Abstract
The calcium-binding protein S100B has been shown to support neuron proliferation, migration and neurite growth in vitro, while the significance of S100B for neuronal development in vivo is controversial. We have investigated the effect of S100B deficiency on cerebellar development in S100B knockout mice at an age of 5 and 10 days after birth (P5 and P10). This time range covers important developmental steps in the cerebellum such as granule cell proliferation and migration, as well as dendritic growth of Purkinje cells. Bergmann glial cells contain a particularly high concentration of S100B and serve as scaffold for both migrating granule cells and growing Purkinje cell dendrites. This renders the postnatal cerebellum ideal as a model system to study the importance of S100B for glial and neuronal development. We measured the length of Bergmann glial processes, the width of the external granule cell layer as a measure of granule cell proliferation, the decrease in width of the external granule cell layer between P5 and P10 as a measure of granule cell migration, and the length of Purkinje cell dendrites in wild-type and S100B knockout mice. None of these parameters showed significant differences between wild-type and knockout mice. In addition, wild-type and knockout mice performed equally in locomotor behaviour tests. The results indicate that S100B-deficient mice have normal development of the cerebellum and no severe impairment of motor function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
S100B is a member of the EF-hand calcium-binding protein family of S100 proteins. In the brain, S100B is mainly expressed by astrocytes [1, 2]. High levels of S100B in cerebrospinal fluid and serum were found to be linked to pathological conditions such as brain damage, neurodegeneration and psychiatric disorders [3–5]. Single nucleotide polymorphisms of the human gene encoding S100B, e.g., have been associated with bipolar effective disorder, and elevated S100B levels have been detected in the brain tissue and cerebrospinal fluid of patients with Alzheimer’s disease [6, 7]. Under physiological conditions, S100B affects various cell functions in the brain, with S100B having both intracellular and extracellular targets [1, 8]. Intracellularly, S100B inhibits protein phosphorylation, decreases the activity of the transcription factor p53 and has been shown to stimulate proliferation, differentiation and migration of astrocytes [9–12]. Extracellularly, S100B acts as a bimodal neurotrophic factor. At nanomolar concentrations, it stimulates neurite growth, whereas at higher concentrations, it can induce apoptosis in neurons and astrocytes [12, 13]. S100B is released by astrocytes and binds to the receptor for advanced glycation endproducts (RAGE), which activates downstream targets such as NF-kappaB [14]. Due to its effect on proliferation, migration and neurite growth, S100B has been considered to play an important role in brain development. However, most of the studies on the significance of S100B for cell differentiation and neurite growth have been performed on cultured cells, and only little information exists about the impact of S100B on brain development in vivo.
Bergmann glial cells are specialized astrocytes in the cerebellum that contain high amounts of S100B and play a major role in cerebellar development [15, 16]. In the cerebellum, a second, postnatal wave of proliferation follows the embryonic proliferation of neural precursors, resulting in a large number of immature granule cells in the external granular layer, the part of the molecular layer underneath the pia mater of the cerebellar cortex [17, 18]. These granule cells use the radially arranged processes of Bergmann glial cells as guidance as they migrate into the deeper layer, the inner granular layer, where they differentiate. At the same time, the cerebellar principle neurons, Purkinje neurons, grow their dendrites to fill the space left open by the migrating granule cells in the molecular layer [19, 20]. Whether the developmental steps of granule cell proliferation and migration as well as Purkinje cell outgrowth are affected by S100B secreted by Bergmann glial cells is not known so far. Therefore, we investigated the effect of S100B on Bergmann glial cell morphology, granule cell migration and dendritic growth of Purkinje neurons in the present study. We analysed the length of Bergmann glial cell processes and Purkinje cell dendrites as well as the decrease in size of the external granular layer as a measure of granule cell migration in wild-type and S100B knockout mice. However, we did not find significant changes in the analysed parameters between wild-type mice and mice deficient in S100B. The results indicate that the cerebellar cortex develops normal in the absence of S100B.
Materials and Methods
Animals and Preparation
Heterozygous S100B-deficient mice (46; kindly provided by A. Marks, Toronto) were mated, and offspring (wild-type, heterozygous, knockout) were raised in the animal facility of the University of Münster Medical School. All experiments were performed according to the regulations of the German and European animal welfare laws. A total of 35 littermates were investigated for histological analysis. The genotype was checked by real-time PCR and verified by antibody staining against S100 (Fig. 1a). Pups were decapitated with scissors at an age of 5 and 10 days after birth (P5 and P10), respectively. Brains were rapidly removed from the skull and processed for Western blot analysis or immunostaining.
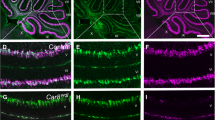
Investigation of cerebella in wild-type and knockout mice. a Anti-S100 immunostaining of cerebellar slices derived from wild-type (left), heterozygous (middle) and knockout mice (right). Both Bergmann glia (arrow) and astrocytes (arrowhead) were labelled in wild-type and heterozygous mice, but not in knockout mice. b Morphology of a sagittal slice of a cerebellum from a wild-type mouse at postnatal day 10 (P10). The square indicates the region which was chosen to analyse cerebellar development. c Anti-GFAP immunostaining of Bergmann glia and astrocytes. The segment A reflects the measurement of the lengths of Bergmann glial processes. d Nuclei stained with propidium iodide. The segment B indicates the width of the granular layer. e Anti-IP3R1 immunostaining of Purkinje neurons. The line C reflects the maximum length of the dendrite, D reflects the proximal dendritic segment from the cell body to the first bifurcation. f Western blot depicting S100B in three independent homogenates of P5 and three independent homogenates of P10 cerebella (each homogenate containing two to five pooled cerebella). Beta-actin was used as a loading control. Scale bars at a, c, d 50 μm, b 500 μm and e 20 μm
Western Blot Analysis
Cerebella were collected from pups at P5 or P10 and placed in ice-cold Tris-buffered saline (pH 7.5) containing protease inhibitors (P8340, Sigma-Aldrich). For each homogenate, two to five cerebella of the same age were pooled. The tissue was homogenized with a glass-Teflon potter, the homogenates were centrifuged at 20,000×g for 30 min and the supernatants were used for analysis. For each age, three independent homogenates were produced and probed by Western blot. The protein content of the homogenate was determined by using the bicinchoninic acid (BCA) protein assay reagent kit (Pierce, Rockford, USA). The BCA reagent was added to the homogenates and incubated for 30 min at 60 °C. The protein content was measured colorimetrically at 540 nm. Solutions with various bovine serum albumin concentrations were used for calibration. All homogenates and calibration solutions were processed simultaneously. Equal amounts of total protein were loaded per lane onto a 4–12 % Bis-Tris gel (NuPAGE, Invitrogen), which was then subject to Western blot analysis using an anti-S100 antibody (Dako, Z0311). The beta-actin content was analysed with anti-β-Actin antibody (Sigma, A5441) as loading control.
Immunohistochemistry
Brains were transferred into 4 % formaldehyde in 0.1 M phosphate-buffered saline (PBS) and fixed over night. Fixed brain tissue was rinsed several times with PBS. The cerebellum was removed, and the vermis was cut out and glued to the base of a vibratome (VT1000, Leica, Bensheim, Germany). Two hundred micrometres thick sagittal sections were cut. Unspecific binding sites were blocked by normal goat serum (5 % in PBS, 0.5 % Triton-X) for 1 h. Primary antibodies were incubated for 24 h at 4 °C at the following concentrations: rabbit-anti-S100 (Dako, Z0311) [21] 1:1,000; rabbit-anti-GFAP (Dako, Z0334) [22] 1:1,000; and rabbit-anti-IP3R1 (Dianova, ABR-01157) [23] 1:500. A secondary antibody (Alexa Fluor 488 goat-anti-rabbit IgG, Life technologies, A-11008) was incubated at a concentration of 1:1,000 in PBS containing 10 μM propidium iodide for 4 h. Tissue sections were transferred to a slide, embedded in VECTASHIELD (H-1400, Vector, Burlingame, USA) and fixed with a coverslip.
Assessment of Gross Motor Function
At the age of 3 months, motor function of 11 wild-type, 11 heterozygous and 12 homozygous knockout males, and 12 wild-type, 12 heterozygous and 11 homozygous knockout females was assessed in the rope test and the vertical pole test. For the rope test, animals were placed in the middle of a 40 cm long rope that was taut between two towers at a height of 26 cm. To succeed this test, animals had either to hold on the rope for 30 s or to reach one of the towers within this time. For the vertical pole test, animals were placed on of a small ball that was located on top of the 30 cm long wooden vertical pole. Mice had to climb down the pole headfirst, with their tails wrapped around the pole.
Open Field Test
A week after testing motor functions, the same animals were tested in the open field. Since there were no differences between the sexes, data from male and female mice were pooled for analysis. The ground floor of the apparatus was 80 × 80 cm in size, surrounded by a 40 cm high wall. The apparatus was dimly lit at 17 lx. As measure of locomotor activity, the distance each animal travelled was assessed by an automated video tracking system (ANY-maze, Stoelting Co., Wood Dale, IL, USA).
Data Analysis
Stacks of images were taken with a confocal microscope (TCS SPE, Leica) equipped with a ×40/1.25 oil immersion objective. The pinhole size was adjusted to one Airy unit. The voxel size (X × Y × Z) was 0.12 × 0.12 × 0.3 μm, and the entire image stack covered 10 μm of depth. Image stacks were projected using the average projection feature of ImageJ [24], and the resulting images were adjusted for brightness and contrast using Photoshop (Adobe). Morphological parameters were measured with ImageJ. The cerebellar cortex was analysed at the base of the fissure between lobule V and IV (Fig. 1b). Parameters that were measured were the length of the Bergmann glia processes (Fig. 1c), the width of the external granular layer (Fig. 1d), the length of the longest dendritic process of Purkinje neurons (Fig. 1e) and the length of the proximal dendritic segment from the cell body to the first bifurcation (Fig. 1e). Data are presented as mean ± standard deviation (SD) with n indicating the number of cells or the number of measurements analysed. Statistical differences between genotypes were calculated using one-way ANOVA with an error probability of p < 0.05.
Results
Developmental Increase in S100B Expression
The content of S100B in the cerebellum in wild-type mice was compared between mice at an age of 5 and 10 days after birth (P5 and P10) by Western blot analysis. As seen in Fig. 1f, the antibody detected a band at approximately 10 kD, corresponding to the molecular weight of the S100B monomer [25]. In two samples, a second band of approximately 20 kD was detected which presumably represents the dimer of S100B. Tissue samples from P5 animals contained less S100B as compared to the tissue samples from P10 animals, indicating that S100B expression is up-regulated in the cerebellum during postnatal development.
Bergmann Glial Cell Morphology and Granular Layer
Around birth, immature granule cells underneath the pia mater proliferate, resulting in an immense number of granule cells which fill the largest part of the developing molecular layer, the external granular layer (Fig. 2a). The molecular layer is pervaded by radially arranged processes of Bergmann glial cells. No obvious differences in the density and general morphology of Bergmann glial cells were observed between wild-type (S100B+/+), heterozygous (S100B+/−) and knockout littermates (S100B−/−) at P5 and P10, respectively (Fig. 2a, b). Bergmann glia endfeet build the glia limitans in the cerebellum, which together with the basal lamina build a barrier insulating the cerebellum [15]. In all three genotypes tested, the glia limitans appeared intact, suggesting that S100B is not required for endfeet development and glia limitans formation (Fig. 2c). In addition, the morphology of astrocytes in the internal granular layer was not affected in S100B-deficient mice (Fig. 2d).
Glial morphology in S100B-expressing and S100B-deficient mice. a Anti-GFAP immunostaining highlighting glial cells (green) and propidium iodide staining of nuclei (red) in wild-type, heterozygous and S100B-deficient mice at P5. The immature molecular layer (ML) and the external granule layer (eGL) are indicated. b Anti-GFAP immunostaining (green) and propidium iodide staining of nuclei (red) at P10. c Endfeet of Bergmann glial cells forming the glia limitans at P10. d Typical astrocytes in the internal granular layer of wild-type, heterozygous and S100B-deficient mice at P10. Scale bars at a, b 50 μm, c 20 μm and d 10 μm (colour figure online)
We measured the length of the Bergmann glial cell processes to test whether lack of S100B has an impact on Bergmann glial cell development in a more detailed way. At P5, wild-type mice had Bergmann glial cells with a length of 84.1 ± 8.2 μm (n = 31) as compared to 81.0 ± 9.3 μm (n = 19) in heterozygous and 81.6 ± 9.0 μm (n = 18) in knockout mice (Fig. 3a). Until P10, the length of Bergmann glial cells increased to 110.7 ± 15.4 μm (n = 15) in wild-type, 110.2 ± 9.1 μm (n = 31) in heterozygous and 108.0 ± 9.5 μm (n = 29) in knockout mice, reflecting the increase in volume of the cerebellum, and particularly of the molecular layer, between P5 and P10. No statistically significant differences were found between different genotypes at P5 and P10, respectively. To estimate the density of Bergmann glial processes, we counted the number of processes along a segment of pial surface of 100 μm. At P5, 14.3 ± 2.8 processes per 100 μm (n = 13) were counted in wild-type mice, as compared to 14.0 ± 2.0 (n = 6) and 14.5 ± 2.5 (n = 4) in heterozygous and knockout mice, respectively (Fig. 3b). This value increased significantly until P10 to 16.5 ± 1.9 (n = 4) in wild-type, 15.5 ± 1.4 (n = 6) in heterozygous and 15.5 ± 1.2 (n = 10) in knockout mice. Within each age group, the values did not differ significantly among genotypes.
Statistical analysis of morphological parameters. a The length of the radial processes of Bergmann glial cells was not significantly different between S100B wild-type (+/+), heterozygous (+/−) and knockout mice (−/−) at P5 and P10, respectively. b The density of Bergmann glia processes as measured by the number of processes per 100 μm of pial surface does not differ among genotypes at P5 and P10, respectively. c The width of the external granular layer was not significantly different among genotypes at both age investigated
In addition to the length and density of Bergmann glia processes, we measured the width of the external granular layer. Since perpetual proliferation of granule cells during the first postnatal days leads to the formation of the external granular layer [18], the width of this layer at P5 can be used as an estimation of the proliferation of granule cells. At P5, the width of the external granular layer in wild-type mice was 68.2 ± 9.3 μm (n = 22), which was not significantly different from the width in heterozygous mice of 60.2 ± 10.5 μm (n = 13) and knockout mice of 64.8 ± 4.8 μm (n = 12) (Fig. 3c). Between P5 and P10, a large number of granule cells migrate from the external granular layer along Bergmann glial cell processes into the internal granular layer. This migration is reflected by a decrease in width of the external granular layer, as more and more granule cells leave the external granular layer. Hence, at P10, the width of the external granular layer was reduced to 41.1 ± 13.9 μm (n = 17) in wild-type mice, 45.0 ± 13.3 μm (n = 21) in heterozygous and 43.3 ± 11.7 μm (n = 21) in knockout mice (Fig. 3c). The differences between different genotypes were not statistically significant. The results suggest that S100B deficiency does not lead to impairment of Bergmann glial cell development as well as granule cell proliferation and migration.
Purkinje Cell Development
As granule cells continue to migrate into the internal granular layer, Purkinje cells grow a dendrite which arborizes in the molecular layer and occupies the space left by the migrating granule cells (Fig. 4a). To investigate the importance of S100B for Purkinje cell development, we compared the morphology of Purkinje cell dendrites in mice of different genotypes at P10. As for Bergmann glial cells, no obvious differences in the general morphology was found between Purkinje cells of wild-type, heterozygous and knockout mice (Fig. 4a). The maximal length of the dendrite was 86.6 ± 9.6 μm (n = 21) in wild-type mice (Fig. 4b). The length of the dendrite in heterozygous and knockout mice was not significantly different and averaged 90.9 ± 11.9 μm (n = 21) and 93.4 ± 9.4 μm (n = 9), respectively. The lengths of the initial segment of the dendrite, i.e. the segment between the cell body and the first bifurcation, were 18.1 ± 5.2 μm (n = 21) in wild-type and 17.6 ± 5.1 μm (n = 21) in heterozygous mice. In knockout mice, the length of the initial segment was 13.1 ± 4.7 μm (n = 9) and was not significantly different as compared to wild-type mice (Fig. 4c).
Purkinje cell morphology. a Anti-IP3R1 immunostaining of Purkinje cells (green) and nuclei labelled by propidium iodide (red) at P10. Scale bars at 50 μm. b The maximum length of the Purkinje cell dendrite was not significantly different between S100B wild-type (+/+), heterozygous (+/−) and knockout mice (−/−). c The length of the initial dendritic segment was not significantly different in knockout mice compared to wild-type and heterozygous mice (colour figure online)
Motor Function and Locomotor Behaviour
Since the cerebellum is associated with motor abilities, we also assessed whether S100B knockout mice displayed abnormal motor function. Mice of all three genotypes did not show any signs of gross motor impairment. All animals were able to successfully pass both, the rope test (Fig. 5a) and the vertical pole test (Fig. 5b). Furthermore, the genotypes did not differ in the distance travelled in the open field covering distances of about 32–38 m during the 5-min test period (Fig. 5c).
Motor function and locomotor behaviour. a All mice independent of their genotype were able to hold on to the rope for 30 s or climb on a tower within this time. b Mice of all three genotypes were also able to climb down the vertical pole headfirst with their tails wrapped around the pole. c The distance travelled in the open field did not differ between the genotypes, either. Bars represent mean + SD, n = 23 per group
Discussion
Calcium is a ubiquitous second messenger that is involved in most if not all cellular events, including developmental steps such as proliferation, migration and differentiation [26, 27]. To perform these tasks, calcium ions bind to a plethora of proteins, e.g. protein kinases, ion channels and calcium-binding proteins, to name a few. In the brain, the calcium-binding protein S100B is mainly expressed in glial cells, and calcium signalling is the major cellular response of glial cells upon neuronal activity, rendering intracellular S100B as a putative link between calcium signalling and cellular responses [28, 29]. S100B not only acts as an intracellular mediator of an increased calcium concentration but can also be secreted into the extracellular space where it functions as a neurotrophic factor [1, 8]. S100B secretion has been demonstrated in cultured cortical and cerebellar glial cells [30, 31]. Hence, S100B is considered to affect not only the development of S100B-expressing glial cells but also the development of adjacent neurons. Bergmann glial cells are among the cells with the highest S100B expression in the brain and fulfil important tasks in cerebellar development [15, 16]. However, when we compared cerebella of S100B-deficient mice with those of wild-type mice to evaluate the significance of S100B for the development of the cerebellar cortex, we did not find severe differences in the morphology of Bergmann glial cells and Purkinje cells between the genotypes, suggesting that the role of S100B in cerebellar development is minor.
Bergmann Glial Cell Development
Bergmann glial cells derive from radial glial cells in the cerebellar parenchyma during embryogenesis [15, 32]. In mice, immature Bergmann glial cells migrate towards the forming Purkinje cell layer and grow several radial processes towards the pia mater during the third trimester of embryonic development. Until P5, the earliest age investigated in the present study, all Bergmann glial cells have arranged around the cell bodies of the Purkinje neurons, and glial processes extent to the pia mater, where their endfeet build the glia limitans [15]. S100B is already expressed in the cerebellum during embryogenesis [33], and there is an increase in S100B content that persists at least until P10, as shown in the present study. Hence, S100B is a possible candidate to control cerebellar development. S100B can interact in different ways with the steps of Bergmann glia development, e.g. by decreasing the activity of the transcription factor p53, and has been implicated in astrocyte migration [9, 10]. In addition, S100B binds free calcium, thereby buffering calcium transients. Hence, evoked calcium transients are larger in S100B-deficient cerebellar glial cells compared to wild-type mice [34], which may affect calcium-dependent processes in Bergmann glial cells, including the release of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) [35]. S100B also activates myo-inositol monophosphatase 1 in Bergmann glial cells and thus can interfere with InsP3-dependent calcium signalling [36]. However, none of the parameters describing Bergmann glia development tested in the present study were influenced in S100B knockout animals. The number and length of Bergmann glial processes are indicative for early postnatal Bergmann glia development, whereas the increase in density and length of the processes until P10 reflects the ongoing development. Since S100B-deficient Bergmann glial cells resemble those of wild-type mice, S100B appears not to play an important role in Bergmann glial cell development up to P10. Lack of impact of S100B deficiency on glial histology is not a general phenomenon, e.g. S100B-deficient mice display chronic astrogliosis in the hippocampus, as indicated by increased branching and expression of GFAP in astrocytes, but reduced serotonin-induced astrogliosis [37]. Since no information about the age of the studied mice is given in that publication [37], it is not possible to assess whether gliosis in S100B knockout mice is found during development or only in adult mice. In our developmental study, however, we did not observe obvious signs of gliosis.
Neuronal Development
Bergmann glial cells build a scaffold for migrating granule cells [16, 18]. Since S100B can function as a neurotrophic factor, proliferation and migration of granule cells could be driven by secretion of S100B from Bergmann glial cells. At P5, immature granule cells in the external granular layer almost completely fill the molecular layer as a result of the high proliferation rate during the first postnatal days [16, 18]. After P5, migration outnumbers proliferation, and the external granular layer shrinks. Both the width of the granular layer at P5 as well as the reduction in width until P10 did not differ between S100B-expressing and S100B-deficient mice, indicating that proliferation and migration of granule cells are not under strict control of S100B. This is also true for the development of Purkinje cell dendrites. Purkinje cells use Bergmann glial processes as a guide as they grow into the base of the external granular layer [20]. S100B has been shown to augment neurite outgrowth in vitro; however, in the present study, dendrite extension of Purkinje cells was not reduced in S100B-deficient mice. BDNF expression is up-regulated in the hippocampus and cortex of S100B-deficient mice [38], and increased BDNF levels could partly compensate for the lack of S100B-mediated neurotrophic effects. Considering the fact that BDNF activates a different class of receptors with a distinct, albeit overlapping range of downstream targets compared to S100B, it is questionable whether an increase in BDNF expression can fully compensate for S100B loss. In addition, BDNF has no intracellular calcium binding and signalling ability as shown for S100B and hence cannot compensate for intracellular impairments of S100B deficiency. Taken together, the results indicate that S100B deficiency does not lead to serious developmental deficits in the cerebellum. This conclusion is supported by the absence of severe deficits in motor function-related behaviour in the present study. This is in line with results of another study, which showed that motor coordination and conditioning of motor behaviour is normal in S100B knockout mice [39], suggesting that the absence of S100B during development of the cerebellum does not lead to severe impairment of cerebellar function. In addition, the development of serotonergic neurons in the raphe nucleus of S100B knockout mice is normal [40]. On the contrary, S100B appears to affect learning and emotional behaviour, since hippocampal long-term potentiation (LTP) is enhanced in S100B-deficient mice, while in S100B-overexpressing mice LTP is reduced and anxiety-related behaviour is more variable compared to wild-type mice [41–43]. In addition, S100B overexpression results in Parkinson’s disease-related deficits such as motor impairment and increased Alzheimer’s disease-related neuroinflammation and cytoskeletal collapse [44–46]. The results of our study and others suggest that S100B is not crucial for neurite outgrowth during development in vivo, despite its supporting effect on neurite outgrowth in cultured neurons [13, 14, 47] but rather for behaviour such as memory formation and anxiety in the mature animal, possibly due to its calcium-binding and calcium-dependent signalling properties.
References
Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, et al. Functions of S100 proteins. Curr Mol Med. 2013;13:24–57.
Marshak DR. S100 beta as a neurotrophic factor. Prog Brain Res. 1990;86:169–81.
Rothermundt M, Ahn JN, Jorgens S. S100B in schizophrenia: an update. Gen Physiol Biophys. 2009;28:F76–81.
Rothermundt M, Peters M, Prehn JHM, Arolt V. S100B in brain damage and neurodegeneration. Microsc Res Tech. 2003;60:614–32.
Steiner J, Bogerts B, Schroeter ML, Bernstein H. S100B protein in neurodegenerative disorders. Clin Chem Lab Med. 2011;49:409–24.
Peskind ER, Griffin WS, Akama KT, Raskind MA, van Eldik LJ. Cerebrospinal fluid S100B is elevated in the earlier stages of Alzheimer's disease. Neurochem Int. 2001;39:409–13.
Roche S, Cassidy F, Zhao C, Badger J, Claffey E, Mooney L, et al. Candidate gene analysis of 21q22: Support for S100B as a susceptibility gene for bipolar affective disorder with psychosis. Am J Med Genet. 2007;144:1094–6.
Donato R, Sorci G, Riuzzi F, Arcuri C, Bianchi R, Brozzi F, et al. S100B's double life: Intracellular regulator and extracellular signal. Biochim Biophys Acta. 2009;1793:1008–22.
Baudier J, Delphin C, Grunwald D, Khochbin S, Lawrence JJ. Characterization of the tumor suppressor protein p53 as a protein kinase C substrate and a S100b-binding protein. Proc Natl Acad Sci U S A. 1992;89:11627–31.
Brozzi F, Arcuri C, Giambanco I, Donato R. S100B Protein Regulates Astrocyte Shape and Migration via Interaction with Src Kinase: Implications for astrocyte development, activation, and tumor growth. J Biol Chem. 2009;284:8797–811.
Ikura M, Osawa M, Ames JB. The role of calcium-binding proteins in the control of transcription: structure to function. Bioessays. 2002;24:625–36.
Sorci G, Riuzzi F, Arcuri C, Tubaro C, Bianchi R, Giambanco I, et al. S100B protein in tissue development, repair and regeneration. WJBC. 2013;4:1.
Saleh A, Smith DR, Tessler L, Mateo AR, Martens C, Schartner E, et al. Receptor for advanced glycation end-products (RAGE) activates divergent signaling pathways to augment neurite outgrowth of adult sensory neurons. Exp Neurol. 2013;249:149–59.
Villarreal A, Aviles Reyes RX, Angelo MF, Reines AG, Ramos AJ. S100B alters neuronal survival and dendrite extension via RAGE-mediated NF-κB signaling. J Neurochem. 2011;117:321–32.
Buffo A, Rossi F. Origin, lineage and function of cerebellar glia. Prog Neurobiol. 2013;109:42–63.
Xu H, Yang Y, Tang X, Zhao M, Liang F, Xu P, et al. Bergmann glia function in granule cell migration during cerebellum development. Mol Neurobiol. 2013;47:833–44.
Heinsen H. Quantitative anatomical studies on the postnatal development of the cerebellum of the albino rat. Anat Embryol (Berl). 1977;151:201–18.
Komuro H, Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol Life Sci. 2003;60:1084–98.
Kapfhammer JP. Cellular and molecular control of dendritic growth and development of cerebellar Purkinje cells. Prog Histochem Cytochem. 2004;39:131–82.
Lordkipanidze T, Dunaevsky A. Purkinje cell dendrites grow in alignment with Bergmann glia. Glia. 2005;51:229–34.
Thyssen A, Stavermann M, Buddrus K, Doengi M, Ekberg JA, St John JA, et al. Spatial and developmental heterogeneity of calcium signaling in olfactory ensheathing cells. Glia. 2013;61:327–37.
Singaravelu K, Lohr C, Deitmer JW. Regulation of store-operated calcium entry by calcium-independent phospholipase A2 in rat cerebellar astrocytes. J Neurosci. 2006;26:9579–92.
Stavermann M, Buddrus K, St John JA, Ekberg JA, Nilius B, Deitmer JW, et al. Temperature-dependent calcium-induced calcium release via InsP3 receptors in mouse olfactory ensheathing glial cells. Cell Calcium. 2012;52:113–23.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Deloulme JC, Gentil BJ, Baudier J. Monitoring of S100 homodimerization and heterodimeric interactions by the yeast two-hybrid system. Microsc Res Tech. 2003;60:560–8.
Lohr C, Heil JE, Deitmer JW. Blockage of voltage-gated calcium signaling impairs migration of glial cells in vivo. Glia. 2005;50:198–211.
Lohr C, Grosche A, Reichenbach A, Hirnet D. Purinergic neuron-glia interactions in sensory systems. Pflugers Arch. 2014;466:1859–72.
Rosenberg SS, Spitzer NC. Calcium signaling in neuronal development. Cold Spring Harbor Perspect Biol. 2011;3:a004259.
Deitmer JW, Verkhratsky AJ, Lohr C. Calcium signalling in glial cells. Cell Calcium. 1998;24:405–16.
Ciccarelli R, Di Iorio P, Bruno V, Battaglia G, D'Alimonte I, D'Onofrio M, et al. Activation of A1 adenosine or mGlu3 metabotropic glutamate receptors enhances the release of nerve growth factor and S-100beta protein from cultured astrocytes. Glia. 1999;27:275–81.
Pinto SS, Gottfried C, Mendez A, Goncalves D, Karl J, Goncalves CA, et al. Immunocontent and secretion of S100B in astrocyte cultures from different brain regions in relation to morphology. FEBS Lett. 2000;486:203–7.
Hanke S, Reichenbach A. Quantitative-morphometric aspects of Bergmann glial (Golgi epithelial) cell development in rats. A Golgi study. Anat Embryol (Berl). 1987;177:183–8.
Hachem S, Laurenson A, Hugnot J, Legraverend C. Expression of S100B during embryonic development of the mouse cerebellum. BMC Dev Biol. 2007;7:17.
Xiong Z, O'Hanlon D, Becker LE, Roder J, MacDonald JF, Marks A. Enhanced calcium transients in glial cells in neonatal cerebellar cultures derived from S100B null mice. Exp Cell Res. 2000;257:281–9.
Jean YY, Lercher LD, Dreyfus CF. Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron Glia Biol. 2008;4:35–42.
Vig PJ, Shao Q, Subramony SH, Lopez ME, Safaya E. Bergmann glial S100B activates myo-inositol monophosphatase 1 and Co-localizes to purkinje cell vacuoles in SCA1 transgenic mice. Cerebellum. 2009;8:231–44.
Chang MS, Ariah LM, Marks A, Azmitia EC. Chronic gliosis induced by loss of S-100B: knockout mice have enhanced GFAP-immunoreactivity but blunted response to a serotonin challenge. Brain Res. 2005;1031:1–9.
Schulte-Herbrüggen O, Hörtnagl H, Ponath G, Rothermundt M, Hellweg R. Distinct regulation of brain-derived neurotrophic factor and noradrenaline in S100B knockout mice. Neurosci Lett. 2008;442:100–3.
Kim HR, Seto-Ohshima A, Nishiyama H, Itohara S. Normal delay eyeblink conditioning in mice devoid of astrocytic S100B. Neurosci Lett. 2011;489:148–53.
Nishiyama H, Takemura M, Takeda T, Itohara S. Normal development of serotonergic neurons in mice lacking S100B. Neurosci Lett. 2002;321:49–52.
Buschert J, Hohoff C, Touma C, Palme R, Rothermundt M, Arolt V, et al. S100B overexpression increases behavioral and neural plasticity in response to the social environment during adolescence. J Psychiatry Res. 2013;47:1791–9.
Gerlai R, Wojtowicz JM, Marks A, Roder J. Overexpression of a calcium-binding protein, S100 beta, in astrocytes alters synaptic plasticity and impairs spatial learning in transgenic mice. Learn Mem. 1995;2:26–39.
Nishiyama H, Knopfel T, Endo S, Itohara S. Glial protein S100B modulates long-term neuronal synaptic plasticity. Proc Natl Acad Sci U S A. 2002;99:4037–42.
Craft JM, Watterson DM, Marks A, Van Eldik LJ. Enhanced susceptibility of S-100B transgenic mice to neuroinflammation and neuronal dysfunction induced by intracerebroventricular infusion of human beta-amyloid. Glia. 2005;51:209–16.
Liu J, Wang H, Zhang L, Xu Y, Deng W, Zhu H, et al. S100B transgenic mice develop features of Parkinson's disease. Arch Med Res. 2011;42:1–7.
Whitaker-Azmitia PM, Wingate M, Borella A, Gerlai R, Roder J, Azmitia EC. Transgenic mice overexpressing the neurotrophic factor S-100 beta show neuronal cytoskeletal and behavioral signs of altered aging processes: implications for Alzheimer's disease and Down's syndrome. Brain Res. 1997;776:51–60.
Winningham-Major F, Staecker JL, Barger SW, Coats S, van Eldik LJ. Neurite extension and neuronal survival activities of recombinant S100 beta proteins that differ in the content and position of cysteine residues. J Cell Biol. 1989;109:3063–71.
Acknowledgments
We thank M. Doengi, C. Schettler (Münster) and A. C. Rakete (Hamburg) for technical assistance. Financial support by the Deutsche Forschungsgemeinschaft (LO 779/6) is gratefully acknowledged. The authors thank Alexander Marks (Toronto) for kindly providing two breeding pairs of S100B knockout mice.
Conflict of Interest
The authors report no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bluhm, B., Laffer, B., Hirnet, D. et al. Normal Cerebellar Development in S100B-Deficient Mice. Cerebellum 14, 119–127 (2015). https://doi.org/10.1007/s12311-014-0606-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-014-0606-z