Abstract
Essential tremor (ET) is among the most prevalent movement disorders, and by some accounts, the most common form of cerebellar degeneration. Over the past 15 years, we have carefully documented a large number of postmortem changes within the cerebellum; these cerebellar changes differ significantly between ET and controls. A recent Consensus Classification of tremor proposed that ET patients with other neurological signs aside from action tremor (e.g., parkinsonism, ataxia, cognitive changes, dystonia) should be segregated off as “ET-plus”. This diagnostic concept has raised considerable controversy and its validity is not yet established. Indeed, “ET-plus” has not been distinguished from ET based on differences in genetics, pathology or prognosis. Here we determine whether ET cases differ from "ET-plus" cases in underlying pathological changes in the postmortem brain. We examined postmortem brains from 50 ET cases (24 ET and 26 ET-plus), using a set of 14 quantitative metrics of cerebellar pathology determined by histologic and immunohistochemical methods. These metrics reflect changes across the Purkinje cell (PC) body (PC counts, empty baskets, heterotopias), PC dendrites (swellings), PC axon (torpedoes and associated axonal changes), basket cell axonal hypertrophy and climbing fiber-PC dendrite synaptic changes. ET and ET-plus were similar with respect to 13 of 14 cerebellar pathologic metrics (p > 0.05). Only one metric, the linear density of thickened PC axon profiles, differed between these groups (ET = 0.529 ± 0.397, ET-plus = 0.777 ± 0.477, p = 0.013), although after correcting for multiple comparisons, there were no differences. If ET-plus were indeed a different entity, then the underlying pathological basis should be distinct from that of ET. This study demonstrated there were no pathological differences in cerebellar cortex between ET versus ET-plus cases. These data do not support the notion that ET and ET-plus represent distinct clinical-pathological entities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential tremor (ET) is among the most prevalent movement disorders [1], and by some accounts, the most common form of cerebellar degeneration [2], with clinical, neuroimaging and postmortem features that place it within the context of other forms of cerebellar degeneration [3, 4]. Research on etiology, pathogenesis, natural history and clinical features of ET has advanced considerably in the past decade. Case definition has been an area of increasing focus, and the term “ET-plus” was introduced in a recent consensus statement to distinguish and separate ET cases with signs of dystonia, cerebellar dysfunction, and rest tremor, among others, from those without such signs [5]. However, this terminology has engendered considerable controversy [6,7,8,9,10,11]. During these discussions, an important issue that has been raised is whether “ET-plus” cases differ from ET cases with respect to underlying changes in brain pathology. That is, is the proposed stratification grounded in an identifiable biological difference? Over the past 15 years, we have carefully documented a large number of postmortem changes within the cerebellum; these cerebellar changes differ significantly between ET and controls [12,13,14,15,16,17,18,19]. Here we systematically compared these cerebellar postmortem features in ET vs. ET-plus cases to determine whether there were any differences between the two proposed diagnostic categories.
Methods
Brain Repository, Study Subjects, and Clinical Assessment
As part of a broad effort to compare postmortem features in ET to other neurological disorders and controls [12], 50 ET brains were acquired through the Essential Tremor Centralized Brain Repository (ETCBR), a joint effort between investigators at University of Texas Southwestern and Columbia University. During life, subjects signed informed consent approved by these University Ethics Boards.
ET diagnoses were carefully assigned by a senior movement disorders neurologist (E.D.L.) utilizing three sequential methods [13]. First, the clinical diagnosis of ET was initially assigned by treating neurologists, and second, confirmed by E.D.L. using questionnaires, review of medical records and review of Archimedes spirals. Third, a detailed, videotaped, neurological examination was performed, action tremor was rated, and a total tremor score assigned [range = 0–36 (maximum)]. Also using questionnaire data, the final diagnosis of each ET case was re-confirmed using published diagnostic criteria [12, 14].
Data were collected on age of tremor onset, family history, medication use, and ET surgery. Comorbidities were assessed using the Cumulative Illness Rating Scale (CIRS) [20], and cognitive status with the Folstein mini-mental state examination (MMSE) [21].
The videotaped neurological examination also included detailed assessments of rest tremor, Parkinsonism, dystonia and intention tremor. Rest tremor was assessed while seated, standing, and walking. Other parkinsonian features were assessed and rated (i.e., Unified Parkinson’s Disease Rating scale) [22], although tone was not assessed on videotaped examination. Dystonia (sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both) was assessed with views of the face, neck, trunk and extremities: while seated, standing, and walking; with posture (arms extended in front of body and in “wing-beating” position); while drawing spirals, and while speaking and reading [23]. Intention tremor was assessed during the finger-nose-finger maneuver (10 repetitions per arm) and graded with rating = 1 if definitely present in either arm [24].
The 50 ET cases were then segregated into ET-plus based on the presence of any of the following features: tremor at rest, other features of Parkinsonism (i.e., a UPDRS rating > 1 on any item), dystonia, or intention tremor. Given the advanced age of the cohort, the extraordinarily high prevalence of tandem mis-steps and mild cognitive changes in advanced age, and the absence of any agreed upon ET-plus cut-offs with respect to these two variables, it was not feasible to use these items to stratify ET cases into ET vs. ET-plus.
Tissue Processing and Initial Examination
All brains had a complete neuropathological assessment at the New York Brain Bank at Columbia University. Brains had standardized measurements of brain weight (grams), postmortem interval (PMI = hours between death and placement of brain in a cold room or upon ice), Braak and Braak AD (Alzheimer’s Disease) staging for neurofibrillary tangles [25], and Consortium to Establish a Registry for AD (CERAD) ratings for neuritic plaques [26]. We did not include ET cases with Lewy body pathology (α-synuclein staining) or PSP pathology [27].
Quantitative Metrics of Cerebellar Pathology
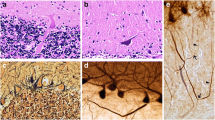
A standard 3 × 20 × 25-mm formalin-fixed tissue block from each brain was obtained from a parasagittal slice located 1–1.5 cm from the cerebellar midline and containing anterior and posterior quadrangulate lobules and the underlying dentate nucleus. As described, this block was used to quantify metrics within eight previously described broad categories of pathological change [12] (Table 1, Fig. 1).
Morphologic metrics to characterize cerebellar pathology. Metrics are shown for each category of pathologic change (see Table 1). (1) PC axonal torpedoes (arrows) identified in LH&E stain (a), Bielschowsky stain (d), and CB immunostain (g, h). (2) Heterotopic PC (arrow) identified in LH&E stain (b). (3) PC loss reflected by empty baskets in calbindinD28k (CB)-GAD dual immunostain (c arrows). (4) PC dendritic swelling (arrow) in Bielschowsky stain (e). (5) Basket cell hypertrophy is rated in a Bielschowsky stain (f, f’), where f basket rating = 1, and f’ basket rating = 3. (6) PC axonal changes, torpedo-related (arrows) identified in CB immunostain [g demonstrates torpedo (black arrow) followed by a thickened axon (white arrow), h demonstrates torpedo (black arrow) with axonal recurrent collateral (white arrow)]. (7) PC axonal changes, other than torpedoes (arrows) with a thickened axon in the CB immunostain (i). (8) Climbing fiber—PC synaptic changes identified by density of VGlut2 positive puncta in the molecular layer (j arrows), and climbing fibers in the outer 20% of the molecular layer (k arrows). Scale bars a, b, c, d, e, f, f’, k 50 μm (in panel k); g, h, i 50 μm (in panel i); j 50 μm
Within these eight broad categories were 37 quantitative metrics of pathological change. We previously performed a detailed analysis of these 37 metrics and identified 14 quantitative metrics of cerebellar pathology that were the key discriminators of severity of cerebellar pathology across ET, other cerebellar disorders, and controls [12]. Four stains (Table 1) were performed on 7 μm thick paraffin sections using methods previously described [12]. In addition, metrics referred to as “CB” were determined by the calbindinD28k immunostain performed on 100 μm thick formalin fixed vibratome sections to quantify Purkinje cell (PC) loss and an array of associated PC axonal changes [12, 14]. Some metrics were normalized to the length of the PC layer (per mm), to account for potential differences in amount of cerebellar cortex in the tissue block and/or microscopic field imaged, or to the PC count in that case (per PC), which in the setting of PC loss may affect the ability to detect the pathologic change.
Statistical Analysis
Data analyses were performed using SPSS (Version 24). The primary analysis was the comparison of two groups: ET vs. ET-plus. A Kolmogorov–Smirnov test was performed to test for normality of each continuous metric (e.g., age, LH&E torpedoes). As the large majority of these were not normally distributed, Mann–Whitney tests were used for group comparisons. Categorical metrics were compared across groups using Chi-square tests. The issue of multiple comparisons is important; hence, we presented both unadjusted and Bonferroni adjusted data in which the significant p value was 0.05/14 = 0.0036. In a secondary set of analyses, we compared subgroups of ET based on phenotypic features (rest tremor, intention tremor). Too few had dystonia for these types of analyses.
Sample size was identical to numerous previous studies of ours (i.e., 20—30 ET cases per subgroup) [28,29,30]; indeed, using data on the ET subgroups and assuming two-sided tests and alpha = 0.05, we calculated that we were powered to detect as little as a 15% difference between groups in numerous metrics (e.g., LH&E PC body linear density, CB PC body linear density).
Results
We compared the ET and ET-plus groups with respect to demographic and clinical features (Table 2). In 17 of 18 features, the two groups did not differ (p > 0.05). There was a higher percentage of males in the ET-plus category. Similarly, there was no significant difference in basic neuropathologic metrics, including PMI, brain weight or Braak AD and CERAD scores between these two groups. ET and ET-plus were similar with respect to 13 of 14 cerebellar pathologic metrics (p > 0.05), with many mean and median values being very similar or identical (Table 2). Only one metric, the CB thickened PC axon profile density, differed between these groups (p = 0.01). After Bonferroni correction, ET and ET-plus differed with respect to none of the 14 cerebellar pathological metrics.
We tested for possible confounding due to the gender difference between ET and ET-plus cases. There was no significant difference between males (n = 17) and females (n = 33) in any of the 14 cerebellar pathologic metrics (Mann Whitney tests, all p > 0.05). Thus, gender was not a significant confounder in this analysis.
We next performed two additional phenotypic analyses. First, all ET cases were grouped by presence (n = 18) or absence (n = 32) of rest tremor while seated, standing, or walking (Table 3). Second, we grouped all ET cases by presence (n = 14) or absence (n = 36) of intention tremor (Table 3). In both analyses, there was no significant difference in any of the 14 cerebellar pathology metrics (p > 0.05) (Table 3).
Discussion
The goal of this study was to determine whether the new ET case definition proposed in a 2018 Consensus Classification on tremor is grounded in a biological difference as assessed by cerebellar pathologic changes in ET postmortem brain cases stratified as ET versus ET-plus. Using a cohort of 50 ET cases who had been characterized in detail both clinically and pathologically, we determined that there were no differences between cases defined as ET versus ET-plus, and none of the cerebellar pathologic metrics significantly differed when ET cases were grouped by rest tremor or intention tremor.
Several aspects of the study design merit additional consideration. First, the postmortem work was performed in a single region of the cerebellar cortex, corresponding to motor cerebellum, and does not represent the activity of the entire cerebellum and/or the cerebello-thalamo-cortical loop. Nonetheless, an abundance of data from this region demonstrate postmortem changes that distinguish ET cases from controls [12,13,14,15,16,17,18,19] (Fig. 2). Second, the UPDRS did not include an assessment of rigidity; nonetheless, it is unlikely that an ET case would have rigidity in the absence of bradykinesia. Third, given the advanced age of the cohort, the high prevalence of tandem mis-steps or mild cognitive changes in the elderly, and the absence of established ET-plus cut-offs with respect to these variables, these items could not be used to stratify ET cases. Fourth, this study assesses pathological characteristics on postmortem examination. It remains a possibility that ET and ET-plus differ solely with respect to neuronal function, and that this might not be evident on postmortem examination. While this is a possibility, it is not likely that the two differ solely on a functional, physiological level, as it has been abundantly documented that there is a rich array of postmortem changes in the ET brain. Indeed, current evidence demonstrates that ET is not characterized solely by functional changes, but rather has an anatomic-pathological signature. It would be reasonable to assume that these postmortem changes, or at least several of these, would differ across valid ET subtypes, particularly as there are clear clinical differences between the supposed subtypes. Finally, our metrics were numerous and were grounded in many controlled studies. In the future, however, additional differentiating metrics could be discovered that would be relevant to these analyses.
Cerebellar morphological changes in control versus ET and ET-plus. Four categories of morphological change are illustrated, including PC cell body linear density (arrows) in LH&E stain (a, b, c), axonal changes including torpedoes (black arrows) and axonal recurrent collaterals (white arrows) in CB immunostain (d, e, f), basket cell hypertrophy in Bielschowsy stain (g, h, i), and climbing fibers in the outer 20% of the molecular layer in VGlut2 immunostain (arrows) (j, k, l). ET and ET-plus have a reduced PC linear density, increased axonal torpedoes and axonal recurrent collaterals, basket cell hypertrophy, and climbing fibers in the outer 20% of the molecular layer compared to control. Scale bars a-f 100 μm (in panel f); g-l 100 μm (in panel l)
Strengths of this study include use of a large cohort of ET cases prospectively followed and carefully phenotypically characterized by a senior movement disorders neurologist. Second, the two groups were similar with respect to nearly all demographic, clinical and basic pathological variables (e.g., PMI, CERAD); the one difference, gender, was assessed as a potential confounder and it was not. Third, the choice of quantitative metrics of cerebellar pathology was based on > 15 years of postmortem studies of ET in which we have carefully documented numerous significant ET case vs. control differences [14,15,16,17,18,19, 28,29,30]. The metrics used a broad array of histological and immunohistochemical methods.
If ET-plus were a different entity, then, logically, the postmortem abnormalities should be distinct from those of ET. This study demonstrated that this was not the case. These data do not provide support for the notion that ET and ET-plus represent distinct clinical-pathological entities.
References
Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41.
Louis ED, Faust PL. Essential tremor: the most common form of cereb ellar degeneration? Cerebellum Ataxias. 2020;7:12. https://doi.org/10.1186/s40673-020-00121-1.
Louis ED, Faust PL. Essential Tremor Within the Broader Context of Other Forms of Cerebellar Degeneration. Cerebellum. 2020;19(6):879–96. https://doi.org/10.1007/s12311-020-01160-4.
Filip P, Lungu OV, Manto MU, Bareš M. Linking Essential Tremor to the Cerebellum: Physiological Evidence. Cerebellum. 2016;15(6):774–80. https://doi.org/10.1007/s12311-015-0740-2.
Bhatia KP, Bain P, Bajaj N, et al. Consensus statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33:75–87.
Pandey S, Bhattad S, Hallett M. The problem of questionable dystonia in the diagnosis of ‘essential tremor-plus’ Tremor Other Hyperkinet Mov (NY). 2020;10:27. https://doi.org/10.5334/tohm.539.
Louis ED. “Essential Tremor Plus”: A Problematic Concept: Implications for Clinical and Epidemiological Studies of Essential Tremor. Neuroepidemiology. 2020;54:180–4.
Louis ED, Bares M, Benito-Leon J, et al. Essential tremor-plus: a controversial new concept. Lancet Neurol. 2020;19:266–70.
Vidailhet M. Essential tremor-plus: a temporary label. Lancet Neurol. 2020;19:202–3.
Louis ED. Essential tremor: “Plus” or “Minus”. Perhaps now is the time to adopt the term “the essential tremors.” Parkinsonism RelatDisord. 2018;56:111–2.
Prasad S, Pal PK. Reclassifying essential tremor: Implications for the future of past research. Mov Disord. 2019;34:437.
Louis ED, Kerridge CA, Chatterjee D, et al. Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach. Acta Neuropathol. 2019;138(5):859–76. https://doi.org/10.1007/s00401-019-02043-7.
Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–307. https://doi.org/10.1093/brain/awm266.
Babij R, Lee M, Cortés E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136(Pt 10):3051–61. https://doi.org/10.1093/brain/awt238.
Lee PJ, Kerridge CA, Chatterjee D, Koeppen AH, Faust PL, Louis ED. A Quantitative Study of Empty Baskets in Essential Tremor and Other Motor Neurodegenerative Diseases. J Neuropathol Exp Neurol. 2019;78(2):113–22. https://doi.org/10.1093/jnen/nly114.
Louis ED, Kuo SH, Tate WJ, et al. Heterotopic Purkinje Cells: a Comparative Postmortem Study of Essential Tremor and Spinocerebellar Ataxias 1, 2, 3, and 6. Cerebellum. 2018;17(2):104–10. https://doi.org/10.1007/s12311-017-0876-3.
Choe M, Cortés E, Vonsattel JP, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: Random sampling quantification and nearest neighbor analysis. Mov Disord. 2016;31(3):393–401. https://doi.org/10.1002/mds.26490.
Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014;137(Pt 12):3142–8. https://doi.org/10.1093/brain/awu314.
Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137(Pt 12):3149–59. https://doi.org/10.1093/brain/awu281.
Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–6. https://doi.org/10.1111/j.1532-5415.1968.tb02103.x.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. https://doi.org/10.1016/0022-3956(75)90026-6.
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. MovDisord. 2003;18(7):738–50. https://doi.org/10.1002/mds.10473.
Louis ED, Hernandez N, Alcalay RN, Tirri DJ, Ottman R, Clark LN. Prevalence and features of unreported dystonia in a family study of “pure” essential tremor. Parkinsonism Relat Disord. 2013;19(3):359–62. https://doi.org/10.1016/j.parkreldis.2012.09.015.
Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: Prevalence and association with disease duration. Mov Disord. 2009;24(4):626–7. https://doi.org/10.1002/mds.22370.
Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. https://doi.org/10.1007/BF00308809.
Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–86. https://doi.org/10.1212/wnl.41.4.479.
Louis ED, Babij R, Ma K, Cortés E, Vonsattel JP. Essential tremor followed by progressive supranuclear palsy: postmortem reports of 11 patients. J Neuropathol Exp Neurol. 2013;72(1):8–17. https://doi.org/10.1097/NEN.0b013e31827ae56e.
Louis ED, Kuo SH, Tate WJ, Kelly GC, Faust PL. Cerebellar pathology in childhood-onset vs. adult-onset essential tremor. NeurosciLett. 2017;659:69–74. https://doi.org/10.1016/j.neulet.2017.08.072.
Kuo SH, Wang J, Tate WJ, Pan MK, Kelly GC, Gutierrez J, Cortes EP, Vonsattel JG, Louis ED, Faust PL. Cerebellar Pathology in Early Onset and Late Onset Essential Tremor. Cerebellum. 2017;16(2):473–82. https://doi.org/10.1007/s12311-016-0826-5.
Louis ED, Kuo SH, Wang J, Tate WJ, Pan MK, Kelly GC, Gutierrez J, Cortes EP, Vonsattel JG, Faust PL. Cerebellar Pathology in Familial vs. Sporadic Essential Tremor Cerebellum. 2017;16(4):786–91. https://doi.org/10.1007/s12311-017-0853-x.
Acknowledgments
This work was supported by the National Institutes of Health (NINDS R01 NS088257).
Author information
Authors and Affiliations
Contributions
The following is a list of all authors and their contributions in the project and the preparation of the manuscript. These include but are not restricted to: (1) research project: A. conception, B. organization, C. execution; (2) statistical analysis: A. design, B. execution, C. review and critique; and (3) manuscript: A. writing of the first draft, B. review and critique.
Mr. Gionco: 1B, 1C, 2B, 2C, 3A, 3B.
Ms. Hartstone: 1C, 3B.
Dr. Martuscello: 1C, 3B.
Dr. Kuo: 1A, 1B, 3B.
Dr. Faust: 1A, 1B, 1C, 2A, 2C, 3B.
Dr. Louis: 1A, 1B, 2A, 2B, 2C, 3B.
Corresponding author
Ethics declarations
Conflicts of Interest
There are no conflicts of interest or competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gionco, J.T., Hartstone, W.G., Martuscello, R.T. et al. Essential Tremor versus “ET-plus”: A Detailed Postmortem Study of Cerebellar Pathology. Cerebellum 20, 904–912 (2021). https://doi.org/10.1007/s12311-021-01263-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-021-01263-6