Abstract
To date, few studies focused on prediction of functional recovery after cerebellar stroke. The main aim of this prospective pilot study was to determine the association between cerebellar lesion location and functional outcome in adults with acute cerebellar infarction. We examined 14 patients with first-ever unilateral cerebellar ischemic stroke within 7 days and at 90 days from the onset of stroke by means of the International Cooperative Ataxia Rating Scale. Cerebellar lesions were traced from magnetic resonance imaging performed within 72 h since stroke and region of interest were generated. The association between the International Cooperative Ataxia Rating Scale score and lesion location was determined with the voxel-based lesion-symptom mapping methods implemented in the MRIcro software. Colored lesion-symptom maps representing the z statistics were generated and overlaid onto the MNI-ICBM 152 linear probabilistic atlas of the human brain and the Johns Hopkins University white matter templates. Our results documented that injuries to the V, VI, VIIA Crus I, VIIA Crus II, VIIB, VIIIA, and VIIIB lobules and the middle cerebellar peduncle are significantly associated with the International Cooperative Ataxia Rating Scale (ICARS) score at 1 week after the onset of stroke. Furthermore, we found that injuries to the VI, VIIA Crus I, VIIA Crus II, VIIB, VIIIA, and VIIIB lobules, the dentate nucleus, and the middle cerebellar peduncle are significantly associated with the ICARS score at 3 months since the cerebellar stroke onset. The findings of this pilot study might improve prognostic accuracy of functional outcome in patients with acute cerebellar infarction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are three major vessels supplying the cerebellum: superior cerebellar artery (SCA), posterior inferior cerebellar artery (PICA), and anterior inferior cerebellar artery (AICA) [1]. Cerebellar infarction can occur in any of these vascular territories, representing approximately 1.5–2 % of strokes [2, 3]. Despite of it being a quite rare condition, the importance of cerebellar ischemic stroke is considerable because of its nonspecific clinical manifestations in the acute stage [1, 3]. Ataxia is a common disabling symptom of cerebellar stroke that involves disturbances in the control of gait (gait ataxia), voluntary limb movements (limb ataxia), speech (dysarthria), and eye movements (nystagmus) [1, 3, 4].
Decisions on the type, duration, and goals of rehabilitation are based on several factors, including estimates of the patient’s potential for recovery of function [5]. Hence, prognostic accuracy of functional outcome after stroke is important to define an efficient treatment plan and allocate resources [6]. To date, few studies focused on prediction of functional recovery after cerebellar stroke, mainly describing a fast motor recovery of upper limb function after the acute phase, as well as the prognostic role of coexisting age-related white matter changes and the functional status at discharge from acute hospitalization [7–9]. Also, the lesion site was found to be critical for motor recovery after cerebellar stroke, with a significant correlation between lesions of the cerebellar nuclei and ataxia scores that was found in chronic ischemic cerebellar patients [10].
To the best of our knowledge, no previous study prospectively evaluated the functional prognosis of patients with cerebellar ischemic stroke on the base of lesion location. Thus, the main aim of this study was to determine the association between cerebellar lesion location and functional outcome in adults with acute cerebellar infarction. Our secondary aims were to follow up the functional outcome of cerebellar stroke patients, also investigating its relationship with age, comorbidities, and lesion volume.
Patients and Methods
This was a single-center, prospective, pilot study. Fourteen adults presenting with acute ischemic cerebellar stroke (mean age 68.5 years) were consecutively recruited from January 2013 to December 2014. Inclusion criteria were as follows: first-ever unilateral cerebellar ischemic stroke (≤72 h from onset) and high-resolution 1.5T anatomical magnetic resonance imaging (MRI) scans with T2-weighted fluid-attenuated inversion-recovery (FLAIR) and diffusion-weighted imaging (DWI) sequences available. Exclusion criteria were as follows: inclusion in other trials, previous stroke, vestibular disorders, paroxysmal vertigo, recurrence of stroke during the study period, and other neurological disorders or musculoskeletal conditions that might bias motor evaluation. All patients gave their informed consent for participation in the current study, which was carried out according to the Declaration of Helsinki and approved by the local Ethics Committee. During the study period, patients were treated according to the current stroke rehabilitation guidelines [11].
Clinical Assessment
All participants were evaluated within 72 h (T0) as well as 7 days (T1) and 90 days (T2) from the onset of stroke by means of the International Cooperative Ataxia Rating Scale (ICARS), which is a validated 100-point ordinal scale that quantifies ataxia in four categories of movement: posture and gait, limb kinetics, speech, and eye movements (higher scores indicate greater ataxia) [12]. According to the previous literature, we used the ICARS as functional outcome measure of cerebellar infarction [10, 13].
Furthermore, comorbid conditions were evaluated with the Charlson Comorbidity Index (CCI) on the basis of hospital discharge ICD-10-CM. The CCI is the most widely used comorbidity adjustment method. It accounts for multiple comorbidities by creating a sum score, weighted according to the presence of various conditions (19 diseases). The CCI provides evaluations by categorizing the score into 0, 1, 2, and ≥3 [14].
Lesion Tracing and Evaluation
Cerebellar lesions were visually identified as having altered FLAIR and DWI signal intensity compared to corresponding contralateral tissue [15]. Lesion tracing was carried out using the ch2bet anatomical brain template provided with the MRIcro software (http://www.mricro.com), and region of interest (ROI) images were generated [15]. Furthermore, ROI images of each patient were converted into volume of interest (VOI) images using MRIcron software (http://www.mricro.com/mricron). All lesions were traced by a trained image analyst and confirmed by an experienced clinical neurologist, who was blind to all clinical data. In order to improve the power of the lesion overlay analysis, ROI images were transformed to the right side [13, 15].
Lesion Mapping and Statistical Analysis
Association between ICARS scores and VOI images was analyzed with the VBM methods implemented in the nonparametric mapping (NPM) software (provided with the MRIcron software) [15]. The nonparametric Brunner-Munzel statistical analysis for continuous data was used [15]. The level of significance was P < 0.05 and corrected for multiple comparisons with the false discovery rate (FDR) threshold [15]. Colored lesion-symptom maps representing the z statistics were generated and overlaid onto the MNI-ICBM 152 linear probabilistic atlas of the human brain (http://www.bic.mni.mcgill.ca/ServicesAtlases/ICBM152NLin6) and the Johns Hopkins University (JHU) white matter templates (provided with MRIcron software) [15].
Additional statistical analysis was performed with the Statistical Package for Social Science, version 21.0, for Macintosh (SPSS Inc, Chicago, IL). The Wilcoxon signed rank test was used to evaluate the ICARS improvement comparing T1 versus T0 and T2 versus T0 scores. The Spearman rank correlation test was performed to assess the association between the ICARS score, age, CCI, and stroke lesion volume (number of voxels). The alpha level for significance was set at P < 0.05. The Bonferroni correction was used when investigating multiple comparisons (P < 0.025).
Results
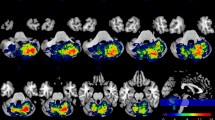
Patients’ demographic and clinical features at T0 are detailed in Table 1. Overlay of lesions for all patients is shown in Fig. 1.
Cerebellar lesion locations that were significantly associated with the ICARS score at T1 and T2 are detailed in Tables 2 and 3 (see also Fig. 2 for the scheme of cerebellar regions).
As to the ICARS, we found a median score of 12 points (interquartile range 9; 20) at T1 and 4 points (interquartile range 1.5; 11.75) at T2, with a significant improvement observed at T1 versus T0 (P = 0.001; Z = −3.301) as well as at T2 versus T0 (P = 0.001; Z = −3.301) comparisons. The Spearman correlation showed a significant direct association between age and the ICARS score at T1 (P = 0.021; ρ = 0.608) and T2 (P = 0.001; ρ = 0.783). No correlation was found between CCI or stroke lesion volume and the ICARS score at T1 and T2.
Discussion
This prospective pilot study was mainly aimed to investigate the anatomy underlying the short-term (7 days) and long-term (90 days) functional outcome (as measured by the ICARS) after acute cerebellar infarction. We documented that injuries to the V, VI, VIIA Crus I, VIIA Crus II, VIIB, VIIIA, and VIIIB lobules and the middle cerebellar peduncle are significantly associated with the ICARS score at 1 week after the onset of stroke. Furthermore, we found that damage to the VI, VIIA Crus I, VIIA Crus II, VIIB, VIIIA, and VIIIB lobules, the dentate nucleus, and the middle cerebellar peduncle is significantly associated with the ICARS score at 3 months after the cerebellar stroke onset. Comparing patients with an infarction of the SCA or the PICA, Bultmann and colleagues reported that within the 3 months after the onset of stroke, patients with infarction of the SCA improved significantly better than those with a stroke in the PICA territory [13]. Furthermore, Schoch and coworkers compared patients with acute (<90 days from onset) and chronic (>90 days since stroke) cerebellar infarction describing that the remaining cerebellar symptoms in the chronic phase of illness were significantly related with lesions of the cerebellar nuclei [10]. These observations are in line with our findings, which showed a greater involvement of cerebellar regions in the PICA territory as well as of the nucleus dentate in the VBM analysis of the association between lesion location and functional outcome (as measured by the ICARS) after cerebellar infarction (see also Tables 2 and 3). Interestingly, we also found that lesions of the middle cerebellar peduncle significantly relate to the ICARS score at T1 and T2 in patients with acute cerebellar ischemic stroke. This was probably because its lesion frequently causes ataxia as well as possibly due to the middle cerebellar peduncle volume, which is greater than other cerebellar peduncles.
As to our secondary aims, in line with Grips and colleagues [9], we found a correlation between age and the ICARS score and we failed to observe a significant role for comorbidity (as measured by the CCI) in the prediction of functional outcome (as measured by the ICARS) after acute cerebellar infarction. Moreover, according to previous literature, the lack of correlation between lesion volume and functional outcome (as measured by the ICARS) observed in our patients further confirms that lesion site may be more important than lesion volume in causing ataxia after ischemic cerebellar stroke [4, 7, 10].
This pilot study has several limitations. First, the sample size was small. Second, we did not recruit patients with infarction in the territory of AICA. Third, we did not include patients with hemorrhagic cerebellar stroke. Fourth, we used the ICARS as the only one functional outcome measure.
Conclusion
Our findings might improve prognostic accuracy of functional outcome in patients with acute cerebellar infarction. Future prospective larger studies including several types of cerebellar stroke and further clinical outcomes are needed to strengthen our results.
References
Datar S, Rabinstein AA. Cerebellar infarction. Neurol Clin. 2014;32:979–91.
Dziadkowiak E, Chojdak-Łukasiewicz J, Guziński M, Noga L, Paradowski B. The usefulness of the TOAST classification and prognostic significance of pyramidal symptoms during the acute phase of cerebellar ischemic stroke. Cerebellum. 2015. doi:10.1007/s12311-015-0676-6.
Kase CS, Norrving B, Levine SR, Babikian VL, Chodosh EH, Wolf PA, et al. Cerebellar infarction. Clinical and anatomic observations in 66 patients. Stroke. 1993;24:76–83.
Deluca C, Moretto G, Di Matteo A, Cappellari M, Basile A, Bonifati DM, et al. Ataxia in posterior circulation stroke: clinical-MRI correlations. J Neurol Sci. 2011;300:39–46.
Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010;9:1228–32.
Picelli A, Tamburin S, Dambruoso F, Midiri A, Girardi P, Santamato A, et al. Topical distribution of initial paresis of the limbs to predict clinically relevant spasticity after ischemic stroke: a retrospective cohort study. Eur J Phys Rehab Med. 2014;50:489–94.
Konczak J, Pierscianek D, Hirsiger S, Bultmann U, Schoch B, Gizewski ER, et al. Recovery of upper limb function after cerebellar stroke: lesion symptom mapping and arm kinematics. Stroke 2010;2191–200.
Kelly PJ, Stein J, Shafqat S, Eskey C, Doherty D, Chang Y, et al. Functional recovery after rehabilitation for cerebellar stroke. Stroke. 2001;32:530–4.
Grips E, Sedlaczek O, Bäzner H, Fritzinger M, Daffertshofer M, Hennerici M. Supratentorial age-related white matter changes predict outcome in cerebellar stroke. Stroke. 2005;36:1988–93.
Schoch B, Dimitrova A, Gizewski ER, Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage. 2006;30:36–51.
Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, et al. Management of adult stroke rehabilitation care: a clinical practice guideline. Stroke. 2005;36:e100–43.
Schoch B, Regel JP, Frings M, Gerwig M, Maschke M, Neuhäuser M, et al. Reliability and validity of ICARS in focal cerebellar lesions. Mov Disord. 2007;22:2162–9.
Bultmann U, Pierscianek D, Gizewski ER, Schoch B, Fritsche N, Timmann D, et al. Functional recovery and rehabilitation of postural impairment and gait ataxia in patients with acute cerebellar stroke. Gait Posture. 2014;39:563–9.
Lim JH, Cheon SH. Analysis of variation of length of stay (LOS) after ischemic and hemorrhagic stroke using the Charlson Comorbidity Index (CCI). J Phys Ther Sci. 2015;27:799–803.
Picelli A, Tamburin S, Gajofatto F, Zanette G, Praitano M, Saltuari L, et al. Association between severe upper limb spasticity and brain lesion location in stroke patients. Biomed Res Int. 2014;2014:162754.
Acknowledgments
We would like to recognize the contribution of Marco Veronese in helping us with the preparation of various figures in the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with Ethical Standards
All patients gave their informed consent for participation in the current study, which was carried out according to the Declaration of Helsinki and approved by the local Ethics Committee.
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Picelli, A., Zuccher, P., Tomelleri, G. et al. Prognostic Importance of Lesion Location on Functional Outcome in Patients with Cerebellar Ischemic Stroke: a Prospective Pilot Study. Cerebellum 16, 257–261 (2017). https://doi.org/10.1007/s12311-015-0757-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0757-6