Abstract
Because the cerebellum plays a role in motor coordination, timing, sequencing, and feedback, it is hypothesized to be involved in swallowing-related functions. The role of the cerebellum in deglutition has become increasing evident, but the exact nature of this role remains inconclusive because of limited data from pure cerebellar lesions. Therefore, we conducted location analysis in isolated cerebellar lesions to complement previous findings and provide additional information. We reviewed 40 stroke patients with isolated cerebellar lesion. Lesion location and volume were measured on brain magnetic resonance images. We generated statistical maps of lesions related to VDS using voxel-based lesion symptom mapping (VLSM). We also created an overlay map of subgroups according to VDS score, those who have low risk and those who have high risk. Patients with cerebellar lesion had difficulty swallowing, both in the oral and pharyngeal phases. Multivariate analysis of cognitive function was selected as an independent predictor. In the group of high-risk patients, the overlay map showed some bilateral asymmetry, with a wider distribution in the left hemisphere and involvement of deep cerebellar nuclei. Using VLSM, we found that lesion location was associated with dysphagia. Although these results were not statistically significant, they showed a lesion pattern with predominant distribution in the left posterior lobe. Our results suggest that damage to the posterior lobe of the left cerebellum tends be related to severity of dysphagia in patients with isolated cerebellar lesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Swallowing is a complex process that involves voluntary and reflexive motor control (Zald and Pardo 1999). Functional swallowing requires coordination of many systems and levels, including several cortical and subcortical structures, the brainstem, five pairs of cranial nerves, and various muscles of the face and neck. Impairment in any of these organs can disrupt the normal process of swallowing, leading to dysphagia (Robbins et al. 1999). Post-stroke dysphagia is a common problem, and the incidence of swallowing problems has been associated with increased risk for pulmonary complications and even mortality (Marik and Kaplan 2003). Therefore, early diagnosis of and therapy options for dysphagia have become increasingly important in medical care in stroke units or neurorehabilitation facilities (Ickenstein et al. 2012).
Even though it is well known that cerebral infarction can induce dysphagia, the regions that impact and cause swallowing problems and the types of brain lesions likely to lead to specific patterns of dysphagia are unknown. Numerous studies have discussed the association between brain lesion and dysphagia (Moon et al. 2012; Robbins et al. 1993; Saito et al. 2016). Right hemispheric lesions of the pre- and post-central gyri, opercular region, supramarginal gyrus, and respective subcortical white matter tracts were related to dysphagia, with post-central lesions especially associated with severe swallowing impairment (Suntrup et al. 2015). However, the effect of lesion location on the pattern of dysphagia is unclear.
The cerebellum acts to monitor execution and adjust to planning motor movement (Brunker and Shah 2001). Given the suggested role of the cerebellum in motor coordination, timing, sequencing, and feedback, a supportive role for the cerebellum is hypothesized in swallowing-related functions. Studies in nonhuman primates have provided evidence about movements associated with swallowing (Mussen 1930; Berntson et al. 1973; Martner 1975; Lisander and Martner 1975). One previous study reported impacts on eating and grooming behavior in cats following stimulation of fastigial nucleus (Berntson et al. 1973), and another study showed that stimulation of the fastigial nucleus elicited oral behaviors critical for grooming and chewing and suggested a role of the cerebellum in masticatory movements in cats (Martner 1975).
In addition, some studies have assessed the possible role of the cerebellum in swallowing in human subjects, most using imaging techniques. Zald and Pardo analyzed regional cerebral blood flow using positron emission tomography (PET) in healthy subjects during swallowing and reported various activation patterns including the left cerebellum, especially the Crus Ia/VI region (Zald and Pardo 1999). Suzuki et al. used functional magnetic resonance imaging (fMRI) to assess volitional saliva swallowing and revealed that bilateral cerebellar activation was associated with prominent activation of the left cerebellar hemisphere (Suzuki et al. 2003). Another study proposed a positive role of the cerebellum in capsule swallowing alone but not in swallowing other bolus consistencies (Shibamoto et al. 2007).
A limited number of studies has been performed to assess dysphagia in patients with cerebellar lesions, and the results are inconclusive. Some of the studies reported that cerebellar lesions were not associated with dysphagia symptoms (Flowers et al. 2011; Moon et al. 2012). The study by Dehaghani et al. in 116 stroke patients did not find cerebellar damage to be significantly associated with dysphagia (Dehaghani et al. 2016). More recent studies have not shown any significant association between cerebellar lesions and dysphagia (Mo et al. 2018; Fernandez-Pombo et al. 2019). However, other studies have reported symptoms of dysphagia in cases with cerebellar lesion (Alberts et al. 1992; Isono et al. 2013).
The role of the cerebellum in deglutition has become increasing evident, but the data focused on cerebellar lesions are limited, and the exact nature of this role remains inconclusive. Therefore, we performed lesion location analysis in isolated cerebellar lesion to complement previous findings and provide additional information. We used the voxel-based lesion symptom mapping (VLSM) technique to statistically assess the effect of a lesion on behavioral scores on a voxel-by-voxel basis. In each voxel within the lesion, a statistical test was conducted to determine if a difference in behavioral scores existed between lesioned and non-lesioned groups. Groups were assigned at each new voxel location based on the presence of lesion (Bates et al. 2003).
The aim of this study was to investigate the characteristics and risk factors of dysphagia. These factors were assessed with the videofluoroscopic dysphagia scale (VDS) using a videofluoroscopic swallowing study (VFSS) in patients with isolated lesion in the cerebellum. We also aimed to define the lesion location that was associated with dysphagia after adjusting for other relevant factors using VLSM.
Materials and methods
Subjects
From January 2005 to May 2019, from the total patients admitted to the department of rehabilitation after stroke, 40 were included in this study. Diagnosis of stroke was limited to cases of cerebellar infarction or hemorrhage verified by magnetic resonance imaging (MRI). We included patients with first-time stroke that was restricted to the cerebellum and who were undergoing inpatient rehabilitation therapy at our institution. The inclusion criteria were as follows: (1) first-ever stroke restricted to the cerebellum, (2) age 18 years or older, and (3) elapsed time of 3 months or less after stroke onset. The exclusion criteria were as follows: (1) missing medical records or brain MRI scans; (2) previous history of stroke, dementia, or other disease that could cause difficulty in swallowing; and (3) severe cognitive deficit (Mini-Mental Status Examination (MMSE) score < 10 points). Ultimately, a total of 40 patients satisfied the criteria and were included for analysis; data from these patients were collected retrospectively. The study protocol was approved by the institutional review board of our institution, and the requirement for informed consent was waived due to the retrospective design of the study.
Methods
Review of medical records: The medical records and VFSS results of the patients were reviewed retrospectively. Patient demographic and clinical characteristics were recorded, including age, sex, brain lesion laterality (left, right, or bilateral), and duration between onset of stroke and VFSS (number of days).
Videofluoroscopic swallowing study: Physicians of the Department of Rehabilitation Medicine conducted the VFSS according to a modified version of Logemann’s procedure (Logemann et al. 1977). Briefly, subjects were seated, and fluoroscopy was performed as the patients swallowed barium mixed with juice, yogurt, thick gruel, or rice. For juice and yogurt, two different volumes were used (2 ml or 5 ml, respectively). Each food type was mixed with undiluted liquid barium to ensure proper bolus observation during the fluoroscopy procedure.
Interpretation of VFSS: In the oral phase, functions such as lip sealing, bolus formation, mastication, early spillage, oral remnants, and oral transit time of the bolus were assessed. In the pharyngeal phase, laryngeal elevation and aspiration or penetration of the respiratory tract were assessed. We evaluated clinical dysphagia characteristics and VFSS findings in each patient group. The severity of dysphagia can be quantitatively evaluated with VDS using a numerical scale (from 0 to 100). Lip closure, bolus formation, mastication, apraxia, tongue-to-palate contact, premature bolus loss, oral transit time, triggering of pharyngeal swallowing, vallecular residue, laryngeal elevation, pyriform sinus residue, pharyngeal wall coating, pharyngeal transit time, and aspiration were evaluated (Kim et al. 2014). The subjects were divided into high- (> 47) and low-risk groups (< 47) based on VDS score.
Image analysis: Lesion location was determined first by visual inspection performed by a single rater. To determine lesion volume, binary lesion masks were produced manually by segmenting the lesion area on all consecutive sections that displayed the lesion. All lesions were mapped using MRIcron software (http://www.mccauslandcenter.sc.edu/mricro/mricron/) and were drawn manually on individual fluid-attenuated inversion recovery (FLAIR) scans by a single researcher. FLAIR hyperintensities with no corresponding diffusion-restriction, representing leukoaraiosis or silent old stroke lesions (with no corresponding DWI lesion), were not included in stroke lesion segmentation. For hemorrhagic lesions, the perilesional edema was included in the ROI if restriction of diffusion was present. We conducted a second consensus review with minor adjustments of lesion margins among researchers. FLAIR images were co-registered with the same subject’s T1 MRI, and the FLAIR lesion maps were normalized to Montreal Neurologic Institute (MNI) spacing using T1 MRI normalization parameters and statistical parametric mapping (SPM) 12 (Wellcome Department of Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). We adopted the widely used template MNI-152, designed by adapting the 3D MRI brain scans of 152 healthy individuals to the Talairach and Tournoux template (Maintz and Viergever 1998). Normalized lesions were subjected to statistical mapping analysis using VLSM algorithms implemented with a statistical non-parametric mapping (SnPM) toolbox (http://www2.warwick.ac.uk/fac/sci/statistics/staff/academic-research/nichols/software/snpm). The VLSM analysis identified clusters of voxels with significant t-values comparing voxel-wise subject clinical measures (VDS) with lesions to those without lesions. Statistical significance was determined following permutation correction at p < 0.05 FWE correction for comparisons and controlling for K-MMSE as a nuisance covariate in the multivariate model. All voxels defining the lesion (1 voxel = 1 mm3) were counted using MRIcron software. Additionally, uncorrected threshold results associated with the VDS for semisolid food were presented to show the trending lesion areas.
Statistical analysis
Descriptive statistics were used to present the clinical data. The patients were divided into two groups according to total VDS score for semisolid food to investigate the characteristics. Group allocation was performed according to VDS score (Han et al. 2008). Han et al. established a cut-off of 47 points, above which most dysphagia patients were not able to orally ingest food at 6 months after onset of stroke. Therefore, we divided the subjects into two groups according to VDS at a cut-off score of 47. The Mann–Whitney U test was conducted for comparative analysis of the two groups. Multivariate logistic regression analysis was performed to analyze the predictors that affect VDS. The 95% confidence intervals and equivalent p values were calculated using linear logistic regression analysis. The SPSS statistical package (IBM SPSS Statistics for Windows, Version 21.0; IBM, Armonk, NY, USA) was used for all statistical analyses. Statistical significance was defined as p < 0.05.
Results
Patient general characteristics
General demographic characteristics and clinical variables of the patients are listed in Table 1. Of the 40 patients, 24 were males, and mean age was 64.02 ± 13.21 years. A mean time duration of 18.9 ± 6.2 days elapsed until the VFSS. The mean baseline K-MMSE score was 17.71 ± 8.45 points. Characteristics of dysphagia shown in VFSS in cerebellar stroke are shown in Table 2. Both oral phase and pharyngeal phase problems were observed in patients with cerebellar lesion.
Predictors of dysphagia
Differences between patients with low risk (VDS < 47) and patients with high risk (VDS > 47) are shown in Table 3. There were no significant differences in the two groups for the following variables at baseline: age, sex, lesion side, lesion volume, and interval between stroke onset and VFSS. However, insertion of a tracheal tube, K-MMSE, and initial FIM score differed significantly between the two subgroups. All significant parameters were adjusted in the multivariate analysis, as shown in Table 3. The K-MMSE parameter independently predisposed patients for dysphagia after stroke in cases with cerebellar lesion (p = 0.002). Patient sex, lesion laterality, interval between stroke onset and VFSS, tracheal tube, lesion volume, and initial FIM score were not included as significant factors in the regression model for high-risk dysphagia (Table 4).
Lesion location association with dysphagia in cerebellar lesions using VLSM
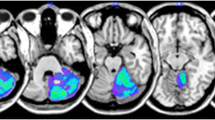
The overall distribution of cerebellar damage among participants is shown in Fig. 1. Based on a design that included K-MMSE score as an interfering covariate, the VLSM results revealed no significant area for dysphagia. Therefore, uncorrected threshold results associated with dysphagia are presented in Fig. 2 to show the trending lesion areas. Although these results did not reach statistical significance, they showed that lesion pattern had a predominant distribution in the left cerebellar posterior lobe. We also created an overlay map of subgroups according to VDS score, those who have low risk (VDS < 47) and those who have high risk (VDS > 47). To characterize the lesions from subgroups according to VDS, a lesion overlay map was created (Fig. 3). There were some disparities in the summed lesion maps of the two groups. The lesion map was wider in the high-risk group of patients. In the group of high-risk patients, the overlay map showed some bilateral asymmetry, with a wider distribution in the left hemisphere and involvement of deep cerebellar nuclei.
Lesion overlay map of 40 patients. A lesion overlay map of 40 patients showing bilateral coverage of most of the cerebellar hemispheres. The lesions appear to be more widely distributed in left hemispheres. Numbers are the z-coordinates of the MNI space, with the patient’s left hemisphere on the right side of the figure. MNI Montreal Neurologic Institute
Lesion patterns associated with VDS. Voxel-based lesion symptom mapping analysis shows the relationship between continuous VDS score for semisolid and brain lesions (uncorrected p value). The results controlling K-MMSE score as nuisance covariates are color-coded, ranging from red to yellow. Numbers are z-coordinates of the MNI space, with the patient’s left hemisphere on the right side of the figure. VDS videofluoroscopic dysphagia scale, MNI Montreal Neurologic Institute
Lesion overlay map for characterization of lesions from subgroups according to VDS. A Lesion overlay map of patients with high risk (VDS > 47). B Lesion overlay map of patients with low risk (VDS < 47). The lesion overlay map of the high-risk group was distributed in the bilateral lobe and showed a tendency to involve deep nuclei of the cerebellum, and the lesion overlay map of the low-risk group was distributed mainly in the right lobe rather than the left lobe. Numbers are the z-coordinates of the MNI space, with the patient’s left hemisphere on the right side of the figure. VDS videofluoroscopic dysphagia scale, MNI Montreal Neurologic Institute
Discussion
This study demonstrates that patients with cerebellar lesion had difficulty swallowing, both in the oral and pharyngeal phases. The cerebellum acts to monitor motor execution, effectively compare intended body movement with actual behavior, and to make adjustments to the motor plan accordingly (Brunker and Shah 2001). The cerebellum has long been known to be involved in movement. Although movements are initiated in the cerebral cortex, the cerebellum plays a key role in ensuring muscular activity is accurate, smooth, and coordinated (Roostaei et al. 2014). Given this function of the cerebellum, swallowing problems can occur in both the oral and pharyngeal phases when cerebellar lesions are present. Impaired coordination of oral muscles can cause problems in mastication and bolus formation, which can cause delays in the oral phase or drooling and difficulties with controlling food in the mouth. A previously reported nonhuman primate study suggested that muscle contraction of the oral cavity was triggered when the ventral parts of the vermis were triggered (Mussen 1930). Additionally, stimulation of the fastigial nucleus elicited oral behaviors critical for chewing, which suggested a role of the cerebellum in mastication in cats (Martner 1975). There can be challenges for synchronization of the pharyngeal muscles during swallowing because of poor coordination, tremor, or erratic contraction, which can lead to penetration or aspiration.
Also, diseases that affect the cerebellum have been demonstrated to cause dysphagia. This is likely due to disruption of the motor modulatory influence of the cerebellum on cerebral motor cortical areas. Chronic neurodegenerative conditions such as cerebellar ataxia, multiple system atrophy (MSA), and multiple sclerosis (MS) have been shown to be associated strongly with dysphagia (Almotairi et al. 2018; Lee et al. 2018; Sulena et al. 2017). Dysphagia is a frequent finding in patients with posterior fossa tumor and has been reported in a few patients with cerebellar involvement (Lapa et al. 2020).
A previous study examined the effect of TMS (transcranial magnetic stimulation) of the cerebellum on pharyngeal constrictor muscles; the study indicated that the cerebellum can act to intensify or modulate cortical firing for swallowing (Jayasekeran et al. 2011). A recent study suggested that bilateral cerebellar rTMS (repetitive transcranial magnetic stimulation) facilitates corticobulbar pathways to the pharynx in healthy adults (Sasegbon et al. 2020). All these aforementioned studies showed that the cerebellum has a role in swallowing both in oral and pharyngeal phases. Likewise, the results of our study indicated that patients with cerebellar lesions showed effects in swallowing phases, both oral and pharyngeal.
In this study, two groups were formed according to VDS, and the results were statistically different based on presence of tracheal tube and cognitive function. There was a difference in FIM between the two groups. This result might be attributable to patient severity. However, when multivariate analysis was performed with factors that were significant in univariate analysis, cognitive function was an independent predictor. In patients with decreased cognition, oral phase delay is common, and there can be difficulties in following instruction during the examination, which can affect the outcome. Based on our observations, a cerebellar cognitive affective disorder is another possibility. Because the cerebellum is involved in not only motor, but also cognition and mood, if there is an injury to the cerebellum, cognitive dysfunction can appear in the patient and can affect dysphagia. In 1998, Schmahmann and Sherman introduced cerebellar cognitive affective syndrome (CCAS), which was characterized by disturbances of executive function, impaired visuospatial cognition, personality change, and linguistic difficulties that resulted in a general decline in intellectual functioning (Schmahmann and Sherman 1998). Compared to controls, cerebellar patients perform significantly worse at processing speed, executive functions, memory, language, and visuospatial functions (Nazarahari et al. 2019). All these components could affect the voluntary process of swallowing. There was a possibility that the symptoms could be associated with apraxia. Accumulating evidence from recent anatomo-clinical studies of patients with neurodevelopmental and acquired neurological disorders affecting the cerebellum indicate that the cerebrocerebellar network is directly implicated in the pathophysiology of forms of apraxia such as apraxia of speech, apraxic agraphia, constructional apraxia, drawing apraxia, and mastication dyspraxia (Marien et al. 2015). We used K-MMSE for cognitive evaluation in this study, however, previous study reported that the patients with cerebellar injury performed within the published normal ranges on both the MMSE and the MoCA. This can be explained by the fact that MMSE and MoCA contain many test items that are insensitive to cognitive functions that are compromised in cerebellar patients (Hoche et al. 2018). Therefore, the use of MMSE to evaluate cognitive function in this study might not have sufficiently detected the deficits associated with CCAS, which is a limitation of this study.
Another possibility is combined presence of hypertrophic olivary degeneration (HOD). HOD presents on MRI with abnormal T2 hyperintense signal in the inferior olive that can be accompanied by abnormal hypertrophy of the olive (Hirano et al. 2015). Lesions involving the Guillain–Mollaret triangle linking the dentate nucleus, red nucleus, and inferior olivary nucleus have been associated with palatal tremor and might be associated with dysphagia. HOD is not unlikely to occur after cerebellar stroke lesions and can lead to palatal tremor with dysphagia (Lee et al. 2021). Unfortunately, we could not evaluate the presence of palatal tremor in a clinical exam due to the retrospective study design; however, such tremor might contribute to development of dysphagia.
Our secondary goal was to investigate whether lesion anatomy predicts dysphagia in isolated cerebellar lesions after adjusting for other related factors using neuroimaging analysis. In the overlay map, the lesions appeared to be more widely distributed in the left hemisphere even though there were fewer patients with left sided cerebellar stroke. A large proportion (37.5%) of the patients had bilateral lesions. Therefore, it is difficult to directly compare the volume of the right and left lesions. The median volumes of the right, left, and bilateral lesion groups were 22, 18, and 26, respectively. It is possible that the group with bilateral lesions showed a more widely biased lesion pattern to the left. The lesion map was wider in the high-risk group of patients. In the group of high-risk patients, the overlay map showed some bilateral asymmetry, with a wider distribution in the left hemisphere, and showed involvement of deep cerebellar nuclei. The cerebellar deep nuclei have been identified to have harmful effects on complex functional cerebrocerebellar connectivity and cerebello-thalamo-cortical networks if damaged. The cerebellar cortex projects to the deep cerebellar nuclei that mediate all output from the cerebellum. The largest of these nuclei, the dentate, is a target of powerful convergence from the lateral cerebellar hemispheres. Most of the output from the dentate nucleus ascends back to the cerebral cortex (Sokolov et al. 2017).
Suzuki et al. reported bilateral activations in the sensorimotor cortex, thalamus, putamen, and globus pallidus during dry swallowing. Insular activation was reported to be more prominent in the right hemisphere, whereas cerebellar activation was more prominent in the left (Suzuki et al. 2003). In terms of motor learning functions, it has been reported that the left cerebellum is more strongly activated in unimanual movements (Matsumura et al. 2004). The hypothesis that the left cerebellum dominates in motor skill learning is supported by a structural comparison of musicians and non-musicians using voxel-based morphometry that showed a greater volume of gray matter in the left cerebellum of pianists (Gaser and Schlaug 2003). Like motor learning functions, cognitive spatial operations are left-lateralized, while linguistic skill is correlated with the right cerebellum (Hu et al. 2008; Berl et al. 2014). Although lateralization of the cerebellum related to swallowing has not been elucidated, it is thought that the same will be the case with swallowing as there is functional lateralization for other functions.
We performed VLSM analysis on the VDS score after adjusting for degree of cognitive impairment as identified by regression analysis. In the VLSM results with FEW (familywise error correction), no brain area was significantly correlated with VDS score. Although they did not reach statistical significance, the VLSM results for VDS (with uncorrected thresholds) showed a lesion pattern mainly distributed in the left posterior region in the cerebellum. Knowing which voxels have a substantially lower association with the experimental variable could provide valuable information representing statistical evidence of effect size in different parts of the map (Jernigan et al. 2003). Few investigations have reported dysphagia in individuals with cerebellar lesions, and the results regarding potential patterns of dysphagia are inconclusive. Some case studies have focused on patients with cerebellar lesion presenting with dysphagia (Min et al. 1999; Zuketto and van Gijn 2002; Massey et al. 1984; Nagahiro et al. 1993). The damage in the cerebellum comprised infarctions in the proximal segment of the vertebral artery, an area VI lesion with tandem intracranial occlusive disease, and lesions to anterior and posterior inferior cerebellar arteries. In our study, the lesion pattern was distributed predominantly in the left cerebellar posterior lobe. According to previous studies on involvement of the cerebellum in normal swallowing, the crus Ia/VI lobule region of the left hemisphere, superior portions of both cerebellar hemispheres, and the bilateral posterior part of the cerebellum were discussed and thought to be correlated with swallowing function (Zald and Pardo 1999; Suzuki et al. 2003; Malandraki et al. 2009; Grabski et al. 2012). In a previous cerebellar lesion analysis, damage to the left cerebellar hemisphere was significantly correlated with post-stroke depression, which can indicate laterality of depression-related cerebellar function (Kim et al. 2017). The result of the previous study could have associations with the results of our study. Dysphagia has been reported to occur frequently in depressed patients with cerebrovascular incident (Saxena et al. 2008). Depressed stroke patients can be at risk for dysphagia as the result of reduced motivation and concentration. Conversely, people with dysphagia can become depressed because they cannot eat as desired. The role of the cerebellum in emotional control is neuroanatomically based on connections between the cerebellum and non-motor cortical and subcortical areas, which have been documented by functional neuroimaging studies (Rapoport et al. 2000).
There are some limitations to our study including the retrospective study design and relatively small sample size. However, although imaging was assessed retrospectively, videofluoroscopic swallowing evaluations were performed according to a standard method. Moreover, clinical data are routinely collected in patient admission records. The major drawback of this study is the lack of flexible endoscopic evaluation of swallowing (FEES) data, which would provide more insight into swallowing problems.
We used K-MMSE, which is a simple screening tool for cognitive impairment. Therefore, we could not analyze the various domains of cognitive function, i.e., attention, memory, and executive functions. Finally, we could not assess subject depression, which could accompany cerebellar lesion and influence the results of VFSS, especially in the oral phase. Thus, further studies with a prospective design are needed to collect serial long-term follow-up data that allow analysis of critical predictive factors of dysphagia in isolated cerebellar lesion.
Conclusion
Our results suggest that damage to the posterior lobe of the left cerebellum tends to be related to severity of dysphagia in patients with isolated cerebellar lesion.
References
Alberts MJ, Horner J, Gray L, Brazer SR (1992) Aspiration after stroke: lesion analysis by brain MRI. Dysphagia 7(3):170–173. https://doi.org/10.1007/BF02493452
Almotairi FS, Andersson M, Andersson O, Skoglund T, Tisell M (2018) Swallowing dysfunction in adult patients with chiari I malformation. J Neurol Surg B Skull Base 79(6):606–613. https://doi.org/10.1055/s-0038-1655758
Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003) Voxel-based lesion-symptom mapping. Nat Neurosci 6(5):448–450. https://doi.org/10.1038/nn1050
Berl MM, Mayo J, Parks EN, Rosenberger LR, VanMeter J, Ratner NB, Vaidya CJ, Gaillard WD (2014) Regional differences in the developmental trajectory of lateralization of the language network. Hum Brain Mapp 35(1):270–284. https://doi.org/10.1002/hbm.22179
Berntson GG, Potolicchio SJ Jr, Miller NE (1973) Evidence for higher functions of the cerebellum: eating and grooming elicited by cerebellar stimulation in cats. Proc Natl Acad Sci U S A 70(9):2497–2499. https://doi.org/10.1073/pnas.70.9.2497
Brunker C, Shah S (2001) Systems and diseases. Exploring normal anatomy and physiology. Nervous system 11: Brain tumours. Nurs times 97(26):43–46
Dehaghani SE, Yadegari F, Asgari A, Chitsaz A, Karami M (2016) Brain regions involved in swallowing: evidence from stroke patients in a cross-sectional study. J Res Med Sci 21:45. https://doi.org/10.4103/1735-1995.183997
Fernandez-Pombo A, Seijo-Raposo IM, Lopez-Osorio N, Canton-Blanco A, Gonzalez-Rodriguez M, Arias-Rivas S, Rodriguez-Yanez M, Santamaria-Nieto A, Diaz-Ortega C, Gomez-Vazquez E, Martinez-Olmos MA (2019) Lesion location and other predictive factors of dysphagia and its complications in acute stroke. Clin Nutr Espen 33:178–182. https://doi.org/10.1016/j.clnesp.2019.05.019
Flowers HL, Skoretz SA, Streiner DL, Silver FL, Martino R (2011) MRI-based neuroanatomical predictors of dysphagia after acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis 32(1):1–10. https://doi.org/10.1159/000324940
Gaser C, Schlaug G (2003) Brain structures differ between musicians and non-musicians. J Neurosci 23(27):9240–9245
Grabski K, Lamalle L, Vilain C, Schwartz JL, Vallee N, Tropres I, Baciu M, Le Bas JF, Sato M (2012) Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp 33(10):2306–2321. https://doi.org/10.1002/hbm.21363
Han TR, Paik NJ, Park JW, Kwon BS (2008) The prediction of persistent dysphagia beyond six months after stroke. Dysphagia 23(1):59–64. https://doi.org/10.1007/s00455-007-9097-0
Hirano M, Hatzoglou V, Karimi S, Young RJ (2015) Hypertrophic olivary degeneration resulting from posterior fossa masses and their treatments. Clin Imaging 39(5):787–790. https://doi.org/10.1016/j.clinimag.2015.05.015
Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD (2018) The cerebellar cognitive affective/Schmahmann syndrome scale. Brain 141(1):248–270. https://doi.org/10.1093/brain/awx317
Hu D, Shen H, Zhou Z (2008) Functional asymmetry in the cerebellum: a brief review. Cerebellum 7(3):304–313. https://doi.org/10.1007/s12311-008-0031-2
Ickenstein GW, Hohlig C, Prosiegel M, Koch H, Dziewas R, Bodechtel U, Muller R, Reichmann H, Riecker A (2012) Prediction of outcome in neurogenic oropharyngeal dysphagia within 72 hours of acute stroke. J Stroke Cerebrovasc Dis 21(7):569–576. https://doi.org/10.1016/j.jstrokecerebrovasdis.2011.01.004
Isono C, Hirano M, Sakamoto H, Ueno S, Kusunoki S, Nakamura Y (2013) Differences in dysphagia between spinocerebellar ataxia type 3 and type 6. Dysphagia 28(3):413–418. https://doi.org/10.1007/s00455-013-9450-4
Jayasekeran V, Rothwell J, Hamdy S (2011) Non-invasive magnetic stimulation of the human cerebellum facilitates cortico-bulbar projections in the swallowing motor system. Neurogastroenterol Motil 23(9):831-e341. https://doi.org/10.1111/j.1365-2982.2011.01747.x
Jernigan TL, Gamst AC, Fennema-Notestine C, Ostergaard AL (2003) More “mapping” in brain mapping: statistical comparison of effects. Hum Brain Mapp 19(2):90–95. https://doi.org/10.1002/hbm.10108
Kim J, Oh BM, Kim JY, Lee GJ, Lee SA, Han TR (2014) Validation of the videofluoroscopic dysphagia scale in various etiologies. Dysphagia 29(4):438–443. https://doi.org/10.1007/s00455-014-9524-y
Kim NY, Lee SC, Shin JC, Park JE, Kim YW (2017) Voxel-based lesion symptom mapping analysis of depressive mood in patients with isolated cerebellar stroke: a pilot study. Neuroimage Clin 13:39–45. https://doi.org/10.1016/j.nicl.2016.11.011
Lapa S, Quick-Weller J, Nasari C, Dziewas R, Gessler F, Wagner M, Warnecke T, Hattingen E, Seifert V, Konczalla J (2020) Pre- and post-surgical dysphagia in adults with tumors of the posterior fossa: a prospective blinded study. Cancers. https://doi.org/10.3390/cancers12092561
Lee HH, Seo HG, Kim KD, Lee SH, Lee WH, Oh BM, Lee WW, Kim Y, Kim A, Kim HJ, Jeon B, Han TR (2018) Characteristics of early oropharyngeal dysphagia in patients with multiple system atrophy. Neurodegener Dis 18(2–3):84–90. https://doi.org/10.1159/000487800
Lee S, Moon HI, Shin JH (2021) Post-stroke palatal tremor as a clinical predictor of dysphagia and its neuroanatomical correlates in patients with midbrain and pontine lesions. J Neural Transm. https://doi.org/10.1007/s00702-021-02417-w
Lisander B, Martner J (1975) Effects on gastric motility from the cerebellar fastigial nucleus. Acta Physiol Scand 94(3):368–377. https://doi.org/10.1111/j.1748-1716.1975.tb05896.x
Logemann JA, Boshes B, Blonsky ER, Fisher HB (1977) Speech and swallowing evaluation in the differential diagnosis of neurologic disease. Neurol Neurocir Psiquiatr 18(2–3 Suppl):71–78
Maintz JB, Viergever MA (1998) A survey of medical image registration. Med Image Anal 2(1):1–36. https://doi.org/10.1016/s1361-8415(01)80026-8
Malandraki GA, Sutton BP, Perlman AL, Karampinos DC, Conway C (2009) Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp 30(10):3209–3226. https://doi.org/10.1002/hbm.20743
Marien P, van Dun K, Verhoeven J (2015) Cerebellum and apraxia. Cerebellum 14(1):39–42. https://doi.org/10.1007/s12311-014-0620-1
Marik PE, Kaplan D (2003) Aspiration pneumonia and dysphagia in the elderly. Chest 124(1):328–336. https://doi.org/10.1378/chest.124.1.328
Martner J (1975) Cerebellar influences on autonomic mechanisms. An experimental study in the cat with special reference to the fastigial nucleus. Acta Physiol Scand Suppl 425:1–42
Massey CE, El Gammal T, Brooks BS (1984) Giant posterior inferior cerebellar artery aneurysm with dysphagia. Surg Neurol 22(5):467–471. https://doi.org/10.1016/0090-3019(84)90304-5
Matsumura M, Sadato N, Kochiyama T, Nakamura S, Naito E, Matsunami K, Kawashima R, Fukuda H, Yonekura Y (2004) Role of the cerebellum in implicit motor skill learning: a PET study. Brain Res Bull 63(6):471–483. https://doi.org/10.1016/j.brainresbull.2004.04.008
Min WK, Kim YS, Kim JY, Park SP, Suh CK (1999) Atherothrombotic cerebellar infarction: vascular lesion-MRI correlation of 31 cases. Stroke 30(11):2376–2381. https://doi.org/10.1161/01.str.30.11.2376
Mo SJ, Jeong HJ, Han YH, Hwang K, Choi JK (2018) Association of brain lesions and videofluoroscopic dysphagia scale parameters on patients with acute cerebral infarctions. Ann Rehabil Med 42(4):560–568. https://doi.org/10.5535/arm.2018.42.4.560
Moon HI, Pyun SB, Kwon HK (2012) Correlation between location of brain lesion and cognitive function and findings of videofluoroscopic swallowing study. Ann Rehabil Med 36(3):347–355. https://doi.org/10.5535/arm.2012.36.3.347
Mussen AT (1930) The cerebellum: a new classification of the lobes based on their reactions to stimulation. Arch Neurol Psychiatry 23(3):411–459. https://doi.org/10.1001/archneurpsyc.1930.02220090002001
Nagahiro S, Goto S, Yoshioka S, Ushio Y (1993) Dissecting aneurysm of the posterior inferior cerebellar artery: case report. Neurosurgery 33(4):739–741. https://doi.org/10.1227/00006123-199310000-00027 (discussion 741–732)
Nazarahari M, Noamani A, Ahmadian N, Rouhani H (2019) Sensor-to-body calibration procedure for clinical motion analysis of lower limb using magnetic and inertial measurement units. J Biomech 85:224–229. https://doi.org/10.1016/j.jbiomech.2019.01.027
Rapoport M, van Reekum R, Mayberg H (2000) The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci 12(2):193–198. https://doi.org/10.1176/jnp.12.2.193
Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB (1993) Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil 74(12):1295–1300. https://doi.org/10.1016/0003-9993(93)90082-l
Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J (1999) Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia 14(4):228–232. https://doi.org/10.1007/PL00009610
Roostaei T, Nazeri A, Sahraian MA, Minagar A (2014) The human cerebellum: a review of physiologic neuroanatomy. Neurol Clin 32(4):859–869. https://doi.org/10.1016/j.ncl.2014.07.013
Saito T, Hayashi K, Nakazawa H, Ota T (2016) Clinical characteristics and lesions responsible for swallowing hesitation after acute cerebral infarction. Dysphagia 31(4):567–573. https://doi.org/10.1007/s00455-016-9716-8
Sasegbon A, Smith CJ, Bath P, Rothwell J, Hamdy S (2020) The effects of unilateral and bilateral cerebellar rTMS on human pharyngeal motor cortical activity and swallowing behavior. Exp Brain Res 238(7–8):1719–1733. https://doi.org/10.1007/s00221-020-05787-x
Saxena SK, Ng TP, Yong D, Fong NP, Koh G (2008) Subthreshold depression and cognitive impairment but not demented in stroke patients during their rehabilitation. Acta Neurol Scand 117(2):133–140. https://doi.org/10.1111/j.1600-0404.2007.00922.x
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121(Pt 4):561–579. https://doi.org/10.1093/brain/121.4.561
Shibamoto I, Tanaka T, Fujishima I, Katagiri N, Uematsu H (2007) Cortical activation during solid bolus swallowing. J Med Dent Sci 54(1):25–30
Sokolov AA, Miall RC, Ivry RB (2017) The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci 21(5):313–332. https://doi.org/10.1016/j.tics.2017.02.005
Sulena GD, Sharma AK, Singh B (2017) Clinical profile of dysphagia in patients with Parkinson’s disease, progressive supranuclear palsy and multiple system atrophy. J Assoc Physicians India 65(8):32–37
Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, Heindel W, Wiendl H, Dziewas R (2015) The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur J Neurol 22(5):832–838. https://doi.org/10.1111/ene.12670
Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H (2003) Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia 18(2):71–77. https://doi.org/10.1007/s00455-002-0088-x
Zald DH, Pardo JV (1999) The functional neuroanatomy of voluntary swallowing. Ann Neurol 46(3):281–286
Zuketto C, van Gijn J (2002) Severe reversible dysphagia caused by herniation of the cerebellar ectopia. Ned Tijdschr Geneeskd 146(16):771–773
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors did not receive support from any organization for the submitted work. The authors have no conflict of interest to declare that are relevant to the content of this article.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Bundang Jeseang Hospital approved this study. File number of the local institutional ethics board was DMC 2021-01-004.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moon, H.I., Jeong, Y.J. & Suh, J.H. Voxel-based lesion symptom mapping analysis for dysphagia in stroke patients with isolated cerebellar lesions. J Neural Transm 129, 65–74 (2022). https://doi.org/10.1007/s00702-021-02438-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02438-5