal affected, this might be also helpful in the clinical examination.
Fourth, posturography did also contribute markedly to the diagnosis. Typically, lesions of the anterior lobe are associated with a cerebellar 3-Hz sway predominating in the anterior-posterior direction, whereas vestibulo-cerebellar lesions tend to produce a low-frequency sway (<1 Hz) in all directions [5, 8]. Only 15.5% of our study cohort with posturographic examination showed the typical cerebellar 3-Hz sway. Furthermore, in the clinical examination, fine motor disturbances and limb ataxia were associated with the finding of a 3-Hz sway. In the neuro-ophthalmological examination, the finding of a 3-Hz sway was associated with pathologies of vertical and horizontal saccades and pointed to a pathophysiological involvement of the dorsal oculomotor vermis and underlying fastigial oculomotor region as well. However, 3-Hz sway was not typical and specific for one cerebellar disease. In contrast, another study with static posturography demonstrated that 97.5% of patients with MSA-C predominantly showed a typical 3-Hz postural sway compared to 24.1% of MSA-P patients, and therefore, this finding was discussed as useful in the differential diagnosis of MSA-C and MSA-P [28]. In our study cohort, the finding of a 3-Hz sway did not help to diagnose a specific disease: 3-Hz sway was found in SAOA (n = 13), idiopathic DBN syndrome (n = 2), hereditary cerebellar ataxia (n = 8), and alcohol-induced cerebellar ataxia (n = 3) as well as in MSA-C (n = 4).
Fifth, in terms of a dysfunction of the vestibular system, caloric irrigation pathologies were found in 35%, most commonly indicative of an accompanying bilateral vestibulopathy (16% of cases). Even more patients (49%) showed a bilateral pathological clinical head-impulse test, more commonly in degenerative and hereditary forms of ataxia. It is well known that pseudo-pathological head impulses can be found in cerebellar disorders, which do not have to be of peripheral-vestibular origin [29]. This is similar to findings in a patient cohort of 31 patients presenting with the combination of gait and stance ataxia, cerebellar oculomotor signs, and a bilateral pathological head-impulse test where only 17 out of the 31 patients showed a diminished caloric response in the bithermal testing [30]. Of the 8% with a unilaterally pathological head-impulse test, all patients had unilateral vestibulopathy in caloric irrigation. A group of 8% of patients showed a caloric hyperactivity. Other vestibular abnormalities that were reported in patients with cerebellar disease are a vestibular hyperresponsiveness with increased VOR gain [10] and increased responsiveness of the cervico-ocular reflex [31]. Vestibular hyperreactivity has been discussed as being caused by a dysfunction of olivocerebellar projections with physostigmine as a anticholinergic drug reducing this reactivity [10]. However, in our study caloric hyperactivity was not a common finding; most often DBN syndrome patients showed this pathology in caloric irrigation examination. This finding was associated with pathologic vertical deviation in distant and near view. As vertical misalignment was discussed to be caused by otolith influences and their cerebellar processing [14], there could be a link to vestibular hyperreactivity. However, this remains speculative due to only small numbers of patients with vestibular hyperreactivity.
This study has several limitations. First, we examined a selected patient population in our study cohort, specifically patients who presented in an outpatient clinic for vertigo, dizziness, balance, and gait disorders. We cannot rule out the possibility that some diagnoses might change during the course of the disease as most diagnoses are clinical. Although great efforts were taken to diagnose patients with acquired ataxia, we cannot exclude that some of the participants had, for example, an immune-mediated ataxia. Furthermore, since not all patients underwent genetic testing and we do not have longitudinal follow-up, we cannot exclude the possibility that a substantial portion of the patients classified either as SAOA or with isolated cerebellar oculor motor deficits suffered from an SCA form or might develop MCA-C or CANVAS in their course of disease. Furthermore, the diagnoses were not confirmed by autopsy; therefore, there remains an uncertainty about the final neuropathological diagnoses. For SCA6 patients, for example, it has been well described that symptoms of vertigo and dizziness occurred in most patients, often pre-dating the development of ataxia and that almost all patients showed central oculomotor abnormalities in careful examination [32]. On the other hand, this group of patients may also represent patients with degenerative cerebellar aging, leading to symptoms, but causing only subtle cerebellar oculomotor disturbances. Therefore, from a clinical point of view, a careful examination of eye movements and nystagmus could often be the key to the diagnosis.
Conclusion
This cohort study shows that “cerebellar vertigo and dizziness” is of clinical relevance because it comprises about 10% of all patients presenting with vertigo and dizziness. This is the first study cohort that has been documented clinically and diagnostically in a standardized manner and therefore provides us with clinical information regarding the symptoms of vertigo and dizziness in cerebellar patients as well as the frequency of typical cerebellar clinical, neuro-opthalmological findings, and other diagnostics. Furthermore, our study reveals that a thorough examination of the oculomotor system by an experienced neurologist might be the key to the correct diagnosis and is indispensable, since other cerebellar signs might be missing. Additional instrumental procedures such as posturographic measurements with artificial network analysis might help in the diagnostic process, but have to be interpreted carefully since particularly a vestibular or phobic pattern can also be found in cerebellar patients.
References
Corrales CE, Bhattacharyya N. Dizziness and death: an imbalance in mortality. Laryngoscope. 2016;126(9):2134–6.
Brandt T, Dieterich M, Strupp M. Vertigo and dizziness - common complaints, Vol. 2. Springer; 2013.
Kim JS, Lee H. Vertigo due to posterior circulation stroke. Semin Neurol. 2013;33(3):179–84.
Lee H. Neuro-otological aspects of cerebellar stroke syndrome. J Clin Neurol. 2009;5(2):65–73.
Grimaldi G, Manto M. Topography of cerebellar deficits in humans. Cerebellum. 2012;11(2):336–51.
Bodranghien F, Bastian A, Casali C, Hallett M, Louis ED, Manto M, et al. Consensus paper: revisiting the symptoms and signs of cerebellar syndrome. Cerebellum. 2016;15(3):369–91.
Leigh RJ, Zee DS, editors. The Neurology of Eye Movements. 5th ed. Oxford, New York: Oxford University Press; 2015.
Diener HC, Dichgans J, Bacher M, Gompf B. Quantification of postural sway in normals and patients with cerebellar diseases. Electroencephalogr Clin Neurophysiol. 1984;57(2):134–42.
Baloh RW, Konrad HR, Honrubia V. Vestibulo-ocular function in patients with cerebellar atrophy. Neurology. 1975;25(2):160–8.
Thurston SE, Leigh RJ, Abel LA, Dell'Osso LF. Hyperactive vestibulo-ocular reflex in cerebellar degeneration: pathogenesis and treatment. Neurology. 1987;37(1):53–7.
Klockgether T. Sporadic adult-onset ataxia. Handb Clin Neurol. 2018;155:217–25.
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–6.
Szmulewicz DJ, Roberts L, McLean CA, MacDougall HG, Halmagyi GM, Storey E. Proposed diagnostic criteria for cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS). Neurol Clin Pract. 2016;6(1):61–8.
Hufner K, et al. Esophoria or esotropia in adulthood: a sign of cerebellar dysfunction? J Neurol. 2015;262(3):585–92.
Schneider E, Villgrattner T, Vockeroth J, Bartl K, Kohlbecher S, Bardins S, et al. EyeSeeCam: an eye movement-driven head camera for the examination of natural visual exploration. Ann N Y Acad Sci. 2009;1164:461–7.
Bremova-Ertl T, et al. Oculomotor and vestibular findings in Gaucher disease type 3 and their correlation with neurological findings. Front Neurol. 2017;8:711.
Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, Rosengren SM, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the classification Committee of the Barany Society. J Vestib Res. 2017;27(4):177–89.
Krafczyk S, Tietze S, Swoboda W, Valkovič P, Brandt T. Artificial neural network: a new diagnostic posturographic tool for disorders of stance. Clin Neurophysiol. 2006;117(8):1692–8.
Subramony SH. SARA--a new clinical scale for the assessment and rating of ataxia. Nat Clin Pract Neurol. 2007;3(3):136–7.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. https://www.R-project.org/.
Claassen J, Feil K, Bardins S, Teufel J, Spiegel R, Kalla R, et al. Dalfampridine in patients with downbeat nystagmus--an observational study. J Neurol. 2013;260(8):1992–6.
Claassen J, Spiegel R, Kalla R, Faldon M, Kennard C, Danchaivijitr C, et al. A randomised double-blind, cross-over trial of 4-aminopyridine for downbeat nystagmus--effects on slowphase eye velocity, postural stability, locomotion and symptoms. J Neurol Neurosurg Psychiatry. 2013;84(12):1392–9.
Claassen J, Teufel J, Kalla R, Spiegel R, Strupp M. Effects of dalfampridine on attacks in patients with episodic ataxia type 2: an observational study. J Neurol. 2013;260(2):668–9.
Strupp M, Kalla R, Claassen J, Adrion C, Mansmann U, Klopstock T, et al. A randomized trial of 4-aminopyridine in EA2 and related familial episodic ataxias. Neurology. 2011;77(3):269–75.
Strupp M, Kalla R, Dichgans M, Freilinger T, Glasauer S, Brandt T. Treatment of episodic ataxia type 2 with the potassium channel blocker 4-aminopyridine. Neurology. 2004;62(9):1623–5.
Strupp M, Schuler O, Krafczyk S, Jahn K, Schautzer F, Buttner U, et al. Treatment of downbeat nystagmus with 3,4-diaminopyridine: a placebo-controlled study. Neurology. 2003;61(2):165–70.
Strupp M, Teufel J, Habs M, Feuerecker R, Muth C, van de Warrenburg BP, et al. Effects of acetyl-DL-leucine in patients with cerebellar ataxia: a case series. J Neurol. 2013;260(10):2556–61.
Li X, Wang Y, Wang Z, Xu Y, Zheng W. 3-Hz postural tremor in multiple system atrophy cerebellar type (MSA-C)-a static posturography study. Neurol Sci. 2018;39(1):71–7.
Kremmyda O, et al. False-positive head-impulse test in cerebellar ataxia. Front Neurol. 2012;3:162.
Kirchner H, Kremmyda O, Hüfner K, Stephan T, Zingler V, Brandt T, et al. Clinical, electrophysiological, and MRI findings in patients with cerebellar ataxia and a bilaterally pathological head-impulse test. Ann N Y Acad Sci. 2011;1233:127–38.
Bronstein AM, Hood JD. Cervical nystagmus due to loss of cerebellar inhibition on the cervico-ocular reflex: a case report. J Neurol Neurosurg Psychiatry. 1985;48(2):128–31.
Yu-Wai-Man P, Gorman G, Bateman DE, Leigh RJ, Chinnery PF. Vertigo and vestibular abnormalities in spinocerebellar ataxia type 6. J Neurol. 2009;256(1):78–82.
Acknowledgments
We thank Katie Göttlinger for copy-editing the manuscript. We thank the team of the neuro-orthoptists for the neuro-ophthalmological examination of the patients.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics committee of the Ludwig-Maximilians University Munich and was performed in accordance with the ethical standards according to the 1964 Declaration of Helsinki and its later amendments.
Competing Interests
K. Feil reports no disclosures.
R. Strobl reports no disclosures.
A. Schindler reports no disclosures.
S: Krafczyk reports no disclosures.
N. Goldschagg received honoraria for lecturing from Actelion.
C. Frenzel reports no disclosures.
M. Glaser reports no disclosures.
F. Schöberl reports no disclosures.
A. Zwergal reports no disclosures.
M. Strupp is Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro-otology, and Section Editor of F1000. He has received speaker’s honoraria from Abbott, Actelion, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, MSD, Otometrics, Pierre-Fabre, TEVA, and UCB. He is a shareholder in IntraBio. He acts as a consultant for Abbott, Actelion, AurisMedical, Heel, IntraBio, and Sensorion.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feil, K., Strobl, R., Schindler, A. et al. What Is Behind Cerebellar Vertigo and Dizziness?. Cerebellum 18, 320–332 (2019). https://doi.org/10.1007/s12311-018-0992-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-018-0992-8