Abstract
Salinity is one of the most severe abiotic stress in the world. Also, the irrigated lands have been treated with second salinity. Canola is one of the most important industrial crops for oil production all over the world which is affected by salinity. Salt stress causes imbalanced ion hemostasis (Na+ and K+) and interrupted mineral absorption in canola. Also, salinity stress leads to oxidative stress (production and accumulation of reactive oxygen species (ROS). Accumulation of ROS is extremely dangerous and lethal for plants. As a consequence, canola production is reduced under salinity stress. So, a suitable approach should be found to deal with salinity stress and prevent the loss of production oilseed. Plant growth-promoting rhizobacteria (PGPR) can colonize on the plant root surface and alleviate the salt stress effect by providing minerals like nitrogen, phosphate, and potassium. Also, they alleviate salt stress by phytohormones like auxin (IAA), cytokinin (CK), and abscisic acid (ABA). This study focus on physiological parameters like leaf area (LA), root length (RL), shoot length (SL), chlorophyll fluorescence indexes (Fv/Fm and Fv/F0), relative water content (RWC), electrolyte leakage index (ELI), photosynthesis pigments (chlorophyll a, b, and carotenoids), Na+, and K+; and biochemical parameters like malondialdehyde (MDA) content, hydrogen peroxide content (H2O2), total protein content, proline, antioxidant capacity, and antioxidant enzyme activities in canola through the inoculation with Enterobacter sp. S16-3 and Pseudomonas sp. C16-2O. This study showed that LA, RL, SL, chlorophyll fluorescence indexes, RWC were significantly increased and ELI was significantly decreased in bacteria inoculated treatments. Also, MDA, H2O2 were decreased, and antioxidant capacity, proline, and antioxidant enzymes were increased due to inoculation with these bacteria. Besides, the amount of K+ as an index of salinity tolerance significantly increased, and leaf Na+ content was significantly decreased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seventy percent of global agricultural productions are lost by abiotic stresses such as high temperature, drought, and salinity. Salinity is the frequent and severe abiotic stress for the plants (Husain et al. 2003). Accumulation of salt occurs in all soil types in many soil classifications, but it is more usual for soils that interact with water. It happens when the evaporation rate exceeds precipitation, and that is why this phenomenon has been named secondary salinity. Secondary salinity directly affected by farm management methods like irrigation system with low-quality water and cutting trees (Bui 2013). It is estimated that in the future more than 50% of cultivatable lands will be affected by salt due to secondary salinity. More than 800 million hectares, approximately 6% of total world’s land, has been affected by salinity and sodicity and 2% of the rainfed lands has become secondary-salinity-induced and 20% of irrigated lands affected by salinity (Lakhdar et al. 2009; Munns and Gilliham 2015; Pan et al. 2019).
Canola (Brassica napus L.) is the second most crucial oil-rich seed containing 40–45% oil and 25% protein. It has been a food resource for both humans and animals, and it has industrial and biofuel usage that has worth overall 41 billion dollars every year. Salinity stress is a harsh hamper to canola production like other plants (Hashem et al. 2019; Lohani et al. 2020). Salt stress inhibits the growth of canola through two mechanisms, firstly the water deficit is induced by the presence of ions, which prevent take up of water by roots, and secondly, the ions enter the transpiration stream, which led to the damaging of cells (Munns 2005). Initially, salt stress affects roots and makes them smaller or thicker. In such conditions, Na+ and Cl− accumulate in leaves so that the ionic balance will be triggering programmed cell death, and phenomena like leaf firing and leaf curling would consequently occur (Shannon et al. 1998). Besides, high levels of these ions lead to malfunction of protein synthesis and lipid metabolism. Moreover, this escalated rate of ions causes an imbalanced energy stream in photosynthetic systems which in consequence lead to production of reactive oxygen species (ROS) (Abid et al. 2020). An increase of reactive oxygen species (ROS) amount made in this way is hazardous and fatal for cells and plants (Farsaraei et al. 2020). Accumulation of ROS also damages the cell membrane. ROS interacts with membrane lipids and bring malondialdehyde (MDA) as the final lipid peroxidation. MDA content is an indicator of oxidative stress (Das and Roychoudhury 2014). Plants alleviate salinity stress through many ways, mainly preventing the accumulation of toxic ions like Na+ in shoots through several mechanisms like the exclusion of ions or preventing ions uptake and transport of them to shoots, via production of protecting osmolytes like proline. Also, proline has an antioxidant role under the abiotic stresses (Parida and Das 2005). Plants have different defense systems against the ROS; based on enzyme antioxidants and non-enzyme antioxidants. The first mechanism includes enzymes like superoxide dismutase (SOD) and catalase (CAT). These enzymes scavenge and prevent damages of O2− and H2O2, respectively (Navarro-León et al. 2020). There are several strategies for alleviating salt stress and decreasing ROS’s toxic effects, such as plant breeding and genetic manipulation (Li et al. 2017). However, many of these methods are challenging from an environmental point of view, and time consuming with limited success. In addition, many of them were not successful. Using plant growth promoting rhizobacteria (PGPR) is an environment friendly method which could be suitable to encounter against abiotic stresses including salinity stress (Glick and Bashan 1997). Recent studies indicated these microorganisms are beneficial for plants and develop plant tolerance against the abiotic stresses through the direct and indirect mechanisms (Olanrewaju et al. 2017). Direct mechanism encompasses solubilizing of nutrients like phosphate and potassium, providing N2 (biological nitrogen fixation), the production of phytohormones (like auxin, cytokinin, abscisic acid, and gibberellic acid), and synthesized enzyme 1-amino cyclopropane-1-carboxylate (ACC) deaminase (that decrease the stressed ethylene level) and siderophores synthesizing. Also, they are capable of removing toxic heavy metals through bacterial exopolysaccharides. Indirect mechanisms enhance the tolerance against biotic stresses, including antibiotics, chitinase (cell wall degradation enzyme), hydrogen cyanide (HCN) synthase, and competition against pathogens (Olanrewaju et al. 2017; Etesami and Maheshwari. 2018).
There is little data on the effect of PGPR bacteria utilization on canola under salinity stress. Therefore, this research aims to unravel the physiological and biochemical response (mainly antioxidant responses) mechanism of canola inoculated with two compatible strains of bacteria, including Enterobacter sp. S16-3 and Pseudomonas sp. C16-2O. Besides, the effect of two bacteria in regards to alleviating salt stress was investigated. Identification of the mechanism employed by two bacteria will enhance our knowledge in the application of PGPR as an efficient supplement for agricultural purposes.
Materials and methods
Bacterial strains
PGPR bacterial strains Enterobacter sp. S16-3 (Genbank Accession No. MN194179) and Pseudomonas sp. C16-2O (Genbank Accession No. MN192122) were obtained from the laboratory of Soil Biology, Department of Soil Science, University of Tabriz (Tabriz, Iran). Selection of these strains for the present research was based on their growth promoting abilities, as demonstrated in previous studies (Oskuei et al. 2018; Sarikhani et al. 2018; Sarikhani et al. 2020). An over-night culture of these bacteria was prepared in nutrient broth (NB), then a carrier-based inoculant of bacteria was prepared using sterile bagasse and perlite (with a ratio of 1:1) to reach a proper number of alive bacteria per gram of the carrier (108 CFU/g). In pot culture, 3 g of this inoculant was used for inoculation of seeds. Each treatment was individually inoculated with these bacteria.
Seed inoculation and plant material
Canola seeds (Brassica napus L. cultivar okapi) were provided from the Department of Agronomy and Plant Breeding Tehran University’s Gene Bank. Canola seeds were sterilized in sodium hypochlorite solution (1.5%) for fifteen minutes, washed five times with autoclaved distilled water (Yasin et al. 2018). Seeds were individually inoculated with Enterobacter sp. S16-3 and Pseudomonas sp. C16-2O, moreover one treatment used without inoculation as a control, all treatments carried out in three replications. C16-2O, After the inoculation, six seeds were sown in plastic pots (20 cm × 14 cm), which were filled with 3 kg of heated sterile soil with clay-loam structure in greenhouse conditions (25–30 °C, daylight: 15 h, humidity: 65%). After seven days, we kept three suitable seedlings and start to simulate salinity stress conditions with irrigation water containing different concentrations of NaCl solution (0, 100, 200 and 300 mM). After 21 days, all plants were harvested and kept in a − 80 oC freezer for further measurements.
Determination of leaf area
Pictures were taken by camera and leaf area (LA) was analyzed with Digimizer software Version 5.4.5 (MedCalc Software Ltd, Ostend, Belgium).
SPAD reading, chlorophyll fluorescence and photosynthetic pigments determination
The average of the four readings measured by the portable chlorophyll meter (SPAD-502, Minolta, Tokyo, Japan) was calculated for middle leaves. Chlorophyll fluorescence indexes were measured by portable chlorophyll fluorimeter (Handy PEA, Hansatech Instruments Ltd, King’s Lynn, UK), so parameters like the initial fluorescence value (F0), maximum fluorescence value (Fm), variable fluorescence value (Fv), and a reminder of the maximum quantum efficiency of Photosystem II (Fv/Fm) were measured after 20 min dark adaptation. Chlorophyll a, b, and total carotenoids content were measured by the method of Arnon (1949).
Na+ and K+ ion determination
The ion content was measured by a flame photometer (JENWAY PFP7/C, Staffordshire, UK) according to the dry ashing method (Yang et al. 2013). 0.2 g of dried shoot powder was put into the porcelain crucible, that porcelain crucible placed into the furnace for 1.5 h at 200 °C, and gradually temperature was raised to 450 °C to make dry ash. Plant shoot ash was digested by HCl (Merck, Darmstadt, Germany) and HNO3 (Merck, Darmstadt, Germany) acids concentration (in a ratio of 1:1). The solution was filtered and used for quantifying ions.
Relative water content (RWC)
Five disks of young and developed leaves were weighted (fresh weight; FW) and put in a defined amount of distilled water for 10 h to determine the turgid weight (TW). Finally, the leaf discs were put in the oven at 70 °C for 24 h and then weighted to measure dry weight (DW) (Abbasi et al. 2020). The percentage of relative water content (RWC) was calculated as follow:
Electrolyte leakage Index (ELI) and lipid peroxidation
Electrolyte leakage Index (ELI) was measured according to the method of Lutts and Guerrier (1995). Lipid peroxidation was estimated by malondialdehyde content (MDA) following the Qiu et al. (2014) method with a little modification. Approximately 0.2 g of crushed leaves were homogenized with 5% trichloroacetic acid (TCA) (Merck, Darmstadt, Germany) and centrifuged at 12000 g for 10 min. The supernatant was mixed with 0.5% thiobarbituric acid (Merck, Darmstadt, Germany) prepared in TCA (20%) after that mixture was transferred to a boiling water bath for 30 min. After instantly cooled in the ice bath for 5 min solution and then was centrifuged at 12000 g for 10 min. Finally, the absorbance of the supernatant was measured at 532 and 600 nm (Shimadzu UV160U UV–Vis spectrophotometer, Kyoto, Japan). The MDA content (µmol mg−1 FW) was calculated using an extinction coefficient of 155 mM−1 cm−1.
H2O2 and DPPH radical scavenging
The concentration of H2O2 was determined according to Velikova et al. (2000) method. 0.5 g of the crushed plant leaves were homogenized with TCA (0.1%) (Merck, Darmstadt, Germany) and then centrifuged at 16000 g for 10 min. 0.5 ml of supernatant was mixed with 0.5 ml phosphate buffer (Merck, Darmstadt, Germany) (10 mM, pH 7) and 1 ml of potassium iodide (1 M) (Merck, Darmstadt, Germany). The absorbance of the solution was read at 390 nm. 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging was measured based on extract potential to reduce the radical (DPPH) by the method of Brand-Williams et al. (1995) with minor modifications. 0.1 g of plant powder was mixed with 2 ml methanol (80% v/v) (Merck, Darmstadt, Germany) and shake for 24 h in 25 °C and then centrifuged at 12000 g for 20 min. The supernatant was mixed with the same proportion of DPPH (0.1 M) (Merck, Darmstadt, Germany) then the reaction mixtures were incubated in the dark for 40 min. The absorbance of the mixtures was measured at 515 nm (Shimadzu UV160U UV–Vis spectrophotometer, Kyoto, Japan). Scavenging activity is expressed as a percent for any sample.
Total protein content and proline
The total protein content of leaves was measured according to Bradford (1976) method. Plant leaves were crushed in liquid nitrogen; after that, the powder was homogenized with Na-phosphate buffer (50 mM, pH 7.6) (Merck, Darmstadt, Germany) and centrifuged at 12000 g for 20 min at 4 °C. The supernatant was used for protein estimation at 532 nm by spectrophotometer (Shimadzu UV160U UV–Vis spectrophotometer, Kyoto, Japan). The supernatant's residue was transferred to another tube in a − 80 °C freezer for antioxidant enzyme measurements. For proline content quantifying 0.5 g of plant tissue was mixed with 5 ml of sulphosalicylic acid (3%) (Merck, Darmstadt, Germany). Then centrifuged at 15,000 g for 20 min 2 ml of supernatant transferred to test tube and then 2 ml acid ninhydrin solution (we mixed 1.25 g of acid ninhydrin in 30 ml of glacial acetic acid and 20 ml orthophosphoric acid 6 M (Merck, Darmstadt, Germany)) was added to the supernatant. Afterward, 2 ml glacial acetic acid (Merck, Darmstadt, Germany) was added to the solution. Tubes were placed in a boiling water bath for one hour and cooled quickly on the ice. Then we added 4 ml toluene (Merck, Darmstadt, Germany) and vortex for 20 s. Proline content was measured at 520 nm (Shimadzu UV160U UV–Vis spectrophotometer, Kyoto, Japan) and calculated by the standard curve of L-proline (Merck, Darmstadt, Germany) and expressed as a nmol g−1 fresh weight (Bates et al. 1973).
2.10. Antioxidant enzyme activities
Superoxide dismutase (SOD, EC 1.15.1.1) activity was determined according to Dhindsa et al. (1981). One unit of the enzyme is the amount of SOD that inhibits 50% of nitroblue tetrazolium at 25 °C. The reaction mixture was contained 50 mM Na-phosphate buffer pH 7.6, 13 mM methionine, 75 µM NBT (Bio Basic Inc., Toronto, Canada), 2 µM riboflavin (Merck, Darmstadt, Germany), 0.1 mM EDTA (Merck, Darmstadt, Germany), and 0–50 µL enzyme extract, riboflavin added lastly. Then the tube was shaken and placed under a 40-w fluorescent lamp for 15 min, and finally, absorbance was read at 560 nm (Shimadzu UV160U UV–Vis spectrophotometer, Kyoto, Japan). Catalase (EC 1.11.1.6) activity estimated by the conversation of H2O2 to water and oxygen (Bianco and Defez 2009). APX (ascorbate peroxidase, EC 1.11.1.11) activity was determined according to Naveed et al. (2020). According to this protocol, the reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.6), 0.5 mM ascorbate (Merck, Darmstadt, Germany), and 0.1 M EDTA, 0.1 M H2O2 (Merck, Darmstadt, Germany), and the reaction was started by adding extract, and absorbance was decreased at 290 nm (Shimadzu UV160U UV–Vis spectrophotometer, Kyoto, Japan). PPO (polyphenol oxidase EC 1.10.3.1) was measured according to Resende et al. (2002) by using pyrogallol (20 mM) (Merck, Darmstadt, Germany) and H2O2 (20 mM) as substrates, so absorbance of the solution was determined at 420 nm.
2.11. Scanning electron microscopy studies
Ten days seedling roots were washed with autoclaved water and fixed in 3% glutaraldehyde solution prepared in a 0.1 M sodium phosphate buffer (Merck, Darmstadt, Germany) for four hours at 4 °C. Roots were washed three times with buffer, then last fixed carry out in 1% OsO4 (prepared in 0.2 M sodium phosphate buffer) (Merck, Darmstadt, Germany), and then samples were dehydrated at ethanol series (20, 40, 60, 80, and 100%). At the end of the fixation procedure, residual ethanol was replaced by isoamyl acetate (Merck, Darmstadt, Germany) (Dastager et al. 2010). Afterward, samples were coated with a thin layer of gold by Ion Sputter Coater (SPT-20 COXEM Co., Ltd, Daejeon, South Korea), and were prepared to analyze by scanning electron microscope (FEI Quanta 200, Oregon, USA).
Statistical analysis
The experiment was designed based on a completely randomized factorial experiment, and all of the data are analyzed by SAS 9.4 software (SAS Institute Inc, North Carolina, USA). Mean value compared by Least Significance Difference (LSD) test and in the all-data P values < 0.05 were considered significant.
3. Results and discussion
3.1. Root and leaf growth
Salt stress decreased root length (RL), root dry weight (RDW), leaf area (LA), and root fresh weight (RFW). Also, Table 1 shows that RL, RDW, LA, and RFW damage were alleviated through bacterial inoculation. The best performance was under 100 mM, and the were performance was under fourth salt level. Moreover, there is no significant difference in RL, RDW, LA, and RFW between plants that inoculated and non-inoculated under 100 mM. Furthermore, under 300 mM no significant difference was observed between bacterial treatment and non-bacterial treatment. In addition, there is no significant difference between inoculation of plants (with Enterobacter sp. S16-3 and Pseudomonas sp. C16-2O) in these characters under 100 and 200 mM concentrations.
Under control condition (without inoculated PGPR), the most serious damage in root length (79.7%) was observed at 300 mM NaCl concentration, and the lowest (36.9%) was at 100 mM NaCl concentration (Table 1). However, bacterial inoculation significantly ameliorated the stress damage by approximately 22% (S16-3) and 23% (C16-2O) under 100 mM NaCl. Also, under 200 mM salt concentration, bacterial treatment increased RL by about 16% compared to non-inoculated plants (Table 1). Root dry weight and root fresh weight showed the same trends as RL. However, in RFW, there is a significant difference between inoculated and non-inoculated under 300 mM salt stress. Root fresh weight under 100 mM decreased 41%, but plants treated with bacteria showed about 20% reduction for each bacterial treatment (S16-3 and C16-2O), so that inoculation with bacteria showed a significant difference in RFW (Table 1). This reduction of damage through bacterial treatment reduced to 9% under 200 mM but was significant. Bacterial treatment led to a significant increase approximately 13% in RFW under 300 mM (Table 1). Treatment with S16-3 and C16-2O lessened stress damage significantly about 26% (S16-3), 25% (C16-2O) under 100 mM and about 11% (S16-3), 12% (C16-2O) under third level of stress (Table 1). There was a significant growth in RDW after treatment with bacteria which was about 26% and 11% higher under 100 and 200 mM, respectively compared to control (Table 1). Leaf area illustrated a significant increase of about 17.7% (S16-3) and 18% (C16-2O) under bacterial treatment in 100 mM NaCl concentration compared to non-inoculated plants (Table 1). However, this amount was reduced under 200 mM concentration of NaCl, so bacterial treatment could alleviate just 9% of stress damage (Table 1). In the following, under 300 mM of NaCl concentration, there was no significant difference between inoculated and non-inoculated plants regarding LA (Fig. 1). Salt stress leads to the accumulation of Na+ and Cl− in plant tissues, and made the plant tissues and cellular media toxic which consequently leads to ROS production and biosynthesis of ethylene (Ahmad and Prasad 2011; Farsaraei et al. 2020). Also, a high level of salt around the root area disturbs the ion uptake balance in the root, therefore, uptake of vital nutritious minerals such as potassium and phosphorus were prevented by roots (Orozco-Mosqueda et al. 2020). Treatment with Enterobacter sp. (S16-3) and Pseudomonas sp. (C16-2O) made an excellent condition nutritionally, especially under salt stress (Table 1). These two bacteria can solubilize K and P and supply the bioavailable form of these minerals, and K and P are the two most important macronutrients for plant growth (Sarikhani et al. 2020). Because providing and absorbing P and K by root has vital importance for plant growth, especially root growth under salt stress while plants treated with PGPRs had a better performance in RL, RDW, and RFW (Table1). The same trend was observed in the previous study on corn; that study showed treatment with Enterobacter sp. (S16-3) and Pseudomonas sp. (C16-2O) increased P and K content in root and shoot (Sarikhani et al. 2020). In addition, phytohormones such as auxins, cytokinin, and gibberellins have a crucial point in plant growth like cell division, stem, and root elongation, especially under stress conditions. Numerous auxins are known in nature, but indole-3-acetic acid (IAA) is the most common structure of this hormone in plants. Many reports admit that PGPRs (about 80%) can produce IAA (Glick 2012). Then this considerable amount of root growth may be related to the production of IAA by PGPRs. Also, these data show LA was expanded significantly. Maybe it is related to the expanded root condition that had a suitable surface to absorb additional nutrients. Root elongation cooperated in providing like K, P, nitrogen (N) that are important to expand LA (Bakhshandeh et al. 2020; Glick et al. 2007; Sarikhani et al. 2019). what is more, IAA, gibberellin, and cytokinin are the most significant factors of plant growth that many reports in the recent literature admitted PGPRs are capable of producing and transfer them to the plant (Etesami et al. 2014; Olanrewaju et al. 2017). One essential phytohormone with a vital role in plant growth, mainly under stress conditions, is ethylene. Ethylene is a gaseous hormone that has a crucial role in low concentration for seed germination, fruit ripening, root elongation, and the beginning of flower. 1-aminocyclopropane-1-carboxylate (ACC) is an ethylene precursor which generates ethylene by AAC oxidase activity. Also, ACC is produced by ACC synthase activity. Plants have multiple copies of ACC oxidase and synthase genes, and they are induced under abiotic stress conditions like salinity. So, plants respond to salt stress by the production of ethylene that called ‘stress ethylene’ this level of ethylene lead to induction of production of defensive and protecting entities against stress, like proteins (Ali et al 2014; Etesami and Maheshwari 2018; Orozco-Mosqueda et al. 2020). It is the first peak of ethylene and is beneficial for plants. The second peak of ethylene appears after some days of stress condition generated by an additional ACC source. This peak is detrimental to plant growth and leads to chlorosis, abscission, and senescence. ACC-deaminase is an enzyme whose activity was observed in a majority of microbiomes in the rhizosphere. Plant ACC content is transferred to PGPRs through the plant roots, and ACC-deaminase degrades ACC to ammonia and α-ketobutyrate and used them as nitrogen sources (Li et al. 2020; Orozco-Mosqueda et al. 2020).
Consequently, perhaps this conclusion that the significant growth of canola root and leaf in this study results from ACC-deaminase activity via S16-3 and C16-2O is logical. Also, many studies proved that these strains with ACC-deaminase activity could promote canola growth (Bakhshandeh et al. 2020; Li et al. 2017).
Potassium and sodium ion contents
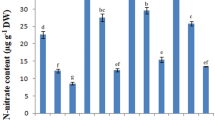
The current study illustrates that inoculation with bacteria significantly alleviates the toxic level of sodium (Na+) and increases the level of potassium (K+) under salt stress that is crucial for plant growth (Fig. 2). Accumulation of Na+ leads to destructive effects on the plant, such as imbalance in absorption of nutrients like K+ and Ca+ that are highly vital for plant growth (Kronzucker et al. 2013). Also, high levels of Na+ prevent plants from access to water (Naveed et al. 2020). The low amount of Na+ vs. the high amount of K+ is one of the critical parameters against salt stress (Pavlović et al. 2017). This study shows that the accumulation of Na+ is significant in control plants under all levels of salt stress (Fig. 2B). However, treatment with bacteria decreased this amount, and the best performance was observed (about 50%) under 100 mM of NaCl concentration, and it is same between the two bacteria (Fig. 2B). After that, results show about 20% reduction under 200 mM NaCl concentration with inoculation of PGPRs (Fig. 2B). There is no significant difference between the two bacteria; also, there is no significant difference between inoculated and non-inoculated plants under 300 mM NaCl concentration (Fig. 2B). Decreasing Na+ in plant leaves treated with S16-3 and C16-2O may be attributed to the secretion of exopolysaccharides (EPS) produced by a wide variety of bacteria. EPS bind with Na+ and prevents the transfer of Na+ to plant tissues and decrease the Na+ high levels as a consequence of toxic effect of the high level of Na+ have been averted (Ashraf et al. 2004). It may also be related to the increase of plasma membrane Na+/H+ antiporter (SOS1) expression level that prevents the absorption of Na+ to plasma. The previous study showed the application of Bacillus subtilis with Brassica campestris under 300 mM NaCl concentration increase of SOS1 expression (Woo et al. 2020). Shahid et al. (2021a, b) observed that the Na+ content of mung beans was decreased through inoculation with Kosakonia sacchari under salt stress. This study shows bacterial treatment lessens the reduction in K+ concentration under salt stress. This increase of K+ level compared to the control plants under 100 mM NaCl is about 21% and 18% for S16-3 and C16-2O, respectively (Fig. 2). Moreover, there is a significant difference in K+ content between inoculated and non-inoculated plant leaves under 200 mM NaCl concentration. After reducing Na+ through EPS binding and increasing K+ in soil media through the presence of PGPRs, it is logical that K+ content increased in plant leaves. The same result was observed in Arachis hypogaea that were inoculated with six different PGPRs (Shukla et al. 2012).
Relative water content (RWC)
Bacterial treatment significantly reduced about 16% damage of sodium destruction effect on RWC, especially under 100 mM salt concentration, and this is the same for two bacteria (Fig. 3). This effect of inoculation with bacteria is significant under 200 mM NaCl concentration (Fig. 3). However, there is no significant difference between inoculated and non-inoculated at the highest level of stress (Fig. 3). Regarding the appropriate root condition under bacterial treatment (Table 1) and high potassium content in inoculated plants (Fig. 2A), it may be logical to conclude that bacterial treatment enhanced relative water content under salt stress. Moreover, stomatal conductance has a vital role to control the water movement under abiotic stress like salt stress, and it is affected by abscisic acid production (Munemasa et al. 2015). Abscisic acid has a crucial role in control and upregulation of stomatal conductance (Barnawal et al. 2017; Woo et al. 2020). And a wide variety of bacteria can trigger plants to produce this phytohormone. Barnawal et al. (2017) observed that Arthrobacter protophormiae and Dietzia natronolimnaea improved the stomatal conductance under salt stress. Bacteria enhance root growth through auxin production and prevent ethylene production. Also, secretion of EPS through bacteria make suitable soil texture for water uptake (Naseem et al. 2018). A previous study showed that sweet pepper inoculation with Bacillus thuringiensis improves RWC significantly under 34 and 68 mM NaCl concentration (ALKahtani et al. 2020).
Chlorophyll pigment content and chlorophyll fluorescence changes
Salt stress decreased SPAD value, photosynthetic pigments and carotenoid contents (Table 2). Lowest decline was observed under 300 mM NaCl concentration for all of photosynthetic traits: SPAD value 67%, Fv/Fm 33%, Fv/F0 70%, chlorophyll a 66%, chlorophyll b 54%, total chlorophyll 50%, carotenoids 43%, and lowest reduction observed under 100 mM salt concentration: SPAD 30%, Fv/Fm 12%, Fv/F0 30%, chlorophyll a 26%, chlorophyll b 18%, total chlorophyll 29%, carotenoids 12% (Table 2).
When plants try to prevent water loss by closing stomata, reduction of carbon dioxide assimilation is inevitable. As a result, there is no output for the electron transfer chain, such as the production of ATP, and it leads to the accumulation of reduced ferredoxins (Zarei et al. 2020); consequently, active oxygen radicals’ build-up was increased. So, the chloroplast is one of the most critical sites for producing reactive oxygen species (ROS) when salt stress happened (Das and Roychoudhury 2014). The cell takes a mechanism to reduce ROS production; thus, chlorophyllase degraded chlorophyll pigments (chlorophyll a and b) are reduced significantly (Harpaz-Saad et al. 2007; Hu et al. 2015). Results showed that plants inoculated with S16-3 and C16-2O had better SPAD values and chlorophyll pigment content (Table 2). The highest SPAD value and chlorophyll pigments were observed under 100 mM salt concentration (Table 2). (S16-3: SPAD value 16%, chlorophyll a 18.2%, chlorophyll b 9%, total chlorophyll 20% and carotenoid 5%. C16-2O: SPAD value 15%, chlorophyll a 11%, chlorophyll b 9%, total chlorophyll 18% carotenoid 4%), compared to non-inoculated plants under 100 mM salt concentration. Also, there is no significant difference between S16-3 and C16-2O (Table 2). The same pattern was observed in soybean treated with Pseudomonas putida H-2–3 under salt stress (Kang et al. 2014). Also, a previous study showed Serratia sp. CP-13 has bioremediation protentional in inducing tolerance against cadmium toxicity in the corn plant (Tanwir et al. 2021).
There are many chlorophyll fluorescence indexes who has highest efficiency of PSII (Fv/Fm) and inferred oxygen-evolving complex activity (Fv/F0), which are the most important (Maxwell and Johnson 2000). These are sensitive parameters to environmental changes like drought and salinity (Sayed 2003). Chlorophyll fluorescence parameters (Fv/Fm, and Fv/F0) may be declined due to inhibition of osmotically driven uptake of water in salt stress, light-harvesting center damages, and D1 protein degradation (Kalaji et al. 2011). Previous studies showed ion imbalance and mineral nutrition shortage in salt stress cause to increase in non-photochemical quenching (Loudari et al. 2020). Results (Table 2) showed that S16-3 and C16-2O significantly ameliorated (P < 0.05) chlorophyll fluorescence parameters especially at 100 mM NaCl concentration (S16-3: Fv/Fm 8.31%, and Fv/F0 15%; C16-2O: Fv/Fm 8.7%, and Fv/F0 14%).
As regard to increasing of photosynthetic area (Table 1) and good condition of water and nutrient uptakes through the plants (Fig. 3) like potassium and phosphorus (Fig. 2), an increase of chlorophyll pigments and chlorophyll indexes are reasonable. A previous study showed that Pseudomonas fluorescens strains ameliorated chlorophyll fluorescence and chlorophyll pigments in sweet corn under water-deficit conditions (Zarei et al. 2020). Also, ALKahtani et al. (2020) observed that sweet pepper plants treated with PGPRs showed a significant increase in chlorophyll fluorescence and chlorophyll pigments.
3.5. Biochemical parameters
Salinity leads to many damages to plants plasma membrane. One of the most important indexes for this damage is electrolyte leakage index (ELI) and this study shows that there are tough damages in plasma membrane stability under salt stress (Fig. 4). ELI was significantly increased about 70% under 300 mM NaCl concentration and the lowest increase of about 30% was observed under 100 mM NaCl concentration (Fig. 4). However, PGPRs significantly subsided the plasma membrane damage in inoculated plants, and decreased ELI about 12% in 100 mM (Fig. 4). A previous study showed that PGPRs belong to Bacillus sp., Exiguobacterium sp. and Enterobacter sp. with phosphorus and potassium solubilizing ability can decrease ELI under salt stress in rice (Prittesh et al. 2020). Sodium concentration at toxic level in plants leads to production of reactive oxygen species (ROS). One of them is hydrogen peroxide (H2O2) and results shows that H2O2 significantly increased under salt stress and the tremendous damage was observed under 300 mM. However, PGPR treatment significantly reduced the level of H2O2 specially under 100 mM. Maybe it is attributed to inhibition of Na+ intake through roots and deceased level of K+ (Prittesh et al. 2020). Moreover, the excellent condition of water content provides a suitable condition in inoculated plants. Under salinity stress, high levels of Na+ content and osmotic stress lead to oxidative stress (Ahmad and Prasad 2011; Waqas et al. 2019). One of the best markers for oxidative stress is malondialdehyde content (Li et al. 2017; Shahid et al. 2021a, b). MDA content is a final product of lipid peroxidation of the plasma membrane by ROS that leads to ion leakage (Javed et al. 2017), and many studies showed that under salt stress, MDA content is increased (Farhangi-Abriz and Torabian 2016; Li et al. 2017; Li et al. 2020).
Mean comparison in canola under salt stress and treated with Enterobacter sp. (S16-3) and Pseudomonas sp. (C16-2O). A electrolyte leakage index B malondialdehyde (MDA) content C hydrogen peroxide content D 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging E proline content F Protein content. Different letters show significance difference (LSD, P < 0.05, n = 3)
Nevertheless, our result illustrates that PGPRs can reduce MDA content significantly under salt stress (Fig. 4B). This reduction is noticeable even under 300 mM of concentration, and under this level, plants inoculated with Enterobacter sp. S16-3 have a lower MDA content (Fig. 4B). In addition, it is evident that a decline in H2O2 level leads to a decrease in membrane damage; consequently, MDA content and ELI decreased. The same pattern was shown by Batool et al. (2020) in Solanum tuberosum inoculated with Bacillus subtilis HAS31. Previous studies showed that wheat inoculation with Bacillus megaterium (Rashid et al. 2021), maize inoculation with Kocuria rhizophila Y1 (Li et al. 2020), and canola inoculation with Enterobacter cloacae HSNJ4 (Li et al. 2017) decrease MDA content under abiotic stress.
Also, DPPH scavenging activity was increased significantly (P < 0.05) under salt stress (Fig. 4D). The highest increase was observed under 300 mM NaCl concentration (Fig. 4D). But the most significant increase (16%) between control and bacterial treatment was observed under 100 mM NaCl concentration and 5% under 200 mM, and 3% under 300 mM (Fig. 4D). DPPH scavenging activity represented antioxidant activity in plants and leaf extracts (Liu et al. 2007). This study showed that bacterial treatment boosted antioxidant capacity (Fig. 4D). It was rational that PGPR treatment leads to low H2O2 and MDA content, and had good membrane stability. A study showed similar results, ELI and DPPH scavenging activity in Pennyroyal (Mentha pulegium L.) inoculated with Azotobacter sp. and Azospirillum sp. stress was decreased and increased, respectively, under water shortage (Asghari et al. 2020).
The present study shows protein content was increased significantly under salt stress (Fig. 4F). Furthermore, there is a significant increase when treated with microbial treatments (S16-3 and C16-2O). The most considerable increase (33%) was seen under 100 mM compared to control (without microbial treatment) (Fig. 4F). However, there is not any significant difference between S16-3 and C16-2O. Maybe it is due to an increase of stress response proteins like Na+/K+ channels. The same results were observed by (Asghariet al. 2020). Also, Batool et al. (2020) showed that Bacillus subtilis treatment increased free amino acid content in Solanum tuberosum. Proline has several functional roles in many abiotic stresses like osmoprotectant, stabilizer in cellular structure, and ROS scavenging (Abbasi et al. 2020). Prittesh et al. (2020) observed that bacteria belong to Bacillus sp., Exiguobacterium sp., Enterobacter sp., Lysinibacillus sp., Stenotrophomonas sp., Microbacterium sp., and Achromobacter sp. can enhance proline content in rice. In the present study, proline content increased under different levels of NaCl concentration (Fig. 4E). The high level of proline content (more than double) was seen under 300 mM, and the minimum level was seen under 100 mM of NaCl concentration. Also, results shows that bacterial treatment had a tremendous effect on the proline level. Moreover, this effect is considerable (about 36% higher) compared to the other levels (Fig. 4E). There is no significant difference between S16-3 and C16-2O like the other parameters. A similar result in proline content was observed in the previous study in wheat inoculation with Bacillus megaterium (Rashid et al. 2021). Furthermore, inoculation with Kocuria rhizophila Y1 (Li et al. 2020) and Enterobacter cloacae HSNJ4 (Li et al. 2017) showed a significant increase in proline content under salinity stress in maize and canola, respectively.
Antioxidant enzymes activity
The present study showed that ROS damages like accumulation of H2O2 and membrane lipid peroxidation were decreased by inoculation with Enterobacter sp. S16-3 and Pseudomonas sp. C16-2O (Fig. 4). These results indicate that plants treated with PGPRs had a powerful antioxidant defense system (Fig. 5D and 6). Superoxide dismutase (SOD) as the first antioxidant enzymatic defense significantly increased under treatment with S16-3 and C16-2O (Fig. 6A). SOD scavenges superoxide and produces H2O2 in the following CAT, APX, and PPO scavenges H2O2 and reduces the ROS damages to the plant cell (Gill and Tuteja 2010; Das and Roychoudhury 2014). Antioxidant enzyme activities were increased under salt stress, whose the highest activity is related to 300 mM of NaCl concentration (Fig. 6). Also, there is a significant difference between inoculated and non-inoculated plants but no significant difference between the two bacterial strain treatments. Biochemical parameters like MDA and H2O2 were decreased in parallel with an increase in antioxidant enzymes. Also, this increase in antioxidant capacity may be related to increasing antioxidant enzyme activities and an expanded level of proline content (Fig. 4 and 6). Previous studies showed that PGPRs Enterobacter cloacae HSNJ4, Pseudomonas fluorescens strains improved canola and sweet corn antioxidant system against salinity stress, respectively (Li et al. 2017; Zarei et al. 2020).
Conclusion
Results illustrate a novel and clear link between bacterial treatment and changes in light-harvesting centers regarding chlorophyll fluorescence parameters under salt stress. Also, this study shows the relation between hydrogen peroxide content produced by the electron transport chain in photosynthesis and the antioxidant system that is boosted by bacterial treatments. Pseudomonas sp. C16-2O and Enterobacter sp. S16-3 can solubilize potassium, and phosphorous which can inhibit NaCl intake by effective cloning on canola roots (Fig. 5). Inhibition of Na+ intake is one of the fundamental ways to avoid salt stress damages, and the second is the antioxidant system. The current study shows antioxidant enzyme activity such as SOD, CAT, APX, and PPO was increased through inoculation with C16-2O and S16-3. The effect of bacterial inoculation to ameliorate salinity stress was significant in all salt stress levels used in this study, but it was considerable under 100 mM NaCl. Also, the bacterial treatment improved the root surface, and it had a significant impact on all aspects of plant growth because water and vital nutrients became available for plants. In conclusion, Pseudomonas sp. C16-2O and Enterobacter sp. S16-3 assuage the destructive effects of salinity. Consequently, they can be used in fertilizers, especially in salty soils.
References
Abbasi S, Sadeghi A, Safaie N (2020) Streptomyces alleviate drought stress in tomato plants and modulate the expression of transcription factors ERF1 and WRKY70 genes. Sci Hortic. https://doi.org/10.1016/j.scienta.2020.109206
Abid M et al (2020) Effect of Salt stress on growth, physiological and biochemical characters of Four kiwifruit genotypes. Sci Hortic 271(May):109473. https://doi.org/10.1016/j.scienta.2020.109473
Ahmad P, Prasad MNV (2011) Environmental adaptations and stress tolerance of plants in the era of climate change. Springer, Berlin
Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem 80:160–167
ALKahtani MD et al (2020) Chlorophyll fluorescence parameters and antioxidant defense system can display salt tolerance of salt acclimated sweet pepper plants treated with chitosan and plant growth promoting rhizobacteria. Agronomy. https://doi.org/10.3390/agronomy10081180
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Asghari B, Khademian R, Sedaghati B (2020) Plant growth promoting rhizobacteria (PGPR) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L) under water shortage condition. Sci Hortic. https://doi.org/10.1016/j.scienta.2019.109132
Ashraf M et al (2004) Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils 40(3):157–162. https://doi.org/10.1007/s00374-004-0766-y
Bakhshandeh E et al (2020) Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul 90(1):123–136. https://doi.org/10.1007/s10725-019-00556-5
Barnawal D et al (2017) Plant growth-promoting rhizobacteria enhance wheat salt and drought stress tolerance by altering endogenous phytohormone levels and TaCTR1/TaDREB2 expression. Physiol Plant 161(4):502–514. https://doi.org/10.1111/ppl.12614
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207. https://doi.org/10.1007/BF00018060
Batool T et al (2020) Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci Rep 10(1):1–19. https://doi.org/10.1038/s41598-020-73489-z
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60(11):3097–3107. https://doi.org/10.1093/jxb/erp140
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brand-Williams W et al (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Tech 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Bui EN (2013) Soil salinity: a neglected factor in plant ecology and biogeography. J Arid Environ 92:14–25. https://doi.org/10.1016/j.jaridenv.2012.12.014
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Fronti Environ Sci. https://doi.org/10.3389/fenvs.2014.00053
Dastager SG, Deepa CK, Pandey A (2010) Isolation and characterization of novel plant growth promoting Micrococcus sp. NII-0909 and its interaction with cowpea. Plant Physiol Biochem 48(12):987–992. https://doi.org/10.1016/j.plaphy.2010.09.006
del Carmen Orozco-Mosqueda M, Glick BR, Santoyo G (2020) ACC deaminase in plant growth-promoting bacteria (PGPB): an efficient mechanism to counter salt stress in crops. Microbiol Res. https://doi.org/10.1016/j.micres.2020.126439
Dhindsa RS, Plumb-dhindsa P, Thorpe TA (1981) Leaf senescence : correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase content in a trusted digital archive. We use information technology and tools to increase produ. J Exp Bot 32(126):93–101
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol Environ Saf 156(January):225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
Etesami H et al (2014) Bacterial biosynthesis of 1-aminocyclopropane-1-carboxylate (ACC) deaminase and indole-3-acetic acid (IAA) as endophytic preferential selection traits by rice plant seedlings. J Plant Growth Regul 33(3):654–670. https://doi.org/10.1007/s00344-014-9415-3
Farhangi-Abriz S, Torabian S (2016) (2017) ‘Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress.’ Ecotoxicol Environ Saf 137:64–70. https://doi.org/10.1016/j.ecoenv.2016.11.029
Farsaraei S, Moghaddam M, Pirbalouti AG (2020) Changes in growth and essential oil composition of sweet basil in response of salinity stress and superabsorbents application. Sci Hortic 271(May):109465. https://doi.org/10.1016/j.scienta.2020.109465
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Glick BR (2012) ‘Plant growth-promoting bacteria: mechanisms and applications.’ Scientifica 1:30–39. https://doi.org/10.1016/j.micres.2013.09.009
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth-promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15(2):353–378. https://doi.org/10.1016/S0734-9750(97)00004-9
Glick BR et al (2007) Promotion of plant growth by bacterial ACC deaminase. Crit Rev Plant Sci 26(5–6):227–242
Harpaz-Saad S et al (2007) Chlorophyllase is a rate-limiting enzyme in chlorophyll catabolism and is posttranslationally regulated. Plant Cell 19(3):1007–1022. https://doi.org/10.1105/tpc.107.050633
Hashem HA et al (2019) The potentiality of marine macro-algae as bio-fertilizers to improve the productivity and salt stress tolerance of canola (Brassica napus L.) plants. Agronomy 9(3):146
Hu X et al (2015) Reexamination of chlorophyllase function implies its involvement in defense against chewing herbivores. Plant Physiol 167(3):660–670. https://doi.org/10.1104/pp.114.252023
Husain S, Munns R, Condon AG (2003) Effect of sodium exclusion trait on chlorophyll retention and growth of durum wheat in saline soil. Aust J Agric Res 54:589–597. https://doi.org/10.1071/AR03032
Javed MT et al (2017) Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol Environ Saf 141:216–225. https://doi.org/10.1016/j.ecoenv.2017.03.027
Kalaji HM et al (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot 73(1):64–72. https://doi.org/10.1016/j.envexpbot.2010.10.009
Kang SM et al (2014) Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact 9(1):673–682. https://doi.org/10.1080/17429145.2014.894587
Kronzucker HJ et al (2013) Sodium as nutrient and toxicant. Plant Soil 369(1–2):1–23. https://doi.org/10.1007/s11104-013-1801-2
Lakhdar A et al (2009) Effectiveness of compost use in salt-affected soil. J Hazard Mater 171(1–3):29–37. https://doi.org/10.1016/j.jhazmat.2009.05.132
Li H et al (2017) Enhanced tolerance to salt stress in canola (Brassica napus L.) seedlings inoculated with the halotolerant Enterobacter cloacae HSNJ4. Appl Soil Ecol. https://doi.org/10.1016/j.apsoil.2017.05.033
Li X et al (2020) A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ Exp Bot 174(January):104023. https://doi.org/10.1016/j.envexpbot.2020.104023
Liu X et al (2007) Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT Food Sci Technol 40(3):552–557. https://doi.org/10.1016/j.lwt.2005.09.007
Lohani N et al (2020) Engineering Multiple Abiotic Stress Tolerance in Canola, Brassica napus. Front Plant Sci 11(February):1–26. https://doi.org/10.3389/fpls.2020.00003
Loudari A et al (2020) Salt stress affects mineral nutrition in shoots and roots and chlorophyll a fluorescence of tomato plants grown in hydroponic culture. J Plant Interact 15(1):398–405. https://doi.org/10.1080/17429145.2020.1841842
Lutts S, Guerrier G (1995) Peroxidase activities of two rice cultivars differing in salinity tolerance as affected by proline and NaCl. Biol Plant 37(4):577–586. https://doi.org/10.1007/BF02908842
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51(345):659–668. https://doi.org/10.1093/jexbot/51.345.659
Munemasa S et al (2015) Mechanisms of abscisic acid-mediated control of stomatal aperture. Curr Opin Plant Biol 28:154–162. https://doi.org/10.1016/j.pbi.2015.10.010
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167(3):645–663. https://doi.org/10.1111/j.1469-8137.2005.01487.x
Munns R, Gilliham M (2015) Salinity tolerance of crops - what is the cost? New Phytol 208(3):668–673. https://doi.org/10.1111/nph.13519
Naseem H et al (2018) Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J Basic Microbiol 58(12):1009–1022. https://doi.org/10.1002/jobm.201800309
Navarro-León E et al (2020) Study of salt-stress tolerance and defensive mechanisms in Brassica rapa CAX1a TILLING mutants. Environ Exp Bot 175(February):104061. https://doi.org/10.1016/j.envexpbot.2020.104061
Naveed M et al (2020) Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability (switzerland) 12(3):1–17. https://doi.org/10.3390/su12030846
Olanrewaju OS, Glick BR, Babalola OO (2017) Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol 33(11):1–16. https://doi.org/10.1007/s11274-017-2364-9
Oskuei BK et al (2018) Protein profiles underlying the effect of plant growth-promoting rhizobacteria on canola under osmotic stress. J Plant Growth Regul 37(2):560–574. https://doi.org/10.1007/s00344-017-9754-y
Pan J et al (2019) The growth promotion of two salt-tolerant plant groups with PGPR inoculation: a meta-analysis. Sustainability 11(2):378. https://doi.org/10.3390/su11020378
Parida AK, Das AB (2005) Salt tolerance and salinity effects on plants: a review. Ecotoxicol Environ Saf 60(3):324–349. https://doi.org/10.1016/j.ecoenv.2004.06.010
Pavlović I et al (2017) (2018) ‘Short-term salt stress in Brassica rapa seedlings causes alterations in auxin metabolism’. Plant Physiol Biochem 125:74–84. https://doi.org/10.1016/j.plaphy.2018.01.026
Prittesh P et al (2020) Amelioration effect of salt-tolerant plant growth-promoting bacteria on growth and physiological properties of rice (Oryza sativa) under salt-stressed conditions. Arch Microbiol 202(9):2419–2428. https://doi.org/10.1007/s00203-020-01962-4
Qiu H et al (2014) Cloning and characterization of a novel dehydrin gene, SiDhn2, from Saussurea involucrata Kar et Kir. Plant Mol Biol. https://doi.org/10.1007/s11103-013-0164-7
Rashid U et al (2021) Drought-tolerant Bacillus megaterium isolated from semi-arid conditions induces systemic tolerance of wheat under drought conditions. Plant Cell Rep. https://doi.org/10.1007/s00299-020-02640-x
Resende MLV et al (2002) Induction of resistance in cocoa against Crinipellis perniciosa and Verticillium dahliae by acibenzolar-S-methyl (ASM). Plant Pathol 51(5):621–628. https://doi.org/10.1046/j.1365-3059.2002.00754.x
Sarikhani MR et al (2018) Isolation and identification of potassium-releasing bacteria in soil and assessment of their ability to release potassium for plants. Eur J Soil Sci 69(6):1078–1086. https://doi.org/10.1111/ejss.12708
Sarikhani MR, Khoshru B, Greiner R (2019) Isolation and identification of temperature tolerant phosphate solubilizing bacteria as a potential microbial fertilizer. World J Microbiol Biotechnol 35(8):126. https://doi.org/10.1007/s11274-019-2702-1
Sarikhani MR, Aliasgharzad N, Khoshru B (2020) P solubilizing potential of some plant growth promoting bacteria used as ingredient in phosphatic biofertilizers with emphasis on growth promotion of zea mays L. Geomicrobiol J. https://doi.org/10.1080/01490451.2019.1700323
Sayed OH (2003) Chlorophyll fluorescence as a tool in cereal crop research. Photosynthetica 41(3):321–330. https://doi.org/10.1023/B:PHOT.0000015454.36367.e2
Shahid M et al (2021a) Colonization of vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, stressor metabolites, antioxidant status and yield under NaCl stress. Appl Soil Ecol 158:103809. https://doi.org/10.1016/j.apsoil.2020.103809
Shahid M et al (2021b) Ressor metabolites, antioxidant status and yield underColonization of Vigna radiata by a halotolerant bacterium Kosakonia sacchari improves the ionic balance, st NaCl stress. Appl Soil Ecol 158:103809. https://doi.org/10.1016/j.apsoil.2020.103809
Shannon MC, Grieve CM (1998) Tolerance of vegetable crops to salinity. Sci Hortic 78(1–4):5–38
Shukla PS, Agarwal PK, Jha B (2012) Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J Plant Growth Regul 31(2):195–206. https://doi.org/10.1007/s00344-011-9231-y
Tanwir K et al (2021) Serratia sp. CP-13 alleviates Cd toxicity by morpho-physio-biochemical improvements, antioxidative potential and diminished Cd uptake in Zea mays L. cultivars differing in Cd tolerance. Ecotoxicol Environ Safety. https://doi.org/10.1016/j.ecoenv.2020.111584
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants protective role of exogenous polyamines. Plant Sci 151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Waqas MA et al (2019) Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Front Plant Sci 10(October):1–14. https://doi.org/10.3389/fpls.2019.01336
Woo OG et al (2020) Bacillus subtilis strain GOT9 confers enhanced tolerance to drought and salt stresses in Arabidopsis thaliana and Brassica campestris. Plant Physiol Biochem 148(January):359–367. https://doi.org/10.1016/j.plaphy.2020.01.032
Yang L et al (2013) Comparison of dry Ashing, wet Ashing and microwave digestion for determination of trace elements in Periostracum Serpentis and Periostracum cicadae by ICP-AES. J Chil Chem Soc 58(3):1876–1879. https://doi.org/10.4067/S0717-97072013000300018
Yasin NA et al (2018) Imperative roles of halotolerant plant growth-promoting rhizobacteria and kinetin in improving salt tolerance and growth of black gram (Phaseolus mungo). Environ Sci Pollut Res 25(5):4491–4505. https://doi.org/10.1007/s11356-017-0761-0
Zarei T et al (2020) The role of ACC deaminase producing bacteria in improving sweet corn (Zea mays L. var saccharata) productivity under limited availability of irrigation water. Sci Rep 10(1):1–12. https://doi.org/10.1038/s41598-020-77305-6
Acknowledgements
The authors thank the agronomy and plant breeding department of the University of Tehran for providing canola seeds and a greenhouse. The authors thank the department of soil science of the University of Tabriz for providing bacteria.
Author information
Authors and Affiliations
Contributions
MN Writing and editing original draft, visualization, formal analysis, resources and investigation research. AA writing and editing, project administration and supervision. AH writing and editing. MRS resources, writing and editing. AR resources, writing and editing. DD resources, writing, editing, and investigation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Neshat, M., Abbasi, A., Hosseinzadeh, A. et al. Plant growth promoting bacteria (PGPR) induce antioxidant tolerance against salinity stress through biochemical and physiological mechanisms. Physiol Mol Biol Plants 28, 347–361 (2022). https://doi.org/10.1007/s12298-022-01128-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-022-01128-0