Abstract
The core symptoms of Autism Spectrum Disorder (ASD) are impairments in social interaction/communication and the presence of stereotyped and repetitive behaviour. The amygdala and hippocampus are involved in core functions in the “social brain” and, thus, may be of particular interest in ASD. Previous studies demonstrated inconsistent results, revealing both increased and reduced volume of these brain structures in individuals with ASD. In this study, we investigated the grey and white matter volumes of amygdala and hippocampus in primary-school-aged children with and without ASD. Also, we assessed the relationships between the volume of brain structures and behavioural measures in children with ASD. A total of 36 children participated in the study: 18 children with ASD (13 boys, age range 8.01–14.01 years, mean age (Mage) = 10.02, standard deviation (SD) = 1.76) and 18 age- and sex-matched typically developing controls (13 boys, age range 7.06–12.03 years, Mage = 10.00, SD = 1.38). The whole-brain structural magnetic resonance imaging (MRI) was applied to acquire T1 images for each child. The results showed a bilateral reduction in grey matter volume of amygdala and hippocampus in children with ASD, but no difference was found in white matter volume. Importantly, pathological reduction in grey matter volume of amygdala was associated with lower language skills and more severe autistic traits; also, a reduced grey matter volume of the left hippocampus was related to lower language skills in the ASD group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Difficulties in social interaction and communication and the presence of stereotyped and repetitive behaviour are among the core symptoms of Autism Spectrum Disorder (ASD; American Psychiatric Association 2013). Recent magnetic resonance imaging (MRI) studies have identified numerous structural brain abnormalities associated with core and co-occurring symptoms of ASD (Ecker 2017). The amygdala in tandem with hippocampus has been consistently investigated in ASD, given their involvement in core functions in the “social bran” and, thus, in core symptoms of this disorder (Banker et al. 2021; Goodman et al. 2014). Previous findings, however, provided contradictory evidence for the alterations in these regions.
Studies in the amygdala have demonstrated both volume enlargement and its reduction in children with ASD (Aylward et al. 1999; Murphy et al. 2012) as well as no difference between the ASD group and typically developing (TD) controls (Haar et al. 2016). Similarly, abnormalities in hippocampus in individuals with ASD were associated with both greater and lower volume (Eilam-Stock et al. 2016; Xu et al. 2020), while also there was an evidence for absence of difference between ASD and TD groups (Aylward et al. 1999). In addition, some studies have shown the atypical asymmetry of amygdala and hippocampus in children with ASD, whereas other findings did not demonstrate it (Monterrey et al. 2017; Richards et al. 2020). The studies also have revealed relationships between pathological increase as well as decrease in the volume of amygdala/hippocampus and social communication deficit, emotional problems, cognitive functioning, and language skills in individuals with ASD (e.g. Bachevalier and Loveland 2006; Banker et al. 2021; DeLong 1992). Inconsistencies in the findings may be caused by the difference in the age range of participants, methodology, highly heterogeneous nature of ASD population, etc.
This exploratory study aimed to investigate a volume of amygdala and hippocampus of primary-school-aged children with ASD compared to age- and sex-matched TD controls as well as patterns of asymmetry of these brain structures. Also, the study addressed the relationships between the volume of amygdala/hippocampus and clinical/behavioural characteristics in ASD group measured in formal assessment (non-verbal IQ, language skills, and the severity of autistic symptoms). The significance of the study is twofold: first, our sample of children included only primary-school-aged participants, whereas most of the studies consisted of children from a broader age range, so we were able to investigate the neurobiological mechanisms of clinical symptoms of a narrow and less-studied group of individuals with ASD. Second, we focused not on the total volume of amygdala and hippocampus (or only grey matter volume) as many studies did but on the grey and white matter volumes of these brain structures separately in the same groups of participants; this could help to understand the roles of grey/white matter in the difference of volume between ASD and TD groups as well as their association with clinical measures separately.
Methods
Participants
The data were collected from 18 children with ASD (13 boys, age range 8.01–14.01 years, mean age (Mage) = 10.02, standard deviation (SD) = 1.76) and 18 age- and sex-matched TD controls (13 boys, age range 7.06–12.03 years, Mage = 10.00, SD = 1.38). The ASD group met the criteria of the International Classification of Diseases—10, and 16 out of 18 children were also assessed with Autism Diagnosis Observation Schedule—Second Edition, ADOS-2 (Lord et al. 2012). A behavioural assessment of both groups of children included the screening of non-verbal intelligence (IQ) (Kaufman and Kaufman 2004; Raven 2000; Wechsler 1991), language abilities (Arutiunian et al. 2022), and severity of autistic symptoms measured with Autism Spectrum Quotient: Children’s Version, AQ (Auyeung et al. 2008). All children had normal hearing (based on the screening with Audiogramm version 4.6.1.3, Professional Audiometric System; Sennheiser HAD 280 audiometry headphones) and normal or corrected-to-normal vision. Table 1 provides the demographic information for both groups.
Structural MRI acquisition and processing
The whole-brain structural MRIs were acquired with a 1.5 T Siemens Avanto scanner with the following parameters: repetition time = 1900 ms, echo time = 3.37 ms, flip angle = 15°, matrix size = 256 × 256 × 176, voxel size = 1.0 × 1.0 × 1.0 mm3. Before MRI acquisition, each child had the opportunity to familiarise himself with the equipment, and an assistant provided a detailed explanation about procedure. No sedation was used during the scanning, and a total scanning time was ~ 7 min.
The MRI data processing was performed with Computational Anatomy Toolbox, CAT12 (http://www.neuro.uni-jena.de/cat/) and Statistical Parametric Mapping 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) on Matlab R2017a, using standard pipeline: an alignment of T1-weighted MRIs with the anterior commissure–posterior commissure (AC–PC) plane; segmentation into native-space grey matter, white matter, and cerebrospinal fluid images (the results of the segmentation of each MRI were inspected for the quality; all images had resolution = 85% ‘good’, two children with ASD had Image Quality Rating (IQR) = ‘sufficient’ and other participants had IQR from ‘good’ to ‘excellent’ based on the image quality measures provided by CAT12); an alignment of brain images from the native-space to the Montreal Neurological Institute standard space MNI-152 template; 4) a standard smoothing procedure with 8 mm FWHM Gaussian kernel. The normalised and smoothed volume data (grey/white matter) were extracted for the left and right amygdalae and hippocampi for further statistical analysis.
Statistical analysis
Linear mixed-effects models with nested contrasts were used to (1) compare volumes of amygdala and hippocampus between ASD and TD groups of children; (2) provide between-hemisphere (left vs. right) comparisons in the volumes of amygdala and hippocampus in children with and without ASD separately; and (3) assess the relationships between volumes of amygdala/hippocampus and behavioural measures in children with ASD. Correlation matrices were applied to explore the association between volumes of brain structures in ASD and TD groups separately.
The models were estimated in R (R Core Team 2019) with the lme4 package (Bates et al. 2015); the data were plotted with ggplot2 (Wickham 2016) and corrplot (Wei and Simko 2021) packages. Supplementary file 1 provides all R codes used in the analysis with the structures/formulae for each model.
Results
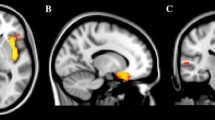
The tables with full model outcomes are provided in Supplementary file 2. A graphical illustration of regions investigated in the study can be seen in Fig. 1A.
The comparison of brain structures in children with and without ASD: A a graphical illustration of brain regions investigated in the study; B between-group differences in grey/white matter volume of amygdala and hippocampus (the significance is labelled with *p < 0.05, **p < 0.01, ns non-significant). Lighter green/yellow colours correspond to the TD group, darker green/yellow colours correspond to the ASD group; C Pearson correlations between all brain structures (upper matrix corresponds to the ASD group, lower matrix corresponds to the TD group). The scale refers to the correlation coefficient. Correlations that were not significant at p = 0.001 (corresponding to a Bonferroni correction for 36 statistical tests) are shown on a white background. L—left hemisphere; R—right hemisphere; gm—grey matter; wm—white matter
Between-group comparisons in the volume of brain structures
The results showed a bilateral reduction in grey matter volume of amygdala and hippocampus in children with ASD (p values are significant at the α = 0.025 level): left amygdala, β = 0.11, SE = 0.04, t = 3.00, p = 0.003; right amygdala, β = 0.10, SE = 0.04, t = 2.91, p = 0.004; left hippocampus, β = 0.25, SE = 0.10, t = 2.60, p = 0.009; right hippocampus, β = 0.22, SE = 0.10, t = 2.33, p = 0.02. There were no differences between groups in white matter volume of these brain structures (see Supplementary file 2, Tables 1–4; Fig. 1B).
Correlation matrices demonstrated that children with ASD have less associated with each other brain regions compared to TD controls (p values are significant at the α = 0.001 level; Fig. 1C).
Asymmetry of brain structures in children with and without ASD
For amygdala, we did not find a difference in both grey and white matter volumes between left and right hemispheres in both groups of children. For hippocampus, there was no difference in white matter volume in children with and without ASD, but there was significant difference in grey matter volume between hemispheres in both groups (p values are significant at the α = 0.025 level): ASD, β = 0.21, SE = 0.02, t = 8.57, p < 0.001; TD, β = 0.18, SE = 0.02, t = 7.48, p < 0.001. The results indicated that grey matter volume was larger in the right hippocampus in both groups of children (see Supplementary file 2, Tables 5–8).
The relationships between grey matter volume of brain structures and behavioural measures in children with ASD
To analyse how pathological reduction in grey matter volume of amygdala and hippocampus in children with ASD is related to clinical and behavioural measures, we fitted models with grey matter volume as a dependent variable and the individual characteristics of children (age, non-verbal IQ, language score, severity of autistic symptoms) as predictors. For amygdala, we found a significant relationship between grey matter volume and both language score and ADOS severity score: lower volume in both hemispheres was associated with lower language abilities and more severe autistic traits. For the left hippocampus, a lower grey matter volume was related to worse language skills (Table 2; see also Supplementary file 2, Tables 9, 10).
Discussion
In the present study, we investigated a grey/white matter volume of amygdala and hippocampus in children with ASD in comparison to age- and sex-matched TD controls. Additionally, we assessed the relationships between the volume of brain structures and behavioural characteristics of children with ASD, such as non-verbal IQ, language skills, and the severity of autistic symptoms. Overall, the results revealed a reduction of grey matter volume in both amygdala and hippocampus in the ASD group and also a significant association between abnormalities in grey matter volume and the severity of autistic symptoms/language skills in children with ASD.
Between-group comparisons showed that children with ASD have a bilateral decrease in grey matter volume of amygdala and hippocampus in comparison to TD controls, but no difference was found in white matter volume of these brain structures. Our results are in line with some of the previous findings showed that school-aged children with ASD had a reduced total brain volume (Baribeau and Anagnostou 2013; Courchesne et al. 2011; Libero et al. 2014) and, specifically, a reduced volume of brain regions related to psychopathology of ASD (Aylward et al. 1999; Eilam-Stock et al. 2016). In contrast to this evidence, most of the studies reported an increased brain volume in ASD; however, the vast majority of this research addressed toddlers and preschoolers with ASD, whereas studies in school-aged children with ASD are limited. Our findings confirmed the hypothesis that children’s age may account for inconsistencies in the results of brain volume in ASD and showed that brain volume can be reduced in older children compared to very young individuals with ASD (Baribeau and Anagnostou 2013). It is important to note, however, that the white matter volume of amygdala and hippocampus did not differ between children with and without ASD.
Although we found a difference in grey matter volumes of both amygdala and hippocampus between ASD and TD groups of children, there was no between-group difference in the pattern of asymmetry in these brain structures. Our results are in line with the comprehensive study of Richards et al. (2020) which showed that school-aged children with ASD were not differed from age-matched TD controls in the asymmetry of total volumes of amygdala and hippocampus. We contributed to these findings highlighting that not only the asymmetry of total volumes of amygdala and hippocampus but also the asymmetry in grey and white matter volumes of these brain structures separately did not differ between groups of children.
Given the known involvement of amygdala and hippocampus in the social communication and language functions, we assessed whether the pathological reduction in grey matter volume of these brain structures observed in the ASD group were associated with behavioural measures of these children. For amygdala, the results indicated that more reduced volume in both hemispheres was related to more impaired language skills and more severe autistic symptoms, which is in line with the previous findings (e.g. Nacewicz et al. 2006). For the hippocampus, we found a significant relationship between volume and language skills only in the left hemisphere, indicating that the lower volume was related to more severe language impairment. This can be explained by the fact that specifically the left hippocampus is connected to the language cortical network and associated with verbal memory (Ezzati et al. 2016). Importantly, the reduction of grey matter volume in both brain structures was not associated with children’s non-verbal IQ. It means that although non-verbal IQ can be related to language abilities and social functioning in ASD (see Arutiunian et al. 2022; Hirosawa et al. 2020), the neuropathology of different behavioural measures is distinct in this population. In addition, it has been demonstrated that amygdala and hippocampus are involved in core functions in the “social brain” and, subsequently, are more related to language and communication functions rather than non-verbal intelligence (Banker et al. 2021; Goodman et al. 2014). It is also important to note that the volume of brain structures was associated with the severity of autistic symptoms measured with ADOS but not AQ. One of the explanations of this discrepancy can be the fact that ADOS is a direct measure of child behaviour, whereas AQ is a parent report.
We acknowledge some limitations of our study. First, we argued that the volume of brain structures in school-aged children with ASD is reduced in comparison to increased volume in younger ASD participants. However, since the present study is not longitudinal, this statement is hypothetical. To test the possible developmental changes in volumes of amygdala and hippocampus, it is necessary to apply a longitudinal design and obtain the MRI images in preschool and school-aged periods. Second, to generalise the findings to the whole ASD population, it is necessary to include a larger sample of participants in the future research.
Conclusion
The present study focused on the grey/white matter volume of amygdala and hippocampus in a group of less-studied primary-school-aged children with ASD. The results showed a reduction of grey matter volume in both brain structures and, importantly, revealed the association between this reduction and the severity of autistic symptoms and language skills but not with non-verbal cognition in children with ASD. This highlighted the specific contribution of amygdala and hippocampus to social/communication skills of children with ASD and their involvement in core functions in the “social brain”.
Data availability
The datasets generated and analysed during the current study are not publicly available as it is human data but are available from the corresponding author on reasonable request.
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5, 5th edn. American Psychiatric Publication, Washington/London
Arutiunian V, Lopukhina A, Minnigulova A, Shlyakhova A, Davydova E, Pereverzeva D, Sorokin A, Tyushkevich S, Mamokhina U, Danilina K, Dragoy O (2022) Language abilities of Russian primary-school-aged children with autism spectrum disorder: evidence from comprehensive assessment. J Autism Dev Disord 52(2):584–599. https://doi.org/10.1007/s10803-021-04967-0
Auyeung B, Baron-Cohen S, Wheelwright S, Allison C (2008) The autism spectrum quotient: Children’s version (AQ-Child). J Autism Dev Disord 38(7):1230–1240. https://doi.org/10.1007/s10803-007-0504-z
Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD (1999) MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology 53(9):2145–2150. https://doi.org/10.1212/WNL.53.9.2145
Bachevalier J, Loveland KA (2006) The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev 30(1):97–117. https://doi.org/10.1016/j.neubiorev.2005.07.002
Banker SM, Gu X, Schiller D, Foss-Feig JH (2021) Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci 44(10):793–807. https://doi.org/10.1016/j.tins.2021.08.005
Baribeau DA, Anagnostou E (2013) A comparison of neuroimaging findings in childhood onset schizophrenia and autism spectrum disorder: a review of the literature. Front Psychol 4:175. https://doi.org/10.3389/fpsyt.2013.00175
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Courchesne E, Campbell K, Solso S (2011) Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 1380:138–145. https://doi.org/10.1016/j.brainres.2010.09.101
DeLong GR (1992) Autism, amnesia, hippocampus, and learning. Neurosci Biobehav Rev 16(1):63–70. https://doi.org/10.1016/S0149-7634(05)80052-1
Ecker C (2017) The neuroanatomy of autism spectrum disorder: an overview of structural neuroimaging findings and their translatability to the clinical settings. Autism 21(1):18–28. https://doi.org/10.1177/1362361315627136
Eilam-Stock T, Wu T, Spagna A, Egan LJ, Fan J (2016) Neuroanatomical alterations in high-functioning adults with autism spectrum disorder. Front Neurosci 10:237. https://doi.org/10.3389/fnins.2016.00237
Ezzati A, Katz MJ, Zammit AR, Lipton ML, Zimmerman ME, Sliwinski MJ, Lipton RB (2016) Differential association of left and right hippocampus volumes with verbal episodic and spatial memory in older adults. Neuropsychologia 93(Part B):380–385. https://doi.org/10.1016/j.neuropsychologia.2016.08.016
Goodman J, Marsh R, Peterson BS, Packard MG (2014) Annual research review: the neurobehavioral development of multiple memory systems—implications for childhood and adolescent psychiatric disorders. J Child Psychol Psychiatry 55(6):582–610. https://doi.org/10.1111/jcpp.12169
Haar S, Berman S, Behrmann M, Dinstein I (2016) Anatomical Abnormalities in Autism? Cereb Cortex 26(4):1440–1452. https://doi.org/10.1093/cercor/bhu242
Hirosawa T, Kontani K, Fukai M, Kameya M, Soma D, Hino S, Kitamura T, Hasegawa C, An K, Takahashi T, Yoshimura Y, Kikuchi M (2020) Different associations between intelligence and social cognition in children with and without autism spectrum disorders. PLoS One 15(8):e0235380. https://doi.org/10.1371/journal.pone.0235380
Kaufman AS, Kaufman NL (2004) Kaufman assessment battery for children, 2nd edn. American Guidance Service, New York
Libero LE, DeRamus TP, Deshpande HD, Kana RK (2014) Surface-based morphometry of the cortical architecture of autism spectrum disorders: volume, thickness, area, and gyrification. Neuropsychologia 62:1–10. https://doi.org/10.1016/j.neuropsychologia.2014.07.001
Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL (2012) Autism diagnostic observation schedule, 2nd edn. Western Psychological Services, New York
Monterrey JC, Philips J, Cleveland S, Tanaka S, Barnes P, Hallmayer JF, Hardan AY (2017) Incidental brain MRI findings in an autism twin study. Autism Res 10(1):113–120. https://doi.org/10.1002/aur.1720
Murphy CM, Deeley Q, Daly EM, Ecker C, O’Brien FM, Hallahan B, Loth E, Toal F, Reed S, Hales S, Robertson DM, Craig MC, Mullins D, Barker GJ, Lavender T, Johnston P, Murphy KC, Murphy DG (2012) Anatomy and aging of the amygdala and hippocampus in autism spectrum disorder: an in vivo magnetic resonance imaging study of Asperger Syndrome. Autism Res 5(1):3–12. https://doi.org/10.1002/aur.227
Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Alexander AL, Davidson RJ (2006) Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry 63(12):1417–1428. https://doi.org/10.1001/archpsyc.63.12.1417
R Core Team (2019) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 23 Mar 2023
Raven J (2000) The Raven’s progressive matrices: change and stability over culture and time. Cogn Psychol 41(1):1–48. https://doi.org/10.1006/cogp.1999.0735
Richards R, Greimel E, Kliemann D, Koerte IK, Schulte-Körne G, Reuter M, Wachinger C (2020) Increased hippocampal shape asymmetry and volumetric ventricular asymmetry in autism spectrum disorder. NeuroImage Clin 26:102207. https://doi.org/10.1016/j.nicl.2020.102207
Wechsler D (1991) The wechsler intelligence scale for children, 3rd edn. The Psychological Corporation, New York
Wei T, Simko V (2021) R package 'corrplot': Visualization of a Correlation Matrix
Wickham H (2016) ggplot 2: elegant graphics for data analysis. Springer, New York
Xu Q, Zuo C, Liao S, Long Y, Wang Y (2020) Abnormal development pattern of the amygdala and hippocampus from childhood to adulthood with autism. J Clin Neurosci 78:327–332. https://doi.org/10.1016/j.jocn.2020.03.049
Acknowledgements
We thank Roman Cheremin for providing access to the resources of the Center for Speech Pathology and Neurorehabilitation, Moscow, Russia. We also thank Olga Buivolova and Natalia Deeva for their assistance with participant recruitment in the MRI part of the study. Special thanks go to all children who participated in the study.
Funding
This work is an output of a research project implemented as a part of the Basic Research Program at the National Research University Higher School of Economics (HSE University).
Author information
Authors and Affiliations
Contributions
VA: conceptualization, methodology, investigation, data curation, formal analysis, writing—original draft, writing—review and editing, project administration; ED: investigation; DP: investigation; AS: investigation; ST: investigation; UM: investigation; KD: investigation; OD: writing—review and editing, resources. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
The approval for this study was obtained from the HSE University Committee on Interuniversity Surveys and the Ethical Assessment of Empirical Research (for the TD group) and the local ethics committee of the Moscow State University of Psychology and Education (for the ASD group). The study was conducted in accordance with the Declaration of Helsinki.
Consent to participate
A parent of each child signed a written consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arutiunian, V., Davydova, E., Pereverzeva, D. et al. Reduced grey matter volume of amygdala and hippocampus is associated with the severity of autistic symptoms and language abilities in school-aged children with Autism Spectrum Disorder: an exploratory study. Brain Struct Funct 228, 1573–1579 (2023). https://doi.org/10.1007/s00429-023-02660-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-023-02660-9