Abstract
Although a decreased number of cerebellar Purkinje cells (PCs) in the autistic brain has been widely reported with a variety of qualitative and quantitative methods, the more accurate method of cell counting with modern stereology has not yet been employed. An additional possible problem with prior reports is the use of Nissl staining to identify the PCs, as this can miss cells due to staining irregularities. In the present study, PCs were immunostained for calbindin-D28k (CB), as this has been shown to be a more reliable marker for PCs than the Nissl stain, with more than 99% of the PCs immunopositive (Whitney, Kemper, Rosene, Bauman, Blatt, J Neurosci Methods 168:42–47, 2008). Using stereology and CB immunostaining, the density of PCs was determined in serial sections from a consistently defined area of the cerebellar hemisphere in four control and six autistic brains, with the density of PCs then correlated with the clinical severity of autism. Overall, there was no significant difference in the density of PCs between the autistic and control groups. However, three of six autistic brains had PC numbers that fell within the control range, whereas the remaining three autistic brains revealed a reduction compared with the control brains. These data demonstrate that a reduction in cerebellar PCs was not a consistent feature of these autistic brains and that it occurred without discernible correlation between their density and the clinical features or severity of autism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism is a behaviorally defined disorder associated with deficits in social interaction and communication as well as restricted repetitive behaviors. There is considerable variability in these clinical features among individuals [1], and to date, the neurobiological substrate of this variability has not been determined. Frequently reported neuropathological abnormalities in the autistic brain include disturbances in neuronal number, size, and packing density in the limbic and cerebellar systems [2–10]. In the cerebellum, decreased numbers of Purkinje cells (PCs) have been frequently reported using both quantitative [4, 7, 9] and qualitative [2, 3, 5, 6, 8, 10] methods, with the cerebellar hemispheres the most consistent area of involvement [3–5, 7, 8, 10]. In a comprehensive review of the neuropathology of autism, Palmen et al. [11] indicated that 21 of 29 autistic cases reported in the literature had reduced PC numbers. Since this publication [11], an additional study reported decreased PC numbers in nine of ten autistic cases [10].
The present study was designed to determine the density of the PCs in a defined area of the cerebellum in control and autistic brains using modern stereological sampling principles and identification of PCs by immunostaining for calbindin-D28k (CB). The previous studies cited above, which report a decreased density of PCs, have not utilized modern stereological methodology, the currently accepted quantitative method for cell counting [12]. Further, these prior studies have identified the PCs using Nissl-stained material [2–9]. Recent work from this laboratory has demonstrated that Nissl-stained material may fail to identify intact PCs, whereas immunostaining for CB is a more reliable marker, with more than 99% of the PCs immunopositive [13]. Further, given the importance of the cerebellum in modulating behavioral [14–19] and cognitive [14–17, 20] functions, we have sought, for the first time, to determine the relationship of any changes in the density of PCs with the clinical severity of autism.
Materials and Methods
Formalin-fixed cerebella were obtained from the Harvard Brain Tissue Resource Center, Kathleen Price Bryan Brain Bank at Duke University Medical Center and University of Maryland Brain Bank. Six autistic and four control brains, ages 13–54 years, were included in the study. All control brains were free from gross pathology and were obtained from individuals with no history of neurological disorders. The case number, age, sex, hemisphere, postmortem interval (PMI), years in formalin, and cause of death are shown in Table 1.
The diagnosis of autism, based on DSM-IV criteria, had been made prior to death in all cases. The classification of the severity of autistic features was determined by review of medical records and by the telephoned administration of the Autism Diagnostic Interview (ADI) by a reliable research assistant under the supervision of one of the authors, MLB (Table 2).
Tissue Processing
In each case, a 2 × 2 × 2-cm parasagittal block of tissue, cut perpendicular to the folia, was obtained from cerebellar hemisphere lobule crus II located inferior to the horizontal fissure [21]. This site was chosen because this area has most consistently demonstrated a reduction in PCs [3–5, 7, 8, 10]. Prior to tissue processing, each case was coded to ensure that the investigators were blind to the case data throughout the experimental process. All tissue blocks were cryoprotected [22] and embedded in an egg albumin gelatin to minimize tissue damage [23]. The blocks were then flash-frozen in −75°C 2-methylbutane and placed in a −80°C freezer for at least 48 h before being serially sectioned on a sliding microtome from medial to lateral. Eighteen series per brain were cut at 30 μm, and two series were cut at 60 μm so that sections within a series were spaced 660 μm apart. Sections were thaw-mounted onto gelatin-subbed slides, air-dried, and stored in a −20°C freezer until the day they were stained.
In each brain, ten 30-μm on-the-slide sections were immunostained for CB using specially designed wells (PolyFab Inc., Avon, MA, USA). All tissue sections from all brains were batch-processed in four groups (two pairs, two triplets) and matched for age and sex to ensure that conditions were identical for autistic and control groups. The optimum anti-CB antibody concentration (mouse monoclonal) was predetermined to be 1:1,000 (Swant Laboratories, Switzerland). Sections were pretreated in a 1% hydrogen peroxide solution to quench endogenous peroxidases and a blocking serum to minimize nonspecific binding. Sections were then incubated in the primary antibody for 48 h at room temperature followed by a 1-h incubation in a biotinylated secondary antibody (Vector Laboratories, Inc., Burlingame, CA, USA). An avidin–biotinylated peroxidase complex solution was then added (Vector Laboratories, Inc.), and the antigen was visualized using 3,3′-diaminobenzidine as the chromogen which is oxidized by incubating in hydrogen peroxide (1.5%) for 10 min. The reaction was stopped by washing sections in dH20. Sections were air-dried overnight, lightly counter-stained with thionin and coverslipped with Permount.
Purkinje Cell Counts
The equipment and computer software was identical for all counting procedures. This included a Nikon Eclipse E600 microscope (Nikon Instruments Inc., Melville, NY, USA), Optronics DEI-750 CE camera control unit (Optronics, Goleta, CA, USA), MAC 2002 motor stage control unit (Ludl Electronic Products, Ltd., Hawthorne, NY, USA), and Neurolucida® computer software from MircoBrightField Inc. (Williston, VT, USA).
Ten sections from each autistic and control brain, equally spaced by 660 μm, were counted. All blocks were cut from hemisphere lobule crus II located inferior to the horizontal fissure [21]. However, because of variability in the tissue samples provided by the brain banks, we were not able to definitively identify the identical folia in all brains that could be counted in their entirety, so data are presented as linear density measures relative to the length of the PC layer. However, in all brains, the superior-most folium in the most medial section was identified for counting, and stereological sampling principles were maintained across all sections [24, 25]. The PC layer was used as the region of interest (ROI) and the PC soma was used as the counting target, since the nucleus and nucleolus are often obscured by the dense immunoreaction product filling the soma. Given the relatively large size of the PC soma [13], PCs in these sections could be reliably counted with the 40× objective lens. This lower magnification and the organized nature of the PC layer made it feasible to count all PCs within the ROI. Using Neurolucida® computer software (MircoBrightField Inc.), PCs were systematically sampled within the ROI using a modified stereological approach. This approach was based on the optical disector method [24, 25] and ensured that all objects, regardless of size, shape, and orientation, had an equal chance of being counted.
In all sections, the length of the PC layer in the region of interest was measured using a 2× objective lens. The magnification was then increased to a 40× objective lens with Nomarski optics, and the length of the PC layer was assessed in its entirety in a stepwise fashion. This is an exhaustive counting scheme that allowed the identification of every PC in the region of interest. In each counting field, however, an exclusionary plane at the top of the section was implemented to avoid double counting in the z-axis. This z-axis exclusionary plane was used instead of z-axis guard volumes because sections with an original thickness of 30 μm, when thaw-mounted onto slides, shrink in the z-axis to 7.5–8.5 μm. At this thickness and with the optics available, it was not possible to implement reliable z-axis guard volumes. While this does introduce problems due to lost caps [26], the same process was used in all cases, and therefore, the relative differences between autistic and control cases were not affected. Along the x- and y-axes, the entire region of interest was measured in a stepwise and systematic manner. Along these dimensions, the leading edge of a PC was counted when it entered the counting field. PCs are easily identifiable by their large size and location along the PC layer. Once a PC was counted, it was clearly marked and was not at risk of being recounted. All CB immunopositive PCs were counted; these data represent the total number of PCs (N T), since previous work has demonstrated that >99% of PCs are CB immunopositive [13]. The total number of PCs counted per brain ranged from 238 to 1,422 with a mean of 694 PCs per brain.
The total number of CB immunopositive PCs (N T) was then used to calculate the density of CB immunopositive PCs (N T-L) along a known length of the PC layer, L(ref) using the following formula:
where N T-L represents the total number of PCs per millimeter (#PCs/mm).
Statistical Analysis
The t test was performed for comparison of the #PCs/mm between the autistic and control brains. Additionally, the variability within each brain was assessed by calculating the standard deviation from the ten sections analyzed per brain. The sampling error in each case was also examined by calculating the standard error of the mean (SE) and coefficient of error (CE). Ninety-five percent confidence intervals were also calculated in each brain using the ten analyzed sections per brain. To examine the relationship between #PCs/mm and years in formalin and #PCs/mm and PMI, Pearson correlation was performed. Finally, the Spearman correlation was used to examine the relationship between #PCs/mm and the severity of autistic features, with both data sets ranked on an ordinal scale.
Results
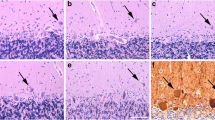
The CB PC counts, which represent total PC counts, ranged from 4.0 to 5.4 PCs/mm with a mean ± SD of 4.7 ± 0.8 PCs/mm in the control brains, whereas cell counts in the autistic brains spanned a wider range, 0.5 to 5.8 PCs/mm with a mean ± SD of 3.5 ± 1.8 PCs/mm (Fig. 1). With the available number of cases, statistical analysis using the t test did not detect a significance difference in the autistic brains as compared with the control brains (p = 0.25). There is clearly a dichotomy in this sample of six autistic brains. Although statistical significance was not reached in this sample of brains, the data suggest that there may be a subset of individuals with autism with reduced PC numbers. Specifically, in three of the autistic brains (2431, 4259, 3511), the number of PCs per millimeter closely approximated the control range, whereas the number of PCs in the other three autistic brains (4414, 3845, 4099) fell below the control range (Fig. 1).
Number of PCs per millimeter (#PCs/mm) in each control (light gray bars) and autistic (black bars) case from the calbindin-D28k-immunostained series. The PC counts in the control group ranges from 4.0 to 5.4 PCs/mm with a mean ± SD of 4.7 ± 0.8 PCs/mm, whereas PC counts in the autistic group ranged from 0.5 to 5.8 PCs/mm with a mean ± SD of 3.5 ± 1.8 PCs/mm. Statistical analysis using the t test did not detect a significant difference between the two groups, with p = 0.25. Photographs from autistic brains 3511, 3845, and 4414 illustrate the variability in PC density. Magnification 25×
Given the limited sample size, the stability of the data within each case was assessed by calculating the intra-case standard deviation using the ten analyzed sections per brain. Results demonstrate that the intra-case variability for the control and autistic groups were similar; the standard deviation in the control group ranged from 0.45 to 0.98, and the autistic group ranged from 0.14 to 0.95 (Table 3). Further, correlation analysis revealed that the intra-case variability, as measured by standard deviation, had a significant relationship to the #PCs/mm (r = 0.77, p = 0.01), but not to group (autistic, control; r = 0.33, p = 0.35). Thus, the greater the #PCs/mm, the greater the intra-case variability regardless of whether the brain was from a control or autistic subject. Additionally, for each case, we calculated the SE and CE (Table 3). These calculations are indicators of sampling error and demonstrate that our overall sampling error was low. Thus, the intra-case variability identified in these cases appears to represent normal variability in the human brain.
Ninety-five percent confidence intervals were also calculated in each case using the ten sections analyzed per brain (Table 3, Fig. 2). These intervals, which delineate the range wherein one is 95% confident that the true mean lies, demonstrate almost identical minimum and maximum range values for the four control brains and the three autistic brains with #PCs/mm in the control range (3511, 4259, 2431). The confidence interval values for the four control brains ranged from 3.4 to 6.2 PC/mm and in autistic brains 3511, 4259 and 2431 ranged from 3.4 to 6.3 PCs/mm. The confidence interval in autistic brain 4099 overlapped with the control range in its upper limit of 3.8 PCs/mm, whereas cases 4144 and 3845 have 95% confidence interval without control brain overlap and confidence intervals of 0.4–0.6 and 2.4–2.8 PCs/mm, respectively.
Number of PCs per millimeter (#PCs/mm) in each case with 95% confidence intervals (control cases, filled square; autistic cases, open diamond). These data reveal confidence intervals with almost identical maximum and minimum ranges for the four control brains (3.4–6.2 PC/mm) and autistic brains 3511, 4259, and 2431 (3.4–6.3 PCs/mm). Autistic brain 4099 overlaps with the control range in its upper limits (2.8–3.8 PC/mm), whereas cases 4144 and 3845 have 95% confidence interval without control brain overlap and confidence intervals of 0.4–0.6 and 2.4–2.8 PCs/mm, respectively
Pearson correlation, used to examine the relationship between #PCs/mm and years in formalin (two control, six autistic), returned a value of r = 0.68 (p = 0.06). These data, although not statistically significant, suggest that 46% of the variability in PC density may be accounted for by the number of years in formalin (r 2 = 0.46). It is important to note, however, that this is a positive correlation; as years in formalin increases, the #PCs/mm also increases. Thus, longer time periods in formalin did not appear to have a detrimental effect on the PCs. Further, to assess whether one brain had a disproportionate influence on the correlation coefficient, the correlations were repeated with one case missing per iteration. These data indicate that one brain did not have a disproportionate influence on the PC numbers with correlation coefficients (r) ranging from 0.56 to 0.78. Finally, although a positive correlation between #PCs/mm and years in formalin exists, qualitative review of the data suggests that this relationship did not have a major impact on the key findings. Specifically, the autistic cases with the fewest #PCs/mm (4414, 3845, 4099) were in formalin for similar timeframes as control case 4334 and autistic case 4259, which had PC numbers in the control range (Table 1).
Pearson correlation was also used to examine the relationship between #PCs/mm and PMI (two control, five autistic), with r = 0.72 (p = 0.06). The correlations were repeated with one case missing per iteration to assess whether one brain had a disproportionate influence on the correlation coefficient. These data indicate that case 4144 did have a disproportionate influence on the correlation coefficient of 0.72. In the one iteration without case 4144, Pearson correlation returned a value of r = 0.12 (p = 0.82). In contrast, the six iterations that included case 4144 returned correlation coefficient values ranging from 0.69 to 0.87. These numbers clearly demonstrate that case 4144 is driving the correlation coefficient. Table 1 shows that this case had the longest PMI and fewest #PC/mm. Given this finding, sections from the anterior lobe of the cerebellum in case 4144 were processed in the identical manner as sections from the posterior lobe. These sections revealed abundant CB immunopositive PCs (Fig. 3), suggesting that PMI is unlikely to be the major factor contributing to reduced PC numbers in case 4144.
Comparison of the density of PCs in the anterior and posterior cerebellar lobes of autistic brain 4414. Calbindin-D28k-immunostained sections from brain 4414 in the anterior lobe (a) and lateral aspect of the posterior lobe (b). Note the abundance of PCs (arrow) in the anterior lobe (lobule V, located superior to the primary fissure) compared with the posterior lobe (lobule crus II, located inferior to the horizontal fissure). Magnification 25×
Spearman correlation, used to examine the relationship between #PCs/mm and classification of autism, returned a value of r = 0.03 (p = 0.50); thus, in this sample of cases, a significant relationship between #PCs/mm and severity of autistic features was not identified.
In addition to obtaining PC numbers, we also gathered information regarding the distribution of PCs across sections in each case. In the brains with reduced PC density (autistic brains 4414, 3845, and 4099), qualitative analysis reveals that PCs tended to be distributed in small clusters throughout the folia. Review of serial sections demonstrates that areas void of PCs in one section were populated with PCs in other sections within the series (Fig. 4); the converse was also true. This indicates that a localized deficit in number of PCs is widely distributed throughout the folia rather than confined to one large region.
Location of PCs remaining along the PC layer in three serial sections from the lateral aspect of the posterior lobe in autistic brain 4414 (serial sections 81, 101, 121). These sections illustrate that a localized deficit in PCs is widely distributed throughout the folia rather than confined to one specific area
Discussion
Cell Counting Data
Based on previous studies indicating a reduction in PCs in the large majority of autistic cases (see [11] for comprehensive review), we were surprised that total PC counts were not lower in the autistic group compared with the control group. Instead, implementation of systematic stereological cell counting principles and CB immunostaining for the first time in the autistic cerebellum revealed striking variability between the six autistic brains in this sample. The within brain variability, in contrast, was similarly stable in the control and autistic cases (Table 3). The mean #PCs/mm in all four control brains and three of the six autistic brains (2431, 4259, 3511) fell within the range of normal PC numbers reported by Hall et al. [27] in a comprehensive study of 90 human brains [27]. Based on this same report [27], autistic brains 4414 and 3845 demonstrated a reduced number of PCs, and possibly autistic brain 4099, which was close to the lower limit of the control range as reported by Hall et al. [27]. If the extent of the 95% confidence intervals are considered, however, then case 4099 may represent the lower range of normal #PCs/mm; this is based on the overlap of confidence intervals in case 4099 with control brains 4334 and BCH-13 and the report by Hall et al. [27]. In normal adult males between the ages of 20 and 50 years, Hall et al. [27] reported a mean PC count of 4.47 PCs/mm with a range of 3.40–6.92 PCs/mm. In normal males 10 years and under, the mean PC count was higher at 5.15 PCs/mm and ranged from 4.40 to 5.95 PCs/mm. This reduction in density of PCs/mm from childhood to adulthood was attributed to an age-related increase in cerebellar size, a difference in size that could account for the decreased density of PCs in the autistic brain reported by Ritvo et al. [4]. In that study, the control group ranged in age from 3 to 13 years (mean, 7.75 years), and their autistic group ranged in age from 10 to 22 years (mean, 17.5 years). When their data are expressed as number of PCs/mm, the difference between the control and the autistic brains appears to be an age-related change that is in agreement with Hall et al. [27] rather than a difference due to autism. In the younger control group, this recalculation reveals a mean of 5.6 PC/mm (range, 5.0 to 6.3) and in the older autistic group a mean of 4.2 PC/mm (range, 3.8 to 4.6).
Other studies, however, provide more convincing evidence of a decreased number of PCs in the autistic brain. Bailey et al. [7], using quantitative analysis, reported reduced number of PCs in the vermis and hemisphere in five of six autistic brains. Qualitatively, Williams et al. [2] reported a decrease in PCs in one of three individuals with autistic features, and Kemper and Bauman [5] qualitatively observed varying degrees of PC reduction in the hemisphere of six autistic brains. More recently, Lee et al. [8] qualitatively reported a decrease in PC number in the vermis and hemisphere of two autistic brains. However, Fatemi et al. [9] found no difference in PC number when comparing five autistic and five control brains.
The above studies, however, have used Nissl-stained material; this is in contrast to our approach using CB-immunostained material. Recent work from this laboratory has demonstrated that Nissl-stained material may fail to identify intact PCs [13], a finding that was first described by Spielmeyer in 1922 [28]. Fever [28], infection [28], general disease [28], hypoglycemia [29], and ischemia [30] have all been suggested as possible causes for altered Nissl staining properties of PCs in human cases. Immunostaining for CB is a more reliable marker of human PCs [13], and thus, although the sample size in this study was of modest size, our methodology demonstrates that possibly only half of autistic cases have a reduction in PCs. This finding highlights that similar to the variable expression in the structural changes in the neocortex, limbic structures, and brainstem as emphasized in the case reports of Bailey et al. [7], there is also a high degree of variability in cerebellar PC density. An appreciation of this variability is of great importance, particularly as researchers work to develop an accurate animal model of autism.
Although there is evidence that a decreased number of PCs is a structural feature of the autistic brain, etiologies for it other then autism itself need to be considered in individual cases. In the present study, both cases 4414 and 3845 experienced seizures that were treated with phenytoin (Dilantin®), a medication that has been reported, in some cases, to induce PC death throughout the anterior, posterior, and floculonodular lobes of the cerebellum [31, 32]. Phenytoin toxicity appears to be an unlikely etiology of the decreased number of PCs in these two brains, since sections from the anterior lobe of the cerebellum in both brains, processed in the identical manner as sections from the posterior lobe, revealed abundant CB immunopositive PCs (Fig. 3). Based on case reports [31, 32], had phenytoin been a factor, there would have been decreased numbers of PCs in the anterior lobe as well. Additionally, PC loss secondary to phenytoin exposure is reported to produce ataxia, dysarthria, hypotonia, and intention tremor [31, 33], clinical manifestations that were not reported in either individual. For example, medical records indicate that case 4414 displayed coordinated movements, had normal tone, normal gait, and maintained the ability to tandem walk.
Another factor to consider is that case 4414 had a prolonged PMI of 48 h. However, if this were the cause of the reduced PC number in the posterior cerebellar lobe, then there should also have been PC loss in the anterior lobe of the cerebellum and, again, this was not the case.
Correlation of Neuropathologic and Clinical Data
The marked variability in PC number among the autistic brains in our data set is a striking finding and provides an opportunity to correlate PC number with clinical phenotype. The cerebellum receives multimodal inputs, via the pons, from numerous cortical regions [34–39] and sends projections, via the thalamus, to several neocortical areas [40] which are involved in a variety of cognitive functions that may be pertinent to autism [14–16]. In previously healthy children, approximately half of those with vermal tumor excision exhibited postoperative deficits in affective behavior [15, 16], with one child meeting the DSM-IV diagnostic criteria for autism [16]. In contrast to the vermis, cerebellar hemisphere tumor resection in children produced impairments in executive functions, auditory and visual learning, time-based attention tasks, expressive language, and visuospatial abilities [15, 16]. Hence, one hypothesis to consider is whether autistic cases with the lowest PC numbers would express more severe autistic features as compared with autistic cases with normal PC numbers. Examination of Table 2, however, does not reveal any a clear relationship between the severity of autism and the reduction in PCs. Additionally, Spearman rank correlation did not reveal a significant relationship between #PCs/mm and severity of autistic features in this sample of brains.
Unlike these studies of resected cerebellar tumors in previously healthy children, the apparent lack of a clinical and neuropathological correlation in the autistic brain may be due to severe behavioral problems that impede testing of the cognitive deficits attributable to the cerebellar hemisphere and/or that clinical testing has not been detailed enough to uncover a more subtle correlation between phenotype and PC number.
These latter points demonstrate that a correlation between neuropathology and phenotype will not be not be as simple as broadly classifying the clinical severity of this disorder, but may require gathering much more detailed clinical information on a larger set of autistic cases. Alternatively, even if there is significant cerebellar pathology in a subset of autistic cases, since it is likely acquired during developmental periods when the brain is capable of constructing alternate innervation patterns [41, 42], there may be sufficient compensation to remove any clear relationship between PC number and autistic phenotype. Further, Yip et al. [43] recently reported a pervasive decrease in GAD67 messenger RNA levels in PCs in the autistic brain, suggesting that there may be alterations in PC physiology without a decrease in PC density.
Conclusion
The sample size obtained for this study is comparable to prior reports of the autistic cerebellum [2–9]; however, in the current study, the more reliable method of CB immunostaining was utilized, since the Nissl stain may fail to adequately stain intact PCs [13, 28–30]. Additionally, modern stereological sampling principles were used for the first time in the autistic cerebellum, with data demonstrating that a decrease in cerebellar PCs is not a universal feature of autism. Our methodology demonstrates that possibly only half of autistic cases have a reduction in PCs. This finding highlights that similar to the varied clinical features observed in autism, there is variability in the density of PCs, with some autistic brains showing a striking decreased density and others with a density comparable to controls. Understanding the scope of PCs reduction among autistic cases is essential as researchers work to develop accurate animal model of autism. Although a decrease in PC density is evident in some cases, the role of PCs in the clinical manifestations of autism remains obscure, since there was no apparent correlation between the density of PCs and the clinical severity of autism.
References
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, DC
Williams RS, Hauser SL, Purpura DP, DeLong GR, Swisher CN (1980) Autism and mental retardation: neuropathologic studies performed in four retarded persons with autistic behavior. Arch Neurol 37:749–753
Bauman ML, Kemper TL (1985) Histoanatomic observations of the brain in early infantile autism. Neurology 35:866–874
Ritvo ER, Freeman BJ, Scheibel AB, Duong T, Robinson H, Guthrie D, Ritvo A (1986) Lower Purkinje cell counts in the cerebella of four autistic subjects: initial findings of the UCLA-NSAC autopsy research report. Am J Psychiatry 146:862–866
Kemper TL, Bauman ML (1993) The contribution of neuropathologic studies to the understanding of autism. Neurol Clin 11:175–187
Guerin P, Lyon G, Barthelemy C, Sostak E, Chevrollier V, Garreau B, Lelord G (1996) Neuropathological study of a case of autistic syndrome with severe mental retardation. Dev Med Child Neurol 38:203–211
Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos PA (1998) Clinicopathological study of autism. Brain 121:889–905
Lee M, Martin-Ruiz C, Graham A, Court J, Jaros E, Perry R, Iversen P, Bauman M, Perry E (2002) Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 15:1483–1495
Fatemi SH, Halt AR, Realmuto G, Earle J, Kist DA, Thuras P, Merz A (2002) Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol 22:171–175
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation in the brain of patients with autism. Ann Neurol 57:67–81
Palmen S, van Engeland H, Hof PR, Schmitz C (2004) Neuropathological findings in autism. Brain 127:2572–2583
Saper CB (1996) Any way you cut it: a new journal policy for the use of unbiased counting methods. J Comp Neurol 364:5
Whitney ER, Kemper TL, Rosene DL, Bauman ML, Blatt GJ (2008) Calbindin-D28k is a more reliable marker of human Purkinje cells than standard Nissl stains: a stereological experiment. J Neurosci Methods 168:42–47
Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121:561–579
Levisohn L, Cronin-Golomb A, Schmahmann JD (2000) Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain 123:1041–1050
Riva D, Giorgi C (2000) The cerebellum contributes to higher functions during development; evidence from a series of children surgically treated for posterior fossa tumours. Brain 123:1051–1061
Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16:367–378
Parvizi J, Joseph J, Press DZ, Schmahmann JD (2007) Pathological laughter and crying in patients with multiple system atrophy-cerebellar type. Mov Disord 22:798–803
Schmahmann JD, Weilburg JB, Sherman JC (2007) The neuropsychiatry of the cerebellum—insights from the clinic. Cerebellum 6:254–267
Grafman J, Litvan I, Massaquoi S, Stewart M, Sirigu A, Hallett M (1992) Cognitive planning deficit in patients with cerebellar atrophy. Neurology 43:1493–1496
Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC (2000) MRI atlas of the human cerebellum. Academic, San Diego, CA, pp 3–20
Rosene DL, Roy NJ, Davis BJ (1986) A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem 34:1301–1315
Crane AM, Goldman PS (1979) An improved method for embedding brain tissue in albumin-gelatin. Stain Technol 54:71–75
Gundersen HJ (1986) Stereology of arbitrary particles. J Microsc 143:3–45
West MJ, Slomianka L, Gundersen HJ (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497
Hedreen JC (1998) Lost caps in histological counting methods. Anat Rec 250:366–372
Hall TC, Miller AKH, Corsellis JAN (1975) Variations in the human Purkinje cell population according to age and sex. Neuropathol Appl Neurobiol 1:267–292
Spielmeyer W (1922) Histopathologie des Nervensystems. Julius Springer, Berlin, pp 56–79
Lawrence RD, Meyer A, Nevin S (1942) The pathological changes in the brain in fatal hypoglycaemia. Quart J Med 35:181–201
Blackwood W, McMenemey WH, Meyer A, Norman RM, Russell DS (1963) Greenfield’s neuropathology. William and Wilkins, Baltimore, pp 29–34
Ghatak NR, Santoso RA, McKinney WM (1976) Cerebellar degeneration following long-term phenytoin therapy. Neurology 26:818–820
Rapport RL, Shaw CM (1977) Phenytoin-related cerebellar degeneration without seizures. Ann Neurol 2:437–439
McLain LW, Martin JT, Allen JH (1980) Cerebellar degeneration due to chronic phenytoin therapy. Ann Neurol 7:18–23
Schmahmann JD, Pandya DN (1989) Anatomical investigation of projections to the basis pontis from posterior parietal association cortices in rhesus monkey. J Comp Neurol 289:53–73
Schmahmann JD, Pandya DN (1991) Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol 308:224–248
Schmahmann JD, Pandya DN (1993) Prelunate, occipitotemporal, and parahippocampal projections to the basis pontis in rhesus monkey. J Comp Neurol 337:94–112
Schmahmann JD, Pandya DN (1995) Prefrontal cortex projections to the basilar pons in rhesus monkey: implications for the cerebellar contribution to higher function. Neurosci Lett 199:175–178
Schmahmann JD, Pandya DN (1997) The cerebrocerebellar system. Int Rev Neurobiol 41:31–60
Schmahmann JD (2001) The cerebrocerebellar system: anatomic substrates of the cerebellar contribution to cognition and emotion. Int Rev Psychiatry 13:247–260
Middleton FA, Strick PL (2001) Cerebellar projections to the prefrontal cortex in the primate. J Neurosci 21:700–712
Zagrebelsky M, Strata P, Hawkes R, Rossi F (1997) Reestablishment of the olivocerebellar projection map by compensatory transcommissural reinnervation following unilateral transection of the inferior cerebellar peduncle in the inferior cerebellar peduncle in the newborn rat. J Comp Neurol 379:283–299
Sugihara I, Lohof AM, Letellier M, Mariani J, Sherrard RM (2003) Post-lesion transcommissural growth of olivary climbing fibres creates functional synaptic microzones. Eur J Neurosci 18:3027–3303
Yip J, Soghomonian JJ, Blatt GJ (2007) Decreased GAD67 mRNA levels in cerebellar Purkinje cells in autism: pathophysiological implications. Acta Neuropathol 113:559–568
Acknowledgment
We gratefully acknowledge the Harvard Brain Tissue Resource Center, Kathleen Price Bryan Brain Bank at Duke University Medical Center, and University of Maryland Brain Bank for providing brain tissue for this study. This work was supported by grants from the NIH-NICHD HD39459, the National Alliance for Autism Research (NAAR], the Nancy Laurie Marks Foundation, and the John and Lisa Hussman Foundation. We are grateful to Michael Bowley for his assistance with the stereology software instruction and maintenance of the system. We also thank several members of the laboratory for their assistance with tissue processing: Rita Marcon, Sandy Thevarkunnel, Melissa Martchek, Matthew Stoker, and Matthew Fields.
Author information
Authors and Affiliations
Corresponding author
Appendix: Autistic Case Histories
Appendix: Autistic Case Histories
Autistic Case 4414
Twenty-six-year-old male born of an uncomplicated birth. He began showing signs of behavioral abnormalities during his toddler years and was diagnosed with autism at the age of five. He graduated from high school and enjoyed writing. He was able to communicate in sentences. At the age of 23, medical records reported that he was fully oriented and followed all simple commands. He did, however, have trouble with more complex commands. His motor exam showed normal strength and tone. Coordination was normal except for slightly slow and clumsy rapid alternating movements in his feet. His gait was normal and he was able to tandem walk. He began taking Prozac at the age of 23, which was reported to be “very effective” in managing behavioral problems. A seizure disorder became apparent at 10 years of age, with eight to ten seizures per year, for which he received Dilantin®. Medications at time of death included Dilantin® and Prozac®.
Autistic Case 3845
Thirty-two-year-old male without complication during the prenatal or perinatal period. During her pregnancy, his mother had no history of infection and did not take any medications. She sensed that something was wrong in the neonatal period, as he was a very unresponsive baby and his general development was slower than that of his siblings. He was formally evaluated at 2.5 years. At that time, he seemed involved in his own world, had no interest in playing with other children, and never wanted to be held. He had not developed language skills; he only grunted. His parents suspected that he might be deaf, but formal education revealed intact hearing. Additionally, he frequently rocked and waved his arms and developed a fascination for spinning tops. As he got older, he was very difficult to manage. He was aggressive towards others, had daily tantrums, and frequently demonstrated self-abusive behaviors. He remained nonverbal throughout his life, but was able to dress and feed himself independently. Physical examination four months prior to his death revealed the absence of tremors and “unremarkable” gait. He began having seizures at the age of 8 years. Seizures were poorly controlled over the years despite receiving numerous medications including Dilantin®, Depakote®, Felbamate®, Gabapentin®, Tegretol®, and Primidone®.
Autistic Case 4099
Nineteen-year-old male born of an uncomplicated birth. His mother became concerned about him at 2 years of age when he began spinning plates. At this age, he also did not speak and was not yet walking. He began walking and speaking at 3 years of age. In addition to his diagnosis of autism, he was diagnosed with Duchenne’s muscular dystrophy when he was 8 years old. He attended a mainstream middle school with special classes for autistic children. At 10 years of age, his vocabulary consisted of 150 words. Medical records revealed that he was minimally cooperative during physician visits but was reported to recognize his mother and show affection toward her, able to answer yes/no questions, and converse in short simple phrases. He never experienced any seizures.
Autistic Case 2431
Fifty-four-year-old male who sustained trauma to the face, ear, and shoulder during a breech delivery. At 9 months of age, it was thought that he was deaf because he paid no attention to sound. Examination of his hearing revealed possible deafness, but his behavior was not in accordance with deafness alone. He attended a school for the deaf until the age of 12 when he was referred for further evaluation; at that time, his behavior was characterized as “withdrawn, mute, violent, destructive…strangely manneristic.” Examination also revealed normal hearing, and a diagnosis of infantile autism was made. He was first hospitalized at 12 years of age after he was found using an ax to destroy furniture in the family home. He remained hospitalized until his death. Reports consistently reference his aggressive behaviors without provocation. Temper tantrums frequently occurred if disruptions in his daily routine occurred. He remained non-verbal throughout his life, but was able to use some gestures to communicate and was able to follow simple three-step commands. Motor exam at the age of 5.7 years reported that he “has marvelous muscle coordination involving the smaller muscles; however, activities demanding use of large muscles seem to be very difficult for him.” He had no history of seizures. Medications at the time of his death included Ascriptin®, Thorazine®, Centrax®, Ativan®, and Serax®.
Autistic Case 4259
Thirteen-year-old female diagnosed with infantile autism as a toddler. Concerns were raised very early in the neonatal period as she did not cry or respond to noise. She had delayed motor milestones and remained non-verbal throughout her life. She displayed self-abusive hand biting but was rarely aggressive toward caregivers. She experienced her first seizure at 5 years of age for which Tegretol® was prescribed.
Autistic Case 3511
Twenty-seven-year-old male who was living in a residential home at the time of death. He was reportedly very difficult to manage behaviorally. He exhibited unpredictable episodes of aggressiveness with yelling, screaming, pacing, scratching his face, and attacking the staff. Routine was very important to him and he would perseverate on an activity until it was completed. He worked for a cleaning and landscaping service and was said to be an excellent worker. He would work for hours without stopping. He could follow very simple commands and was able to express simple needs in two- to three-word sentences. He had a history of two seizures during his lifetime. At the time of his death, he was taking Haloperidol® and Phenobarbital®.
Rights and permissions
About this article
Cite this article
Whitney, E.R., Kemper, T.L., Bauman, M.L. et al. Cerebellar Purkinje Cells are Reduced in a Subpopulation of Autistic Brains: A Stereological Experiment Using Calbindin-D28k. Cerebellum 7, 406–416 (2008). https://doi.org/10.1007/s12311-008-0043-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-008-0043-y