Abstract
Voxel-based morphometry (VBM) studies have reported abnormalities in brain regions involved in functions that are commonly impaired in autism spectrum disorders (ASD). However, little is known about brain structure anomalies in low-functioning (LF) young children with ASD. A VBM analysis was carried out to assess brain regions involved in ASD LF children, and a multiple regression analysis was used to examine the relationship between regional volume changes and autism symptom measures. Twenty-six LF ASD children (2–10 years) were compared with 21 controls. A VBM-Diffeomorphic Anatomical Registration analysis using Exponentiated Lie algebra (DARTEL) was used to evaluate gray matter (GM) and white matter alterations, covaried with Intelligence Quotient, age, and total brain volume. The resulting altered regions were correlated with Autism Diagnostic Interview (ADI)-Revised and Autism Diagnostic Observation Schedule (ADOS)-Generic scores. GM bilateral reduction was noted in the cerebellum (Crus II and vermis) and in the hippocampi in ASD group. GM reduction was also detected in the inferior and superior frontal gyri, in the occipital medial and superior gyri, and in the inferior temporal gyrus of the left cerebral hemisphere. In the right hemisphere, GM reduction was found in the post-central cortex and in the occipital inferior gyrus. Multiple regression analysis showed a correlation between alterations in GM volume in the cerebellum (Crus II and vermis) and ADI-communication and ADOS-total (communication and interaction) scores. These findings seem to confirm that the cerebellum is involved in integrating and regulating emotional and cognitive functions which are impaired in ASD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism spectrum disorders (ASD) are lifelong neurodevelopmental disorders that include extremely different phenotypes characterised by impaired reciprocal social communication and stereotyped patterns of interests and activities. Due to frequent comorbidities (epilepsy, mental retardation, and psychiatric disorders), it is difficult to isolate the neuropathological substrate of autism, and the underlying biological causes have yet to be established. At present, it can be hypothesized that genetic, immunological, and environmental factors are involved in its etiology, although their complete definition is pending.
The range of variability in clinical phenotype is consequently very wide and nowadays includes polymorphous clinical profiles combining early, lasting, and extensive abnormalities in network underlying communication (verbal and non-verbal), reciprocal social interactions, and restricted, stereotyped behaviors; these areas vary in volume and in neuropathology over the time course of brain development [1, 2].
Global and regional morphological alterations in ASD have been demonstrated by neuroimaging [3] and pathological studies [4], and abnormal brain growth and head enlargement have been evidenced by head circumferences [5] and autoptic studies [6].
Those alterations appear to be especially pronounced during very early development and have also been found in adults, although results are inconsistent. Some studies have reported an increase in total gray matter (GM) volume [7] in some specific areas (caudate nucleus and fronto/temporal regions) [8], while others have described reduction in regional GM volumes in the frontal-striatal and cerebellar regions [9] and the superior temporal sulcus [10]. Enlarged GM volumes in some areas and smaller ones in others have also been reported [11, 12]. As hypothesized by Amaral [2], these contradictory data suggest that it is the time course of brain development rather than the final product that is most disturbed in ASD.
The marked differences among these studies include heterogeneity in the cohorts studied, in the scanner hardware utilized, and in the analysis methods and statistical approaches employed to assess brain volumes. There are also a variety of potentially confounding, not MRI-specific factors characterizing research on autism such as age, gender, IQ, and the inherent heterogeneity of the disorder. Some authors argue that IQ must be strictly matched or statistically factored out in autism studies, while others are convinced that in attempting to “control” for a domain non-specific construct such as IQ, a variability truly associated with autism could be discarded as “non-specific.” In agreement with other authors [13, 14], we believe that both the use of IQ as a covariate and the use of a heterogeneous control group with intellectual disability matched for mental age can be confounding factors during data analysis. To study low-functioning (LF) ASD subjects, a compromise must ultimately be reached, and we are therefore persuaded that it is better to compare the brain volumes of ASD subjects with those of typically developing children.
Apart from brain volume differences in ASD, the correlation between regional brain volumetric abnormalities and clinical features has begun to elicit growing concern among investigators. To date, only two studies have actually attempted to explore this relationship. Rojas et al. [11] found significant correlations between the volumes of the caudate nuclei, multiple frontal and temporal regions, cerebellum, and a measure of repetitive behavior in a sample of adults and children with ASD (age range, 7–47 years). Kosaka et al. [15] compared ASD adults (age range, 17–32 years) with normal controls and found that considerably smaller volumes in the right insula and inferior frontal gyrus were negatively correlated with Spectrum Quotient scores. Considering ASD as a neurodevelopment disorder with a dynamic evolution [2], it is important to search for brain volume abnormalities in young children within a restricted age range. It should also be pointed out both studies mentioned were assessing high-functioning autistic and Asperger patients, while the majority of autistics are intellectually impaired with about 80 % falling in the LF classification, i.e., they have an IQ less than 70 [16]. Despite the high prevalence of intellectual disability in the ASD population, LF subjects are rarely investigated [10, 17, 18] perhaps because of the difficulty of eliciting their cooperation during neuroimaging acquisition.
In view of the hypothesis that the volumes of the cerebral cortices in ASD are different from those in typical children and that the brain volume abnormalities are correlated to core deficits of autism, our study was aimed at comparing the brain volumes in LF ASD and controls using the voxel-based morphometry (VBM)-Diffeomorphic Anatomical Registration analysis using Exponentiated Lie algebra (DARTEL), which is an unbiased, operator-independent method. Multiple regression analysis was used to examine the relationship between regional volume changes and autism symptom measures. To the best of our knowledge, this is the first study using the VBM-DARTEL analysis technique to investigate the correlation between regional volumetric abnormalities and autistic deficits in a LF ASD pediatric population.
Material and Methods
Participants
Twenty-six LF ASD children without associated seizures or other neurological diseases were recruited at the Developmental Neurology Division of the Carlo Besta Neurological Institute in Milan (Italy). Patients with known infectious, metabolic, or genetic diseases, chromosomal abnormalities or disintegrative disorders were excluded. Patients in whom conventional MRI demonstrated structural abnormalities were also excluded. Of the 26 patients (3 females; 23 males; age range from 2 years, 7 months to 10 years,10 months; mean, 5 years,10 months; SD, 2 years, 6 months), 18 had been diagnosed with autism and 8 with Pervasive Developmental Disorder-Not Otherwise Specified. In all cases, the diagnosis was established in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria. The diagnosis was confirmed by the Autism Diagnostic Observation Schedule-Generic (ADOS-G), a semi-structured standardized assessment of social interaction, communication, and play [19] and by the Autism Diagnostic Interview-Revised (ADI-R) which is a semi-structured standardized, investigator-based interview for parents or caregivers [20]. These instruments continue to be the recommended “gold standards” for the diagnosis of ASD [21]. These tools are DSM-oriented and investigate the three domains of typically impaired functioning in ASD: language/communication; social interaction; and restricted, repetitive, and stereotyped behaviors and interests. Each item is scored on a three-point scale, with 0 indicating no evidence of abnormal behaviors linked to autism, 2 indicating that abnormal behaviors are present, and 1 indicating that the subject has shown abnormal behavior, but it is not clear if it is sufficiently severe, frequent, or marked to warrant a 2. The mean scores and the SD for each domain were obtained by dividing the total score by the number of items included (i.e., to accommodate for differences in the modules and diagnostic algorithms which are differentiated depending on developmental and language levels). The ADI-R mean scores were 1.5 ± 0.3 for social interaction; 1.4 ± 0.4 for language/communication; and 0.9 ± 0.4 for restricted, repetitive behaviors. The ADOS-G mean scores were 1.4 ± 0.3 for social interaction; 1.1 ± 0.3 for language/communication; and 1.1 ± 0.5 for restricted, repetitive behaviors. Since the ADOS-G uses only the first two domains for diagnostic purposes, it provides a total score (language/communication + reciprocal social interaction) that in our sample was 1.3 ± 0.3.
The cognitive functioning of the children was assessed using the Wechsler Intelligence Scale according to their age, the Leiter International Performance Scale Revised for non-verbal children, and the Griffiths Mental Developmental Scale for those subjects whose chronological or mental age was below 4 years. The IQ range was 38.7–70.1 (mean, 51.6 ± 9.3).
The control group consisted of 21 normally developing children (8 females; 13 males; age range from 3 years, 9 months to 10 years, 3 months; mean, 6 years,10 months; SD, 2 years, 1 month), with normal IQ, no sign of autistic behavior, psychiatric or neurological disorders, and had negative family history for neurological/psychiatric illnesses among their first-degree relatives. The study was approved by the local ethics committee, and all the patients’ parents gave written informed consent.
MR Imaging Acquisition
All ASD subjects were examined under propofol sedation (1 mg/kg). The controls under age 6 were also examined under propofol sedation. They were recruited among inpatients with suspected spinal cord abnormalities and included in the study if the brain and spine examination was normal. The controls over age 6 were examined without sedation; they were recruited among the children of the medical and technical staff involved in the study.
T1-weighted volumetric MRI images were acquired on a 1.5 T MRI system (Siemens–Erlangen, Germany) using a magnetization-prepared gradient-echo sequence (TR = 1,640 ms, TE = 2.48 ms, TI = 552 ms, FOV = 256 × 256 mm, matrix = 256 × 256, 160 sagittal slices, voxel size = 1 × 1 × 1 mm).
Structural imaging included axial PD/T2-weighted images (TR = 3,500 ms, TE = 17 ms/84 ms, FOV = 208 × 256 mm, matrix = 208 × 256, slice thickness = 5 mm), coronal turbo spin-echo T2-weighted images (TR = 4,100 ms, TE = 143 ms, FOV = 324 × 384 mm, matrix = 324 × 384, slice thickness = 5 mm). Structural imaging was assessed by a senior neuroradiologist for the presence or absence of supratentorial and infratentorial abnormalities and signal changes, based on visual inspection. The MRI images of the controls were similarly reviewed. Moreover, each scan was visually inspected by a senior neuroradiologist, and only scans deemed to have no or minimal movement artefacts were included in analysis.
Image Processing
Voxel-based morphometry analysis was used to investigate the differences in the GM and white matter (WM) volumes in the ASD and control groups studied using the SPM8 package (Welcome Trust Center for Neuroimaging, London, UK, http://www.filion.ucl.ac.uk/spm/software/spm8/) implemented in Matlab 2008a (Math Works, Natick, MA, USA). The T1-weighted volumetric images were analyzed using the VBM protocol with modulation.
The preprocessing was based on the brain probability pediatric (5–9.5 years) templates provided by the Cincinnati Children’s Hospital Medical Center (available at https://irc.cchmc.org) and the DARTEL algorithm to achieve an accurate inter-subject registration with an improved realignment of small inner structures [22]. The following steps were followed: (1) checking for scanner artifacts and gross anatomical abnormalities for each subject; (2) setting the image origin to the anterior commissure; (3) segmenting the images into the GM and WM images using the SPM8 toolbox; (4) importing the parameter files produced by the tissue segmentation in the DARTEL procedure; (5) affine transform of segmented brain maps into the MNI space [23]; (6) the segmented images were modulated with the Jacobian determinants derived from the spatial normalization [24]; and (7) checking for homogeneity across the sample and using standard smoothing by an 8-mm-full width-half maximum Gaussian kernel. In order to exclude normalization fails or suboptimal overlapping, a senior neuroradiologist visually checked the normalization process results.
This preprocessing yielded the smoothed modulated normalized data (in the MNI space) used for the statistical analysis. Due to the modulation step, the final VBM statistics reflected “volume” rather than “concentration” differences in the tissue segments [25].
Statistical Analysis
Comparison of Volumes
Group differences were evaluated for GM, WM, cerebrospinal fluid (CSF) absolute volumes, and total intracranial volume (TIV), obtained in the brain segmentation step of the VBM-DARTEL preprocessing. TIV was calculated as the sum of the GM, WM, and CSF partitions estimated by means of SPM Easy Volume toolbox (http://www.sbirc.ed.ac.uk/lcl/LCL_M1.html). A t test was performed to identify any significant between group difference in the global tissue volumes.
VBM Analysis
A general linear model based on the Gaussian field theory was used to statistically assess differences in the normalized, modulated, and smoothed GM images in the two groups. Covariates such as IQ, age, sex, and TIV were taken into account. A conventional VBM analysis was employed using a p < 0.05 significance threshold, False Discovery Rate (FDR) corrected with an extent threshold of 20 voxels. An absolute threshold mask of 0.3 was used on both the GM and WM to avoid possible edge effects around the border between the two.
Correlation Between ADI-ADOS Scores and GM Volume
Multiple regression analyses were computed in SPM8 to explore the relationship between the ADI and ADOS scores and regional volume changes. The statistical threshold used was p < 0.05 (FDR corrected).
Results
Quantitative Structural MRI
No significant differences were found between patients and controls with regard to total brain volumes (p = 0.199), GM (p = 0.065), WM (p = 0.141), and CSF (p = 0.066). These results are summarized in Table 1.
Regional WM Differences
There were no regional WM volume differences in the two groups.
Regional GM Differences
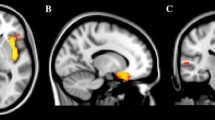
GM volume was reduced in 15 three-dimensional clusters in the ASD subjects (significance threshold p < 0.05, FDR corrected). Both the cerebellum (crus II and vermis) and the hippocampus showed bilateral GM reduction. There were reduced GM volumes in the inferior and superior frontal gyrus, occipital medial and superior gyrus, and inferior temporal gyrus of the left cerebral hemisphere. There were reduced GM volumes in the post-central and pre-central cortex, in the rectus gyrus, and in the occipital inferior gyrus of the right hemisphere (Table 2 and Fig. 1).
Differences in gray matter (GM) volume in the ASD and control groups. GM volume differences between groups outlined over T1 images. The relative decrease in GM volume found in the autistic subjects compared with controls is represented in a red-orange color scale. The statistical threshold is p < 0.05 (FDR corrected)
Multiple Regression Analysis
A significant negative correlation between GM volume and autistic symptoms was found in the cerebellum. In particular, a correlation was found between reduced GM volume of the cerebellum (bilateral hemispheric Crus II and the vermis) and the total ADOS score (the sum of the social interaction and communication; Table 3). A negative correlation was also found between the same areas of cerebellum and ADI-R communication score (Table 4).
Discussion
In this study, we applied unbiased whole brain VBM-DARTEL method to assess neuroanatomical differences in LF ASD children compared to normally developing controls and we compute multiple regression analysis to correlate ADI-R and ADOS scores with brain volume abnormalities.
The main findings of this study were: (1) absence of WM abnormalities; (2) reduction in GM volume in several brain areas involved in social/communication behaviors and other symptoms typical of ASD; and (3) significant correlations between ADI-R-communication and the total ADOS (communication and interaction) scores and the reduction of GM cerebellar volumes in the vermis and hemispheric Crus II.
The fact that there were no WM abnormalities disagrees with data presented by Radua et al. [26], but agrees with the findings of other investigators [11, 15, 27]. These apparently contradictory results can be explained by the heterogeneity of the samples studied (size, type of ASD, and ages) and the methods employed. Diffusion tensor imaging has been found to be more sensitive to WM abnormalities [28].
Reduction in GM volume was found in the inferior and superior frontal gyri, the occipital medial and superior gyri and the inferior temporal gyrus of the left hemisphere, in the postcentral gyrus and the occipital inferior gyrus of the right hemisphere, in the hippocampus and the cerebellum bilaterally. The main finding was a significant correlation between communication/social interaction scores and brain volume loss in the cerebellum (vermis and Crus II).
Previously considered a complex associative center for motor coordination and execution, the cerebellum has begun to be seen as a center involved in higher cognitive and emotional functions even in the developing brain [29]. Its functional specificity is linked to an internal modular organization which harmonizes cognitive and emotional behaviors automatically by means of a complex system of segregated pathways between the cerebellum and other brain regions (the cerebrocerebellar system) [30, 31]. The cerebellum is thus incorporated into the distributed neural circuits subserving cognition and emotion. The human cerebellum has a topographic organization. Cognitive impairments occur when posterior lobe lesions affect lobules VI and VII (including Crus I, Crus II, and lobule VIIB), disrupting cerebellar modulation of cognitive loops involving cerebral associative cortices. Behavioral disorders manifest, instead, when vermis lesions deprive cerebro-cerebellar-limbic loops of cerebellar input [31].
The cerebellum was the first structure to be studied in autistics and the most consistent site of MRI abnormality in ASD patients [2, 32]. Autoptic studies found a decreased number of Purkinje cells in the cerebellar hemispheres and the vermis of autistic subjects [1, 4], and a 24 % decrease has been reported in mean Purkinje cell size [33]. Two studies measured the levels of Reelin and Bcl-2 (two signaling proteins involved, respectively, in the control of neuronal migration and apoptosis) in the cerebellar cortex. The results showed more than 40 % reduction in Reelin and 34–51 % reduction in Bcl-2 in autistic cerebellum [34, 35]. Several recent immunological studies have suggested the involvement of cerebellum in the pathophysiology of autism [36–38]. Self-generated or maternally derived autoantibodies against cerebellar neural cells including Purkinje cells have been detected in the serum of ASD children [39]. A recent study showed that children with those autoantibodies had a higher degree of aberrant behavior and lower cognitive and adaptive functioning compared to children without [40].
Studies focusing on acquired lesions in children have uncovered behavioral features such as autistic-like stereotypical performance, obsessive rituals, and difficulty in understanding social cues whenever the lesion involved the vermis [41–43]. A meta-analysis of structural MRI studies on autism revealed that there is a reduction in the size of the VI, VII, and VIII, X vermal lobules although with significant heterogeneity. The slight differences found in the vermal lobules VIII, X could not be explained by age or IQ. Differences in the vermal lobules VI, VII were explained by the patients’ ages: the size of vermal lobules VI, VII was reduced in younger autistic individuals, while the reduction found in older ones appeared to be an artifact of IQ differences between case and control groups [44].
Anatomical studies have not conclusively identified the vermis as the principal target of limbic connections. Previous physiological and behavioral studies seemed to provide support for the postulated link between midline cerebellar structures and emotional modulation [45, 46]. Stimulation of the cerebellar vermis, for example, modulates firing patterns in the hippocampus, amygdala, and septum nuclei [47]. These observations suggest that there is a link between developmental anomalies of the vermis and neurobehavior. The contribution of the cerebellum to non-motor processes has in recent years been increasingly recognized by different neuroimaging techniques. Resting-state functional connectivity analysis in healthy subjects has shown that activity in the cerebellar anterior lobe is correlated with sensorimotor regions of the cerebral cortex, and the activity in lobule VII correlates with association areas in the prefrontal cortex [48], the posterior parietal regions, and the superior and middle temporal gyri [49]. These studies have indicated that the anterior lobe, lobules VI and VIII are engaged in tasks involving overt motor processing (finger tapping, articulation), while activation in lobules VI and VII is evoked during language production, spatial tasks, executive function, and affective processing [48–50]. A recent resting state fMRI study found that there are links between vermal lobules I–V, hemispheric VIII, IX and the hippocampus and amygdale [51]. A positron emission tomography study investigating the theory of mind in normal volunteers demonstrated that extensive cortical areas, including the vermis, the frontal lobes, and the limbic system, are activated [52]. According to the findings of that study, the cerebellum works in concert with the cerebral cortex to coordinate the process of imagining and describing the internal state of another person, interactively understanding, checking, and monitoring that state. A cerebellar dysfunction could presumably lead to an impaired ability to understand other person’s thoughts and feelings [52]. Studies examining cognitive features in patients with acquired cerebellar lesions [41, 42, 53] have described a range of deficits delineating a typical “cerebellar cognitive affective syndrome” [54] characterized by impairment of executive function, visual-spatial memory, and verbal fluency.
Even if the cerebellum is one of the most consistent abnormal brain region together with the areas connected to it, VBM studies on autism have described controversial findings concerning the increase [17, 55, 56] or reduction [12, 57, 58] in cerebellar volume. The contradictory findings reported could be due to the relatively small size of the samples studied and/or to differences in the inclusion criteria from one study to another (e.g. in age, intelligence quotient, presence of epilepsy, and endophenotypes of ASD). In particular, different phenotypes could explain the differences found in the volume of the vermis. Some studies have, in fact, described a more hypoplasic vermis in subjects with mental retardation and epilepsy and in particular when there is an involvement of lobules VI and VII in younger subjects [44].
Another region whose volume appeared to be decreased according to our findings was the Crus II of both cerebellar hemispheres. This area has never been found to be altered in imaging studies on ASD populations (both adults and children). Crus II is located in the lateral-posterior lobe of cerebellar hemispheres and is part of the cognitive cerebellum.
Together with the cerebral association areas and the ventral part of the dentate nucleus, the lateral cerebellar hemispheres have expanded markedly across evolution [30, 59]. Much of the lateral cerebellar region (the neocerebellum) comprises the hemispheric extensions of lobules VI and VII. A functional dichotomy has been described according to which the anterior lobe (lobules I–V) and lobule VIII are predominantly sensorimotor, while lobules VI and VII (including Crus I and II and lobule VIIB) contribute to higher-level functions [31, 45, 60]. Resting state fMRI studies detected functional connections between lobule Crus I, hemispheric Crus II, and the fronto-parietal network [48, 49, 51, 61]. The tractography maps indicate that the Crus I/II in the posterior cerebellum are linked to the lateral prefrontal areas which are activated by cognitive load increase, but this link was not found for the anterior cerebellar lobe [62]. A functional connectivity between the Crus II and the associative areas of the frontal and prefrontal cortex has become evident; these areas are involved in cognitive and planning tasks, problem solving, mental flexibility, as well as for monitoring complex behavior [63].
Volume loss has been found in the hippocampus bilaterally. The hippocampus is part of the limbic system that is involved in memory tasks, social cognition, and affective functions. Hippocampal volume alterations were described by neuropathological studies carried out by Bauman and Kemper (1985) who reported increased cell density and reduced cell size. Data from other studies measuring hippocampal volume in adults and children do not appear to be in agreement [64, 65].
Unilateral involvement of different areas of the cerebral hemispheres was also uncovered by our findings. In accordance with previous studies, we found reduction in the left inferior frontal cortex [9, 15, 55, 57, 58] and in the left postcentral gyrus [11, 66–68]. These areas are part of the Mirror Neuron System, which is activated during observation and imitation of others’ actions [69, 70] and is thought to facilitate early imitation of actions, development of language and executive functions, i.e., precursors of the theory of mind in children [71, 72]. Another area that seems to be involved in those tasks is the left superior frontal gyrus. Rarely found to be altered in individuals with ASD, it is thought to be involved in emotion evaluation [73], in active discrimination between the self and others [74, 75], and in facial emotion processing [76].
Just as in our previous VMB study [68], a reduction in GM was also found in the left inferior temporal gyrus. According to a growing body of evidence, ASD patients seem to be impaired in perceiving faces and objects. Together with the parahippocampal and lateral occipital gyri, the inferior temporal gyrus appears to be involved in common object perception. In autistic subjects, Schultz et al. [77] found fMRI alterations in the inferior temporal and fusiform gyri in face discrimination tasks.
A reduction in GM volume was also found in the right inferior occipital gyrus. Damage to this area leads to deficits in the recognition of facial expression [78]. Other areas linked to visual perception and recognition of faces such as the left occipital cortex (medial and superior gyrus, BA 17–18–19) were also found to be altered. These findings could cast light on alterations in facial expression recognition noted in ASD patients [67, 79].
Inconsistent results of previous volumetric studies underline, on the one hand, the extreme heterogenity of ASD and, on the other, differences in methodology (characteristics of the sample and post-processing methods). For instance, the relation between alterations of the vermis and behavior in autistic subjects using different post-processing approaches has been analyzed, such as manual [80] or semi-automated methods [81], which have produced contrasting results. In view of these considerations, it is quite difficult to compare the data from different studies, including our previous one [68]. The two studies that examined the correlation between behavioral scores and brain areas volumes [11, 15] investigated high-functioning subjects older than our patients. One used the VBM-DARTEL method and reported a reduction in GM volumes only in the insula and inferior frontal volumes [15].
The strength of this study includes the enrolment of a sizeable group of young ASD LF children, highly representative of the autistic population in whom intellectual disability is present on the average in 80 % [16]. Rigorous and validated imaging methodologies, comprehensive clinical and cognitive assessment of the patients, and novel image analysis techniques (never applied before) are all strengths of the study. The work can be considered limited because of the lack of homogeneity with regard to the ASD endophenotypes. Furthermore, autistic children have only been compared to normally developing subjects, and not to children with intellectual disability of homogenous etiology (like Fra-X Syndrome, Joubert Syndrome, etc.).
Conclusion
The correlation found between reduced GM volumes and ADI-R and ADOS interaction/social scores in young ASD LF children is an extremely interesting finding, especially in view of the cerebellum’s role in higher cognitive and social functions, and the frequency of cerebellar alterations in ASD. Our results not only confirm the involvement of cerebellum in ASD but also indicate that there is a significant correlation between reduction in cerebellar GM volume (vermis and Crus II) and reciprocal social communication measures.
References
Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35(6):866–74.
Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;3(31):137–45.
Dapretto M, Davies MS, Pfeifer JH. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:29–30.
Bauman ML, Kemper TL. Neuroanatomic observations of the brain in autism: a review and future directions. Int J Dev Neurosci. 2005;23(2–3):183–7.
Courchesne E, Redcay E, Kennedy DP. The autistic brain: birth through adulthood. Curr Opin Neurol. 2004;17:489–96.
Courchesne E, Müller RA, Saitoh O. Brain weight in autism: normal in the majority of cases, megalencephalic in rare cases. Neurology. 1999;52:1057–59.
Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58(1):1–9.
Langen M, Durston S, Staal WG, Palmen SJ, van Engeland H. Caudate nucleus is enlarged in high-functioning medication-naive subjects with autism. Biol Psychiatry. 2007;62(3):262–66.
McAlonan GM, Daly E, Kumari V, Critchley HD, van Amelsvoort T, Suckling J, et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain. 2002;125:1594–606.
Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, et al. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage. 2004;23:364–69.
Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, Tregellas JR. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56–68.
Ke X, Hong S, Tang T, Zou B, Li H, Hang Y, et al. Voxel-based morphometry study on brain structure in children with high-functioning autism. NeuroReport. 2008;9(19):921–25.
Palmen SJ, van Engeland H. Review on structural neuroimaging findings in autism. J Neural Transm. 2004;111:903–29.
Piven J, Arndt S. The cerebellum and autism. Neurology. 1995;45:398–402.
Kosaka H, Omori M, Munesue T, Ishitobi M, Matsumura Y, Takahashi T, et al. Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. NeuroImage. 2010;4(50):1357–63.
Fombonne E. Epidemiology of pervasive developmental disorders. Pediatr Res. 2009;65(6):591–98.
Spencer MD, Moorhead TW, Lymer GK, Job DE, Muir WJ, Hoare P, et al. Structural correlates of intellectual impairment and autistic features in adolescents. NeuroImage. 2006;4(33):1136–44.
Bonilha L, Cendes F, Rorden C, Eckert M, Dalgalarrondo P, Li LM, et al. Gray and white matter imbalance—typical structural abnormality underlying classic autism? Brain Dev. 2008;6(30):396–401.
Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–23.
Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–85.
Huerta M, Lord C. Diagnostic evaluation of autism spectrum disorders. Pediatr Clin North Am. 2012;59(1):103–11.
Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. NeuroImage. 2009;44(2):319–27.
Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38(1):95–113.
Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2011;14:21–36.
Ashburner J, Friston KJ. Voxel-based morphometry: the methods. NeuroImage. 2000;11:805–21.
Radua J, Via E, Catani M, Mataix-Cols D. Voxel-based meta-analysis of regional white-matter volume differences in autism spectrum disorder versus healthy controls. Psychol Med. 2011;41(7):1539–50.
Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. NeuroImage. 2004;2(22):619–25.
Bode MK, Mattila ML, Kiviniemi V, Rahko J, Moilanen I, Ebeling H, et al. White matter in autism spectrum disorders—evidence of impaired fiber formation. Acta Radiol. 2011;52(10):1169–74.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34.
Leiner H, Leiner A, Dow R. Does the cerebellum contribute to mental skills? Behav Neurosci. 1986;100:443–54.
Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiat Clin Neurosci. 2004;16(3):367–78.
Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, et al. Mapping early brain development in autism. Neuron. 2007;56(2):399–13.
Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22(2):171–75.
Fatemi SH, Stary JM, Halt AR, Realmuto GR. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J Autism Dev Disord. 2001;31(6):529–35.
Verhoeven JS, De Cock P, Lagae L, Sunaert S. Neuroimaging of autism. Neuroradiology. 2010;1(52):3–14.
Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, et al. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53(4):533–37.
Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178(1–2):149–55.
Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23(1):64–74.
Steinlein M. Cerebellar disorders in childhood: cognitive problems. Cerebellum. 2008;7(4):607–10.
Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25(3):514–23.
Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–50.
Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000;123:1051–61.
Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum. 2007;6(3):254–67.
Stanfield AC, McIntosh AM, Spencer M, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008;4(23):289–99.
Schmahmann JD. An emerging concept. The cerebellar contribution to higher function. Arch Neurol. 1991;48(11):1178–87.
Schmahmann JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. 2000;13:189–214.
Bobée S, Mariette E, Tremblay-Leveau H, Caston J. Effects of early midline cerebellar lesion on cognitive and emotional functions in the rat. Behav Brain Res. 2000;112(1–2):107–17.
Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19(10):2485–97.
Habas C, Kamdar N, Nguyen D, Prater K, Beckmann CF, Menon V, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29(26):8586–94.
Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–44.
Sang L, Qin W, Liu Y, Han W, Zhang Y, Jiang T, et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage. 2012;61(4):1213–25.
Calarge C, Andreasen NC, O’Leary DS. Visualizing how one brain understands another: a PET study of theory of mind. Am J Psychiatry. 2003;160(11):1954–64.
Scott RB, Stoodley CJ, Anslow P, Paul C, Stein JF, Sugden EM, et al. Lateralized cognitive deficits in children following cerebellar lesions. Dev Med Child Neurol. 2001;43(10):685–91.
Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–79.
McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, et al. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128:268–76.
Toal F, Daly EM, Page L, Dee ley Q, Hallahan B, Bloemen O, et al. Clinical and anatomical heterogeneity in autistic spectrum disorder: a structural MRI study. Psychol Med. 2010;7(40):1171–81.
Abell F, Krams M, Ashburner J, Passingham R, Friston K, Frackowiak R, et al. The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. NeuroReport. 1999;10:1647–51.
Salmond CH, Vargha-Khadem F, Gadian DG, de Haan M, Baldeweg T. Heterogeneity in the patterns of neural abnormality in autistic spectrum disorders: evidence from ERP and MRI. Cortex. 2007;6(43):686–99.
Dow R. The evolution and anatomy of the cerebellum. Biol Rev. 1942;17:179–20.
Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4(3):174–98.
O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20(4):953–65.
Salmi J, Pallesen KJ, Neuvonen T, Brattico E, Korvenoja A, Salonen O, et al. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22(11):2663–76.
Teffer K, Semendeferi K. Human prefrontal cortex: evolution, development, and pathology. Prog Brain Res. 2012;195:191–218.
Rojas DC, Smith JA, Benkers TL, Camou SL, Reite ML, Rogers SJ. Hippocampus and amygdala volumes in parents of children with autistic disorder. Am J Psychiatry. 2004;161:2038–44.
Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; The hippocampus is enlarged at all ages. J Neurosci. 2004;28(24):6392–01.
McAlonan GM, Suckling J, Wong N, Cheung V, Lienenkaemper N, Cheung C, et al. Distinct patterns of grey matter abnormality in high-functioning autism and Asperger’s syndrome. J Child Psychol Psychiatry. 2008;49(12):1287–95.
Hyde KL, Samson F, Evans AC, Mottron L. Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Hum Brain Mapp. 2010;4(31):556–66.
Riva D, Bulgheroni S, Aquino D, Di Salle F, Savoiardo M, Erbetta A. Basal forebrain involvement in low functioning autistic children: a voxel-based morphometry study. AJNR Am J Neuroradiol. 2011;32(8):1430–35.
Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–92.
Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7(12):942–51.
Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286:1692–95.
Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25(4):287–95.
Adolphs R. The social brain: neural basis of social knowledge. Ann Rev Psychol. 2009;60:693–16.
Sugiura M, Kawashima R, Nakamura K, Okada K, Kato T, Nakamura A, et al. Passive and active recognition of one’s own face. Neuroimag. 2000;11(1):36–48.
Sui J, Chechlacz M, Humphreys GW. Dividing the self: distinct neural substrates of task-based and automatic self-prioritization after brain damage. Cognition. 2012;122(2):150–62.
Li HJ, Chan RC, Gong QY, Liu Y, Liu SM, Shum D, et al. Facial emotion processing in patients with schizophrenia and their non-psychotic siblings: a functional magnetic resonance imaging study. Schizophr Res. 2012;134(2–3):143–50.
Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;4(57):331–40.
Adolphs R, Damasco H, Tranel D, Damasco AR. Cortical systems for the recognition of emotion in facial expressions. J Neurosci. 1996;23(16):7678–87.
Hadjikhanim N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;5(28):441–49.
Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49(8):655–64.
Webb SJ, Sparks BF, Friedman SD, Shaw DW, Giedd J, Dawson G, et al. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 2009;172(1):61–7.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riva, D., Annunziata, S., Contarino, V. et al. Gray Matter Reduction in the Vermis and CRUS-II Is Associated with Social and Interaction Deficits in Low-Functioning Children with Autistic Spectrum Disorders: a VBM-DARTEL Study. Cerebellum 12, 676–685 (2013). https://doi.org/10.1007/s12311-013-0469-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-013-0469-8