Abstract
Background
The cerebellum controls descending motor commands by outputs to primary motor cortex (M1) and the brainstem in response to sensory feedback. The cerebellum may also modulate afferent input en route to M1 and the brainstem.
Objective
The objective of this study is to determine if anodal transcranial direct current stimulation (tDCS) to the cerebellum influences cerebellar brain inhibition (CBI), short afferent inhibition (SAI) and trigeminal reflexes (TRs) in healthy adults.
Methods
Data from two studies evaluating effects of cerebellar anodal and sham tDCS are presented. The first study used a twin coil transcranial magnetic stimulation (TMS) protocol to investigate CBI and combined TMS and cutaneous stimulation of the digit to assess SAI. The second study evaluated effects on trigemino-cervical and trigemino-masseter reflexes using peripheral nerve stimulation of the face.
Results
Fourteen right-handed healthy adults participated in experiment 1. CBI was observed at baseline and was reduced by anodal cerebellar DCS only (P < 0.01). There was SAI at interstimulus intervals of 25 and 30 ms at baseline (both P < 0.0001), but cerebellar tDCS had no effect. Thirteen right-handed healthy adults participated in experiment 2. Inhibitory reflexes were evoked in the ipsilateral masseter and sternocleidomastoid muscles. There was no effect of cerebellar DCS on either reflex.
Conclusions
Anodal DCS reduced CBI but did not change SAI or TRs in healthy adults. These results require confirmation in individuals with neurological impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cerebellum plays a key role in human motor control and learning via extensive projections to sensory and motor areas of the cortex and brainstem [1]. Dysfunction of the cerebellum may, therefore, contribute to motor deficits in movement disorders by defective modulation of its projection targets [2–4]. Non-invasive stimulation of the cerebellum can modify the connectivity between the cerebellum and primary motor cortex (M1), by putative polarisation of Purkinje cells in the cerebellar cortex [5, 6]. Small clinical studies have indicated that non-invasive cerebellar stimulation has potential as a novel treatment intervention in neurological conditions exhibiting cerebellar pathology [7–12]. However, the mechanisms of action are incompletely understood [6, 10, 13]. Direct current stimulation (DCS) electrodes or a magnetic stimulating coil targets the lateral cerebellum, an area of the cerebellar cortex that specifically projects to the dentate nucleus of the deep cerebellar nuclei [14]. Fibres project from the dentate nucleus to the ventrolateral motor thalamus via the superior cerebellar peduncle and onto M1 as the dentate-thalamo-cortical pathway. The intermediate hemisphere and vermis of the cerebellum project to brainstem motor nuclei [14]. Interestingly, modulation of M1 by the cerebellum can also occur via modulation of sensory inputs en route to the cortex. This was evidenced by findings that paired-associative stimulation (PAS) of M1 is enhanced or suppressed by non-invasive stimulation of the cerebellum in a polarity-specific manner [13, 15, 16]. PAS is a neuroplastic M1 response to repetitive paired peripheral and cortical stimuli, inducing long-term potentiation or depression-like effects that are N-methyl-d-aspartate receptor-dependent [17, 18]. It appears that the cerebellum may prime the degree of neuroplastic reorganisation in M1 by modulating ascending somatosensory inputs at a subcortical level [15, 16]. The control of motor output by the cerebellum is likely a complex balance between direct efferent control of cortical and brainstem motor nuclei and modification of afferent input to the same structures.
The question is raised as to whether the cerebellum can prime M1 by modulating activity in other sensory pathways known to be abnormal in movement disorders, such as short afferent inhibition (SAI) [19–21] or trigeminal reflexes (TRs) [22–24]. This may be important, as greater knowledge of cerebellar modulation of sensorimotor circuits might improve understanding of pathophysiology of motor deficits underlying movement disorders. Single pulses of transcranial magnetic stimulation (TMS) paired with electrical stimulation to the median nerve at the wrist or cutaneous stimulation of a digit can be used to probe the degree of M1 inhibition imposed by afferent input [25–27]. Afferent inhibition is mediated by cholinergic and GABAA-dependent circuits [28, 29]. TRs are evoked by stimulation of trigeminal sensory branches of the face, and responses are recorded in facial and neck muscles using electromyography [30–32]. There has been no report of cerebellar modification of TR reflexes although an anatomical pathway exists [4]. Understanding the influence of the cerebellum on SAI and TRs may support non-invasive stimulation of the cerebellum in movement disorders with dysfunction of somatosensory processing, such as dystonia [33, 34].
Projections between the cerebellum and primary motor cortex (M1) can be probed in humans using a twin coil TMS protocol, known as cerebellar brain inhibition (CBI) [35]. Furthermore, excitability of the CBI pathway can be modulated by non-invasive stimulation in healthy adults [5] and in neurological disorders [8, 9, 11]. The aim of the present studies was to test whether cerebellar anodal DCS can modify CBI, SAI and TRs in healthy humans. The objective was to evaluate the potential of cerebellar DCS as a novel intervention in neurological disorders with cerebellar dysfunction. We hypothesised CBI would be reduced by anodal DCS consistent with our results in a previous study [8]. Based on previous findings from a PAS study [16], we hypothesised that anodal cerebellar DCS would increase SAI. Because anodal cerebellar DCS enhances activity in inhibitory cerebellar output pathways [5, 6], we hypothesised that TRs would be increased by a putative effect on cerebellar-trigeminal brainstem pathways.
Materials and Methods
A total of 27 healthy adults participated in two separate studies. All participants were right-handed without a neurological or musculoskeletal disorder affecting the upper limb (experiment 1) or face and neck (experiment 2). The studies were approved by the local ethics committee, and all participants provided informed written consent. Each participant completed a screening questionnaire, which was reviewed by a study doctor prior to participation, to ensure safety for TMS and DCS. In each study, participants attended two sessions, separated by at least 5 days as per previous protocols in healthy adults [8, 36, 37]. Participants underwent anodal or sham DCS, in counterbalanced order, and all were blinded to the intervention at each session. In both experiments, participants sat comfortably in a chair with their hands relaxed on a pillow placed on their lap during all procedures.
Experiment 1
Fourteen healthy adults (age range 24–83 years, five male) participated in experiment 1. The aim of the study was to investigate the effect of cerebellar DCS on CBI and SAI using TMS. The older adult had been recruited as a control subject in a similar experiment and exhibited similar responses to younger adults and experienced no adverse effects and so was accepted for inclusion in the current study. Other studies have found that TMS measures can be reliably obtained in elderly populations [38], with little adverse side effects [39, 40]. Moreover, non-invasive brain stimulation is able to promote plasticity in the older brain [41].
Electromyography
Surface electromyography (EMG) was recorded from the right first dorsal interosseous (FDI) using 10-mm-diameter Ag/AgCl electrodes (Ambu, Ballerup, Denmark). Electrodes were placed over the muscle belly and the metacarpophalangeal joint. A 20-mm-diameter reference Ag/AgCl electrode was placed over back of the hand (3M Health Care, St. Paul, MN, USA). EMG signals were sampled at 2000 Hz (CED 1401; Cambridge Electronic Design, Cambridge, UK), amplified (CED 1902; Cambridge Electronic Design, Cambridge, UK), band-pass-filtered (20–1000 Hz) and stored for offline analysis (Signal v5.09, Cambridge Electronic Design, Cambridge, UK).
Transcranial Magnetic Stimulation
Single-pulse TMS was delivered with a figure-of-eight coil (70-mm wing diameter) (Magstim Co., Wales) positioned over M1 to induce a posterior to anterior directed current in the underlying motor cortex. The hotspot for evoking motor-evoked potentials (MEPs) in the right FDI muscle was located and marked on the scalp. Resting motor threshold (RMT) for the FDI was determined as the minimum stimulus intensity to elicit a 50-μV MEP in four out of eight trials [42].
Cerebellar Brain Inhibition and Short Afferent Inhibition
The TMS test stimulus used to probe CBI and SAI was determined by increasing stimulus intensity until the maximum MEP amplitude at rest in the target FDI was observed. The stimulus intensity was then adjusted to evoke a MEP in FDI of approximately 50 % of the maximum amplitude (50 % MEPmax). For CBI, the stimulus intensity to evoke 50 % MEPmax was used as the test stimulus and was conditioned by TMS over the contralateral lateral cerebellum, using a figure-of-eight flat coil, 3 cm lateral and 1 cm inferior to the inion with the handle pointing up [43]. The conditioning stimulus (CS) intensity was set to 100 % of RMT of the FDI representation in contralateral M1, and the interstimulus interval (ISI) was set to 5 ms [43–46]. For SAI, cutaneous digital stimulation was applied to the index finger using wire ring electrodes with the cathode placed over the proximal, and anode over the distal, phalanx, respectively. Stimulation was delivered with a 1-ms square wave pulse by a constant current stimulator (DS7A, Digitimer Ltd). The stimulus intensity was set at three times perceptual threshold and delivered at two ISIs (25 and 30 ms). Sixteen non-conditioned MEPs and 16 conditioned MEPs were evoked in randomised order at a rate of 0.2 Hz to assess CBI and then SAI at each ISI.
Transcranial Direct Current Stimulation
Transcranial DCS was delivered at 2 mA for 20 min via two 25-cm2 saline-soaked sponge electrodes (Chattanooga Ionto, Hixon, TN), one positioned 3 cm lateral and 1 cm inferior to the inion (right lateral cerebellum) and the other over the ipsilateral buccinator muscle [5]. At the onset of both (anodal and sham) DCS applications, the current was increased in a ramp-like manner. A total current of 2 mA/cm2 with a current density of 0.08 mA/cm2 was applied, keeping below the recommended threshold to avoid tissue damage. Following DCS, participants sat quietly for 5 min before the post-intervention outcome measures were collected. Sham DCS was delivered in an identical manner; however, current intensity was ramped down to zero after 30 s in accordance with established protocols [47].
Experiment 2
Thirteen healthy adults (age range 20–55 years, 7 male) participated in the second experiment, where the effect of cerebellar DCS on TRs was assessed using peripheral nerve stimulation to trigeminal nerves of the face.
Electromyography
Surface EMG was recorded from the right masseter and sternocleidomastoid muscles using pairs of disposable adhesive 10-mm-diameter Ag/AgCl electrodes (Ambu, Ballerup, Denmark), positioned 1 cm apart in a bipolar montage. A ground electrode (Red Dot, 3 m) was placed over the right clavicle [32]. During the experiment, participants contracted their right masseter (by teeth clenching and smiling wide) or right sternocleidomastoid muscle (SCM) (by turning their head to the left side and tilting the head down to the right) at their maximum strength [48]. Participants practiced contracting the target muscles before data collection and received online visual feedback regarding the degree of muscle contraction during the experiment. The EMG signals were amplified [CED 1902; Cambridge Electronic Design (CED), Cambridge, UK], band-pass-filtered (20–1000 Hz) and sampled at 2 kHz (CED 1401).
Peripheral Nerve Stimulation
Peripheral nerve stimulation (PNS) was delivered by a Digitimer DS7A constant current stimulator (Digitimer, Hertfordshire, UK) using a 1-ms square wave pulse via adhesive electrodes positioned over the right infraorbital nerve just below the centre of the orbit on the front of the face. The cathode was placed over the point where the nerve emerges from the foramen, and the anode was placed 1 cm lateral to the cathode. Stimulation intensity was set to three times perceptual threshold for each participant. Sixty responses (delivered 3 s apart) were recorded from the masseter and sternocleidomastoid muscles in separate blocks. Participants were given short rest periods as required (every 20 or 30 responses) in order to minimise the effect of muscle fatigue.
Transcranial Direct Current Stimulation
Transcranial DCS was delivered in an identical manner to experiment 1, except that the cathode was positioned over the ipsilateral cortex, on the forehead. This position was necessary as the electrodes recording muscle activity were already affixed to the ipsilateral side of the face. Anodal or sham DCS was delivered by a TCT DCS Stimulator (TCT Technologies Ltd).
Data Analysis
For TMS data in experiment 1, MEP amplitudes were measured peak to peak (mV) and averaged. A ratio of conditioned to non-conditioned MEPs was used to calculate CBI and SAI. Data were tested for normality with the Shapiro-Wilk test. One-sample t tests were used to determine CBI and SAI in the pre-DCS data. The effect of cerebellar DCS on CBI and non-conditioned MEPs evoked during CBI and SAI was analysed using a two stimulation (anodal, sham DCS) by a two TIME (pre, post) repeated measures ANOVA (rmANOVA). The effect of cerebellar DCS on SAI was assessed using a two stimulation (anodal, sham DCS) by two ISI (SAI25, SAI30) × two-TIME (pre, post) rmANOVA. Root-mean-square EMG (rmsEMG) during CBI and SAI was analysed using a two stimulation (anodal, sham), four MEP (three conditioned and one non-conditioned), by two time (pre, post) rmANOVA. In experiment 2, individual EMG traces from each trial were full wave rectified and averaged. The onset latency (ms) and the area (mV ms) of the average reflex response for the masseter and sternocleidomastoid were measured separately. The onset latency was determined as the time after the stimulus artefact when the EMG dropped below the average pre-stimulus EMG for greater than 10 ms, starting around 50 ms after stimulus onset [49]. Reflex area was measured between the onset and the offset (when the EMG returned to average pre-stimulus values) and expressed as a proportion of the area below the pre-stimulus mean activity [50]. Data were analysed for each muscle using separate two stimulation (anodal, sham DCS) by two Time (pre, post) rmANOVAs. Statistical Package for the Social Sciences (SPSS) software (v22 SPSS Inc, Chicago, USA) was used for statistical analysis in both studies with the level of significance set to P < 0.05. Data were checked for sphericity and corrected where necessary using the Greenhouse Geisser test. Post hoc t tests were used to explore main effects and interactions and were corrected for multiple comparisons [51].
Results
There were no adverse effects of DCS reported by participants in either study. EMG traces from representative participants for CBI, SAI and TRs are provided in Fig. 1.
Experimental data from one representative individual of each study 1 and 2. Upper trace depicts MEPs prior to DCS, and lower trace depicts MEPs after anodal DCS. a Effect of anodal tDCS on cerebellar brain inhibition. b Effect of anodal DCS on short afferent inhibition. C conditioned response, NC non-conditioned responses. c Effect of anodal DCS on trigeminal reflexes. Upper trace represents TRs prior to DCS, and lower trace represents TRs after DCS. The sternocleidomastoid muscle is on the left, and the masseter muscle is on the right. Approximate onset and offset of each reflex are indicated by the arrows
Experiment 1
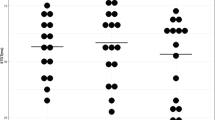
One-sample t tests revealed that there was CBI at baseline (anodal session 0.85 ± 0.31, P < 0.0001; sham session 0.89 ± 0.048, P < 0.05). Following DCS, there was a time × condition interaction (F 1,13 = 5.46, P < 0.05). Post hoc, paired t tests revealed that CBI was reduced by anodal DCS (pre 0.85 ± 0.31, post 1.04 ± 0.07, P < 0.01) but not by sham DCS (P > 0.5) (Fig. 2a). There were no main effects (both P > 0.07). One-sample t tests revealed that there was SAI at baseline at ISI 20 (anodal session 0.74 ± 0.074; sham session 0.78 ± 0.041, both P < 0.0001) and ISI 30 (anodal session 0.76 ± 0.082; sham session 0.80 ± 0.072, both P < 0.0001). There were no main effects or interactions for SAI following DCS (all P > 0.22) (Fig. 2b). There were no main effects or interactions for non-conditioned MEPs evoked during CBI (pre-DCS, 1.64 ± 0.47 mV, post-DCS 1.80 ± 0.50 mV; all P > 0.43) or SAI (pre-DCS, 1.72 ± 0.38 mV, post-DCS 1.85 ± 0.38 mV; all P > 0.25). There was no change in pre-stimulus rmsEMG for CBI (pre-DCS 0.009 ± 0.002 mV, post-DCS 0.009 ± 0.001 mV; all P > 0.66) or SAI (pre-DCS 0.008 ± 0.001 mV, post-DCS 0.009 ± 0.001 mV; all P > 0.54). Experimental conditions therefore, remained consistent during CBI and SAI.
Results of anodal and sham DCS a Effect of anodal and sham DCS on cerebellar brain inhibition. b Effect of anodal and sham DCS on short afferent inhibition. c Effect of anodal and sham DCS on trigeminal reflexes. The left figure is for the sternocleidomastoid muscle, and the right figure is for the masseter muscle. Asterisk indicates significance at P < 0.05 with one-sample t tests (pre-DCS) or a pre-post difference using a post hoc paired samples t test. Error bars signify standard error of the mean (SEM). CBI cerebellar brain inhibition, C conditioned MEP, NC non-conditioned MEP, SAI short afferent inhibition, EMG electromyography, ISI interstimulus interval, tDCS transcranial direct current stimulation
Experiment 2
At baseline, there was an inhibitory reflex with an average onset latency of 62.2 ± 15.9 ms and an area of −10.14 ± 8.19 mV ms in the sternocleidomastoid muscle and an inhibitory reflex with an average onset latency of 44.7 ± 9.2 ms and an area of −11.95 ± 11.28 mV ms in the masseter muscle. For the sternocleidomastoid muscle, there were no main effects and no interaction (all P > 0.22) of DCS on the area of the reflex. Likewise, for the masseter muscle, there were no significant main effects and no interaction on reflex area (all P > 0.11) (Fig. 2c).
Discussion
These experiments demonstrate that anodal DCS did not modulate SAI or inhibitory TRs in healthy adults, at least using the methods applied in the present studies. Excitability of the cerebellum was influenced by anodal DCS, as indicated by a reduction in CBI. Therefore, the lack of effect on SAI and TRs cannot be explained by a failure of DCS to modify activity within the cerebellum. Our findings provide preliminary evidence that the cerebellum may not significantly modulate the afferent pathway responsible for producing SAI in the M1 or brainstem circuits modulating TRs in healthy adults. The pathways under examination are, therefore, likely not responsible for the benefits of cerebellar non-invasive brain stimulation reported in small clinical populations [7–9, 11, 12].
Non-Invasive Cerebellar Stimulation
The neural mechanisms underlying neuromodulation of the cerebellar cortex are still uncertain. Studies from basic science indicate that electrical currents applied to the cortical surface of the cerebellum in the turtle influence the cell body and both proximal and apical dendrites of the Purkinje cells [52]. Furthermore, epidural anodal DCS of the cerebellum of rodents induces reorganisation of M1, reducing its excitability along with that of the motoneurons in the anterior horn of the spinal cord [53]. Investigations aimed at modelling the DCS electric field in humans demonstrated that maximal activation was localised to the targeted cerebellar hemisphere [54]. In contrast, repetitive TMS protocols in Parkinson’s disease patients were found to alter metabolic activity in both cerebellar cortex and the deep cerebellar nuclei [55]. Similarly, cerebellar DCS does not alter the excitability of M1 to single pulse TMS, while cerebellar repetitive TMS increased the MEP response [56]. Differences in neural and non-neural structures within the cerebellum that may be influenced by DCS and rTMS might explain these discrepancies.
Cerebellar Brain Inhibition
The lateral cerebellum controls M1 excitability by inhibiting activity of the disynaptic dento-thalamo-cortical pathway [14]. It might be expected that cerebellar neuromodulation would follow the polarity-dependent effect on output pathways similar to that observed for M1 [57]. However, modulation by excitatory non-invasive brain stimulation on cerebellar output in the form of CBI has been inconsistent to date. Initial reports were of an increase in CBI after anodal DCS [5]. In contrast, CBI was reduced in the current result and our previous study in people with focal hand dystonia [8]. Furthermore, another study using intermittent theta-burst stimulation in patients with cerebellar stroke also reported a reduction in CBI [9]. While a reduction of CBI secondary to greater activation of inhibitory Purkinje cells in the cerebellar cortex appears counter-intuitive, there are a number of possible explanations. There are multiple layers of cells in the cerebellar cortex and, in turn, synaptic connections with the potential to undergo neuroplastic modulation by non-invasive stimulation. Superficially located inhibitory interneurons project onto Purkinje cells and are modulated by parallel fibres from granule cells [58]. If in the present study, anodal DCS produced LTP-like plasticity at the parallel fibre-inhibitory interneuron synapse, Purkinje cells would receive greater inhibition. In turn, less excitable Purkinje cells would disinhibit the dentate nucleus and reduce inhibition imposed upon M1, as we observed. Alternatively, there are both excitatory and inhibitory interneurons within M1 that are targets for cerebello-thalamo-cortical projections [43, 45, 46]. Anodal DCS may have directly excited the Purkinje cells themselves and reduced activity along the cerebello-thalamo-cortical pathway. However, if the cerebello-thalamo-cortical projections terminate onto M1 GABA-ergic intracortical inhibitory neurons, then corticomotor neurons would be disinhibited in turn. We did not assess GABA-ergic intracortical inhibition in the current study and so cannot determine for certain which explanation is more likely. Regardless, the fact that cerebellar inhibition of M1 was reduced by anodal DCS provided evidence that cerebellar excitability was modulated in the present study.
Short Afferent Inhibition
Contrary to our hypothesis, there was no effect of cerebellar DCS on SAI in these healthy adults. This finding contrasts with previous studies using the PAS paradigm, where anodal DCS or intermittent theta-burst stimulation to cerebellum reduced PAS responses in primary motor cortex [15, 16]. The authors suggested that non-invasive cerebellar stimulation may prime M1 responses to PAS by “gating” activity in ascending pathways within the olivary nucleus or thalamus [16]. Alternatively, M1 excitability evoked by PAS may be directly modulated by extra-thalamic projections from the cerebellum [15]. Our result suggests that there is a difference in how cerebellar stimulation affects neurons intercalated in the afferent pathways mediating SAI and PAS in the M1 of healthy adults. One explanation could be that NMDA, but not cholinergic, circuits are modulated by the cerebellum or its projection targets. In agreement, a previous study also reported no effect on SAI evoked by median nerve stimulation after cerebellar theta-burst stimulation in healthy adults [21]. In the same study, cerebellar TBS did partially normalise the reduced SAI observed in patients with Alzheimer’s disease, a disorder associated with pronounced deficits in cortical cholinergic function [21]. It might be that SAI is modified by cerebellar stimulation only when there is cholinergic dysfunction [21]. Future studies are warranted to assess responses to non-invasive cerebellar stimulation in other patient populations where SAI is reduced, but who do not exhibit overt deficits in cholinergic function such as cervical dystonia [59], Parkinson’s disease patients on dopaminergic medication [20] or after stroke [19].
Trigeminal Reflexes
Trigemino-cervical reflexes are mediated by trigeminal sensory afferents and cervical motoneurons. Pathophysiological modification of these reflexes has been reported in cervical dystonia [22, 60] and other neurological disorders [24, 32]. We hypothesised that excitability of these reflexes might be mediated by the cerebellum, as there are direct projections between cerebellum and the trigeminal nuclear complex [4, 61]. Our aim was to examine whether cerebellar stimulation would alter TR activity in healthy individuals, to determine if there might be benefits for translation into a neurological population. The reason why TRs were unaffected in the present study is not clear. One potential explanation may be that other nuclei in the cerebello-trigeminal network, such as the basal ganglia and superior colliculus, may also modulate the TRs [4, 60]. Thus, stimulation of the cerebellum alone may not have been sufficient to alter TR activity in the healthy brainstem. Alternatively, activity in the aforementioned nuclei may have adjusted in compensation for altered cerebellar activity, producing a null net effect. It may be worth investigating whether cerebellar stimulation can modify TRs when there is widespread disruption of motor networks including the basal ganglia and the cerebellum such as dystonia and Parkinson’s disease [2, 3].
Conclusion
Non-invasive cerebellar stimulation is a safe and potentially effective method to modulate cerebellar function [6]. We demonstrate that anodal DCS reduces CBI but has little effect on SAI or TRs in healthy adults. From these data, it appears that cerebellar anodal DCS might not be a useful intervention in neurological disorders exhibiting aberrant sensory processing in the cortex (SAI) or brainstem (TRs). However, these findings require confirmation in individuals with neurological impairment such as cerebellar stroke, ataxia, dystonia or Parkinson’s disease, as our hypothesised effects may only become apparent under certain impaired neurological conditions.
References
Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886(1–2):237–45.
Prudente CN, Hess EJ, Jinnah HA. Dystonia as a network disorder: what is the role of the cerebellum? Neuroscience. 2014;260:23–35.
Wu T, Hallett M. The cerebellum in Parkinson’s disease. Brain. 2013;136(Pt 3):696–709.
Bradnam L, Barry C. The role of the trigeminal sensory nuclear complex in the pathophysiology of craniocervical dystonia. J Neurosci. 2013;33(47):18358–67.
Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29(28):9115–22.
Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, et al. Non-invasive cerebellar stimulation—a consensus paper. Cerebellum. 2013;13:121–38.
Bradnam LV, Frasca J, Kimberley TJ. Direct current stimulation of primary motor cortex and cerebellum and botulinum toxin a injections in a person with cervical dystonia. Brain Stimul. 2014;7(6):909–11.
Bradnam LV, Graetz LJ, McDonnell MN, Ridding MC. Anodal transcranial direct current stimulation to the cerebellum improves handwriting and cyclic drawing kinematics in focal hand dystonia. Front Hum Neurosci. 2015;9:286.
Bonni S, Ponzo V, Caltagirone C, Koch G. Cerebellar theta burst stimulation in stroke patients with ataxia. Funct Neurol. 2014;1–5.
Sadnicka A, Kassavetis P, Saifee TA, Parees I, Rothwell JC, Edwards MJ. Cerebellar transcranial direct current stimulation does not alter motor surround inhibition. Int J Neurosci. 2013;123(6):425–32.
Koch G, Porcacchia P, Ponzo V, Carrillo F, Caceres-Redondo MT, Brusa L, et al. Effects of two weeks of cerebellar theta burst stimulation in cervical dystonia patients. Brain Stimul. 2014;7(4):564–72.
Grimaldi G, Oulad Ben Taib N, Manto M, Bodranghien F. Marked reduction of cerebellar deficits in upper limbs following transcranial cerebello-cerebral DC stimulation: tremor reduction and re-programming of the timing of antagonist commands. Front Syst Neurosci. 2014;8:9.
Sadnicka A, Hamada M, Bhatia KP, Rothwell JC, Edwards MJ. Cerebellar stimulation fails to modulate motor cortex plasticity in writing dystonia. Mov Disord. 2014;29:1304–7.
Holdefer RN, Miller LE, Chen LL, Houk JC. Functional connectivity between cerebellum and primary motor cortex in the awake monkey. J Neurophysiol. 2000;84(1):585–90.
Hamada M, Strigaro G, Murase N, Sadnicka A, Galea JM, Edwards MJ, et al. Cerebellar modulation of human associative plasticity. J Physiol. 2012;590(Pt 10):2365–74.
Popa T, Velayudhan B, Hubsch C, Pradeep S, Roze E, Vidailhet M, et al. Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb Cortex. 2012;23:305–14.
Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543(Pt 2):699–708.
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–84.
Di Lazzaro V, Profice P, Pilato F, Capone F, Ranieri F, Florio L, et al. The level of cortical afferent inhibition in acute stroke correlates with long-term functional recovery in humans. Stroke. 2012;43(1):250–2.
Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson’s disease. Brain. 2003;126(Pt 8):1883–94.
Di Lorenzo F, Martorana A, Ponzo V, Bonni S, D’Angelo E, Caltagirone C, et al. Cerebellar theta burst stimulation modulates short latency afferent inhibition in Alzheimer’s disease patients. Front Aging Neurosci. 2013;5:2.
Nakashima K, Thompson PD, Rothwell JC, Day BL, Stell R, Marsden CD. An exteroceptive reflex in the sternocleidomastoid muscle produced by electrical stimulation of the supraorbital nerve in normal subjects and patients with spasmodic torticollis. Neurology. 1989;39(10):1354–8.
Quartarone A, Girlanda P, Di Lazzaro V, Majorana G, Battaglia F, Messina C. Short latency trigemino-sternocleidomastoid response in muscles in patients with spasmodic torticollis and blepharospasm. Clin Neurophysiol. 2000;111(9):1672–7.
Nakashima K, Takahashi K, Azumi T, Ishida G. Exteroceptive suppression of the masseter and temporalis muscles produced by electrical stimulation of the mental nerve in patients with Parkinson’s disease. Acta Neurol Scand. 1990;81(5):407–10.
Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523(Pt 2):503–13.
Helmich RC, Baumer T, Siebner HR, Bloem BR, Munchau A. Hemispheric asymmetry and somatotopy of afferent inhibition in healthy humans. Exp Brain Res. 2005;167(2):211–9.
Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, et al. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130(1):48–59.
Di Lazzaro V, Pilato F, Dileone M, Tonali PA, Ziemann U. Dissociated effects of diazepam and lorazepam on short-latency afferent inhibition. J Physiol. 2005;569(Pt 1):315–23.
Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, et al. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005;564(Pt 2):661–8.
Di Lazzaro V, Guney F, Akpinar Z, Yuruten B, Oliviero A, Pilato F, et al. Trigemino-cervical reflexes: clinical applications and neuroradiological correlations. Suppl Clin Neurophysiol. 2006;58:110–9.
Di Lazzaro V, Quartarone A, Higuchi K, Rothwell JC. Short-latency trigemino-cervical reflexes in man. Exp Brain Res. 1995;102(3):474–82.
Di Lazzaro V, Restuccia D, Nardone R, Tartaglione T, Quartarone A, Tonali P, et al. Preliminary clinical observations on a new trigeminal reflex: the trigemino-cervical reflex. Neurology. 1996;46(2):479–85.
Meunier S, Russmann H, Shamim E, Lamy JC, Hallett M. Plasticity of cortical inhibition in dystonia is impaired after motor learning and paired-associative stimulation. Eur J Neurosci. 2012;35(6):975–86.
Stamelou M, Edwards MJ, Hallett M, Bhatia KP. The non-motor syndrome of primary dystonia: clinical and pathophysiological implications. Brain. 2012;135(Pt 6):1668–81.
Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37(6):703–13.
McCambridge AB, Bradnam LV, Stinear CM, Byblow WD. Cathodal transcranial direct current stimulation of the primary motor cortex improves selective muscle activation in the ipsilateral arm. J Neurophysiol. 2011;105(6):2937–42.
Bradnam LV, Stinear CM, Byblow WD. Theta burst stimulation of human primary motor cortex degrades selective muscle activation in the ipsilateral arm. J Neurophysiol. 2010;104(5):2594–602.
Christie A, Kamen G. Cortical inhibition is reduced following short-term training in young and older adults. Age. 2014;36(2):749–58.
Abraham G, Milev R, Lazowski L, Jokic R, du Toit R, Lowe A. Repetitive transcranial magnetic stimulation for treatment of elderly patients with depression—an open label trial. Neuropsychiatr Dis Treat. 2007;3(6):919–24.
Milev R, Abraham G, Hasey G, Cabaj JL. Repetitive transcranial magnetic stimulation for treatment of medication-resistant depression in older adults: a case series. J ECT. 2009;25(1):44–9.
Dickins DS, Sale MV, Kamke MR. Plasticity induced by intermittent theta burst stimulation in bilateral motor cortices is not altered in older adults. Neural Plast. 2015;2015:323409.
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91(2):79–92.
Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo E, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res. 2007;179(2):291–9.
Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Ferlazzo F, et al. Changes in cerebello-motor connectivity during procedural learning by actual execution and observation. J Cogn Neurosci. 2011;23(2):338–48.
Lu MK, Tsai CH, Ziemann U. Cerebellum to motor cortex paired associative stimulation induces bidirectional STDP-like plasticity in human motor cortex. Front Hum Neurosci. 2012;6:260.
Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557(Pt 2):689–700.
Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50.
Serrao M, Rossi P, Parisi L, Perrotta A, Bartolo M, Cardinali P, et al. Trigemino-cervical-spinal reflexes in humans. Clin Neurophysiol. 2003;114(9):1697–703.
Ertekin C, Celebisoy N, Uludag B. Trigemino-cervical reflexes in normal subjects. J Neurol Sci. 1996;143(1–2):84–90.
Matthews D, Murtagh P, Risso A, Jones G, Alexander CM. Does interhemispheric communication relate to the bilateral function of muscles? A study of scapulothoracic muscles using transcranial magnetic stimulation. J Electromyogr Kinesiol. 2013;23(6):1370–4.
Rom DM. A sequentially rejective test procedure based on a modified Bonferroni inequality. Biometrika. 1990;77(3):663–5.
Chan CY, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986;371:89–114.
Oulad Ben Taib N, Manto M. Trains of epidural DC stimulation of the cerebellum tune corticomotor excitability. Neural Plast. 2013;2013:613197.
Rampersad SM, Janssen AM, Lucka F, Aydin U, Lanfer B, Lew S, et al. Simulating transcranial direct current stimulation with a detailed anisotropic human head model. IEEE Trans Neural Syst Rehabil Eng. 2014;22(3):441–52.
Brusa L, Ceravolo R, Kiferle L, Monteleone F, Iani C, Schillaci O, et al. Metabolic changes induced by theta burst stimulation of the cerebellum in dyskinetic Parkinson’s disease patients. Parkinsonism Relat Disord. 2012;18(1):59–62.
Oliveri M, Koch G, Torriero S, Caltagirone C. Increased facilitation of the primary motor cortex following 1 Hz repetitive transcranial magnetic stimulation of the contralateral cerebellum in normal humans. Neurosci Lett. 2005;376(3):188–93.
Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9.
Lamont MG, Weber JT. The role of calcium in synaptic plasticity and motor learning in the cerebellar cortex. Neurosci Biobehav Rev. 2012;36(4):1153–62.
Kojovic M, Caronni A, Bologna M, Rothwell JC, Bhatia KP, Edwards MJ. Botulinum toxin injections reduce associative plasticity in patients with primary dystonia. Mov Disord. 2011;26(7):1282–9.
Gunduz A, Ergin H, Kiziltan ME. Long latency trigemino-cervical reflex in patients with cervical dystonia. Neurol Sci. 2015;36(1):103–8.
Billig I, Yatim N, Compoint C, Buisseret-Delmas C, Buisseret P. Cerebellar afferences from the mesencephalic trigeminal nucleus in the rat. Neuroreport. 1995;6(17):2293–6.
Acknowledgments
The authors wish to thank Dr Brenton Hordacre, Mr Lynton Graetz, Ms Sadina Hasmofu and Ms Donna Alessio for assistance with data collection. This project was partially supported by a Flinders Medical Foundation Research Grant (SD and LB).
Conflict of Interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doeltgen, S.H., Young, J. & Bradnam, L.V. Anodal Direct Current Stimulation of the Cerebellum Reduces Cerebellar Brain Inhibition but Does Not Influence Afferent Input from the Hand or Face in Healthy Adults. Cerebellum 15, 466–474 (2016). https://doi.org/10.1007/s12311-015-0713-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-015-0713-5