Abstract
Cassava is an important source of food security and livelihoods for millions of consumers daily. Water deficit conditions are one of the major factors that affect the development of root system architecture (RSA) and consequently, crop productivity, and yet, due to its long maturity periods and bulky storage root systems, RSA studies in cassava are uncommon. The objective of this study was to identify traits that are responsible for the variability and plastic responses of cassava in response to drought at the juvenile stage of growth. Eight cassava genotypes were grown in soil-filled pots under well-watered and droughted conditions for up to 45 days and multivariate analyses employed to determine the major contributory traits to variability and the relative distance plasticity index (RDPI) was computed to evaluate plasticity. There were significant genotypic variations for most of the traits measured. Drought generally inhibited root production and development and the degree of inhibition was between 2 and 22%. Regardless of the soil moisture condition, traits which differentiated the RSA included root biomass, root numbers, root branching density, and total root length, and these were also the important contributory traits to variability under well-watered soil conditions. Important contributory traits to variability traits under drought were shoot-related traits such as leaf area and shoot biomass, and also root system traits such as nodal root number, root biomass, diameter and branching density. Phenotypic plasticity was found in most traits where the number, branching density and diameter of upper nodal roots presented the highest RDPI. These traits corresponded with the traits contributing greatly to variation. Plastic responses of cassava to drought were dependent on trait and genotype. It is concluded that upper nodal roots-related traits could have importance in breeding cassava to better tolerate water deficit conditions. The secondary growth and ability to maintain or increase the upper nodal root count or density under limited soil moisture may be related to good growth and yield performance of cassava under drought conditions. Upper nodal roots could be used to screen and select cassava genotypes adapted to drought at the juvenile stage but as a potential indirect selection strategy, the persistence and pertinence of these traits and their relationship with yield and yield components under drought conditions in the field must be confirmed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As a crop that provides an energy output per hectare per day which is much higher than that of most crops, cassava is an important food security crop, also providing livelihoods for millions of people in sub-Saharan Africa (SSA), Asia and Latin America (Adenle et al. 2012; Adu et al. 2020). Cassava is mostly produced in tropical areas with pronounced dry periods and infertile soils, yet much of the world’s cassava is grown with no irrigation and external nutrient inputs (FAO 2013). The crop is said to be drought-tolerant, reasonably resistance to diseases, and flexible in its cultivation, management and harvesting periods (Bayitse et al. 2017; FAO 2002). Even so, actual yields of cassava are still below potential yields (Okogbenin et al. 2013) due principally to the pervasive use of unimproved genetic materials by the majority of farmers, poor soils, biotic and abiotic stresses (El-Sharkawy 2005; Okogbenin et al. 2013). In the last few years, the national average yield of cassava in Ghana has been approximately 13.8 t ha−1 compared to yield potential of 48.7 t ha−1. In 2017, Ghana achieved 45.96% of the achievable cassava yield (SRID-MOFA 2011; MoFA 2017).
Although relatively drought-tolerant, cassava is very sensitive to soil water deficit during early growth stages where biomass production and yields can be substantially reduced (Pardales and Esquisel 1996, El-Sharkawy 2003). Under water deficit conditions, commercial cassava varieties exhibited a significant reduction in productivity (Oliveira et al. 2015). The development of genotypes with traits that increase water and nutrient acquisition could considerably increase yields on infertile soils and drier areas. While this will require the determination of traits that contribute most to variation between genotypes, there is also, increasingly, a persuasive need to understand the factors that regulate the development of cassava roots because of the role it plays in soil resource acquisition under edaphic stress (Adu et al. 2018a, b; Duque and Villordon 2019; Thorup-Kristensen and Kirkegaard 2016). Yet, relatively fewer studies have generally considered root system architectures (RSAs) of root and tuber crops such as cassava (Adu et al. 2018a; Duque and Villordon 2019). Besides, there is a paucity of studies addressing physiological responses of cassava under water deficit conditions and the key contributing traits to the responses (Daryanto et al. 2016; Shan et al. 2018).
In tropical and regions with prolonged dry spells, one of the approaches to improving the performance of cassava under drought could be the development and cultivation of genotypes with improved root systems, able to acquire limited soil water. For the fact that RSA describes the spatial arrangement of roots within the soil, drought tolerance in cassava might be correlated to cassava RSA. For example, narrower and vertically oriented RSA is normally more drought tolerant. Root growth rate and angle are also key factors contributing to water and nutrient uptake (Rogers and Benfey 2015). It has been reported that well-developed branching pattern, comprising of increased root numbers and lengths of axile, lateral and the total root system, is associated with water acquisition (Izumi et al. 1999). Studying the role of cassava RSA in soil resource acquisition under abiotic and edaphic stress could thus improve food security (Duque and Villordon 2019), particularly in the regions where the ecological conditions often combine with a poor resource base to reinforce low agricultural productivity, food insecurity, and poverty (Adu et al. 2018b).
Opportunely, genetic variability in the ability of cassava to tolerate drought has been reported (dos Santos Silva et al. 2019; Okogbenin et al. 2013), paving the way for the identification of accessions, with high root yield under water deficit conditions, that can be used as parent material in breeding programs (de Oliveira et al. 2017). Early-maturing cultivars of cassava could be harvested at 7 months, while late-maturing types are ready 12 months after planting, protracting conventional breeding that involves spatially and temporally repetitive trials. In cassava breeding programmes, complex traits are often evaluated very late in the growth cycle and these late measurements often deviate from the objectives of modern breeding that seeks a rapid screening procedure and fast elimination of lines to reduce the size of the population. Plant breeding efforts are currently seeking rapid methods to reduce the length of the breeding scheme by using efficient phenotyping strategies (Okogbenin et al. 2013). A substantial amount of time can be saved if desirable traits from juvenile plants can be used to predict final yield, without having to measure the actual yield traits directly at maturity. Moreover, cassava roots could grow as long as 2 m deep, making measurements of fibrous root very difficult (Okogbenin et al. 2013).
The ability to phenotype cassava RSA at the juvenile stage satisfies some of the goals of modern plant breeding, given that it will accelerate breeding for crop improvement in response to environmental stresses and higher productivity. The present study provides a time-saving and less laborious option for a multi-trait selection in breeding for improved genotypes in cassava for drought stress conditions. One of the key contributions of this study is the potential to increase selection intensity (i) in cassava improvement programmes, as previously highlighted by Adu et al. (2020). The four main factors of the genetic gain equation (ΔG) that influence breeding progress, include the phenotypic variation in the population (σp), the heritability (h2), selection intensity (i) and the length of time necessary to complete a cycle of selection (L) (Adu et al. 2020). Being able to screen a larger population or more genotypes of cassava at the juvenile growth stage in pots would lead to increasing the i considerably. Moreover, in between harvests and planting time, selected genotypes can be planted in the field following the regular breeding scheme and having a gain by the indirect selection process that was incorporated at the juvenile stage. The objectives of the current study were therefore to (i) examine the effect of reduced water supply on RSA of juvenile cassava, (ii) identify the responsible traits for variability in juvenile cassava genotypes grown under drought and non-drought conditions and (iii) determine the traits that enable plastic responses of juvenile cassava plants in response to reduced soil moisture.
Materials and methods
Genetic material
The cassava genotypes used in the present study have previously been described in Adu et al. (2018a, 2020). They include a panel of eight cassava genotypes, including a cultivar released in 2005 [‘Cape Vars Bankye’ (Catalogue of Crop Varieties Released in Ghana, 2015); designated as H8 in this study] and seven other genotypes. The seven genotypes, designated as 1A, 2B, 3C, 4D, 5E, 6F, and 7G, were generally bred for high carotenoids contents, host plant resistance to Cassava mosaic disease (CMD), improved yield and ‘poundability’ or ‘mealiness’ of boiled cassava storage root (Table 1). Five of the seven new genotypes have recently been recommended for release. These seven genotypes are composed of 2 white-fleshed and 5 yellow-fleshed cassava genotypes. All 8 genotypes thrive well in the Coastal Savannah, Forest/Savannah transitional zone, and the Forest zones of Ghana. All the genotypes have conical-cylindrical root shape and are characterized by similar tuber initiation and maturity durations, maturing in 12 months after planting. Table 1 shows additional characteristics of the 7 new genotypes included in the study.
Soil and environmental conditions
The experiment was conducted at the Teaching and Research Farm of the School of Agriculture, University of Cape Coast (UCC; 5.1155°N, 1.2909°W) with 0–15 cm topsoil collected from an arable field near the experimental location. The soil was a clay-rich subsoil haplic acrisol. The chemical properties of the soil have previously been described in Adu et al. (2019). This was a pot experiment conducted using 30, 000 cm3 perforated polybags as pots. The pots were filled with air-dried soil and tapped to achieve a bulked density of approximately 1.1 g cm−3. The pots were arranged on a wooden platform raised 30 cm above the floor and kept under an 8 × 12 × 2.4 m rigid rain shelter constructed with a galvanized iron pipe with a 0.1 mm thick ultraviolet (UV)-resistant transparent polyethene film roof. Site temperature and relative humidity ranged between 24 and 32 °C and 60% to 80%, respectively. Day length at the experimental site ranges from approximately 11.30 to 12.40 h while solar radiation ranges from 3151 kJ cm−2 day−1 to 3804 kJ cm−2 day−1, respectively (Adu et al. 2017; 2018a).
Planting, watering and harvesting
The setup in Adu et al. (2018a, 2020) was adopted for this study. Cassava hardwood stem cuttings obtained from the middle third of stems from 12-month-old plants were used, ensuring that the diameter of cuttings was greater than one half the diameter of the thickest part of the stem of respective genotypes (Adu et al. 2018a, 2020). Cuttings of approximately 20 cm in length of each genotype were planted in an inclined position (about 45o) into the soil at the centre of the pots, making sure that about two-thirds of each stem cutting, accommodating at least six nodes, were within the soil. One cutting was planted into each pot and a randomized design was employed. The pots were positioned side by side with the assumption that for growth of up to 45 days after planting (DAP), this spacing would be sufficient and would not cause any mutual shading of plants (Adu et al. 2018a, 2020). Positions of pots were rotated every 10 days to reduce the effects of possible environmental gradients. Two days before planting, the soil in each pot was watered with tap water to 100% field capacity (FC) determined gravimetrically and allowed to drain and evaporate. The soils were subsequently maintained at either 30% or 80% FC during the growth period by watering to weight with tap water every 3–4 days. It was assumed that 30% FC would be sufficient to impose drought stress. No other soil amendment was applied. There were two samplings in this study, at 30 DAP, and 45 DAP. At sampling, plants were harvested by carefully cutting the polyethene pots longitudinally along the two fused sides with a blade to extract roots. Roots were gently washed free of soil under slow-running tap water, using a water hose, taking care to minimize root damage. Three replications per genotype and water supply level were sampled at each of the two harvesting periods.
Shoot and root system measurements
The number of days it took for each propagule to sprout was visually recorded, as days-to-sprout (DtS). Shoot and root system traits were measured as described in Adu et al. (2018a, 2020). Shoots with leaves were detached from the stem cuttings and the number of sprouted shoots (NS) determined. The number of leaves (NL) were determined after leaves have been severed from the shoots. The leaves were then carefully positioned flat and arranged end-to-end on a surface which provided a good contrast. Images of the leaves were subsequently captured with a Canon EOS 70D DSLR camera held stationary on tripod 50 cm above the leaves. Binarization and thresholding feature extraction routines in ImageJ image processing software (US National Institutes of Health, Bethesda, MD, USA, https://imagej.nih. gov/ij/) were used to extract leaf area (LA) (Adu et al. 2018a, 2020). Shoots and leaves were weighed and oven-dried at 80 °C to constant weight to determine shoot fresh weight (SFW) and shoot dry weight (SDW), respectively.
Root system traits were classified into three categories consisting of traits from two types of nodal roots [upper nodal roots (UNRs) and lower nodal roots (LNRs)], and traits from basal roots (BRs), as described in Adu et al. (2018a, 2020) and Izumi et al. (1999). Diameters of basal roots (DBR), of UNRs (DUNR) and LNRs (DLNRS) were measured with Venier Callipers from three sampled roots and at 3 cm from the stem cutting. The inter-branch distance of BRs (IBBR), of UNRs (IBUNR) and LNRs (IBLNR) was measured as the distance between two successive 1st order roots, using a measuring rule. Branching density of basal roots (BDBR), of upper nodal roots (BDUNR) and lower nodal roots (BDLNR) were determined from three randomly sampled roots of each category, 2 cm from the cutting and within 6 cm of each parent root. Numbers of basal roots (NBR), upper nodal roots (NUNR) and lower nodal roots (NLNRs) were manually counted. Sum of NUNR and NLNR gave the total number of nodal roots (TNNR) and the sum of BR and TNNR gave the total number of roots (TRN). Total root length (TRL) was measured by spreading and suspending the roots in water and imaging with a Canon EOS 70D DSLR camera held stationary on tripod 50 cm above roots. Root length was then extracted with skeletonization routines (Adu et al. 2018a, 2020) in ImageJ software. Following the measurements of root system traits, roots were weighed and oven-dried at 80 °C to constant weight to determine root fresh weight (RFW) and root dry weight (RDW), respectively. The quotient of RDW and SDW was Root-to-shoot ratio (R: S). Specific root length (SRL) was calculated as the quotient of RDW and TRL.
Data analyses
Mean data of the two sampling times were used for analyses. Descriptive statistics including mean and range for all traits were calculated. The coefficient of variation (CoV) was determined as the quotient of the standard deviation and the mean and expressed as a percentage. General analysis of variance was used to determine variation between genotype and treatment means and possible interactive effects of genotype and water supply. To identify the traits responsible for variability in juvenile cassava genotypes grown under different soils moisture conditions, multivariate analysis, employing principal component analysis (PCA) was performed using (i) the mean values of the replications of the traits for all the data, (i.e.: traits measured from drought and well-watered plants) and (ii) independently for data from plants grown under reduced water (30% FC) and those grown under well-watered conditions (80% FC), as suggested by Subere et al. (2009). To compare root responses to drought and non-drought among the genotypes and to further determine the traits that were responsible for variation in RSA in response to reduced water, the ratios of the traits from plants grown at 30% FC and those from plants grown at 80% FC (D/W) were calculated and also used to perform a PCA (Subere et al. 2009). Principal components with an eigenvalue > 1.00 were retained as significant PCs (Adu et al. 2018a). The squared cosine (cos2) and trait contribution (contrib) were computed for each trait after the PCA, which gave the quality of representation of the variables on the factor map and the total contribution of individual traits, respectively (Adu et al. 2019). Subsequently, to identify traits responsible for plastic responses of cassava to reduced water supply, the relative distance plasticity index method (RDPI) proposed by Valladares et al. (2006) was employed. Here, the RDPI, which ranges from 0 (no plasticity) to 1 (maximum plasticity) (Fry et al. 2018; Marchiori et al. 2017), specified the relative phenotypic distance or amount of change in a given trait between plants of the same genotype exposed to 30 or 80% field capacity. All replicates of each genotype by soil moisture treatment was compared for each trait, generating a list of indices for each genotype, which were subsequently analysed using analyses of variance. The ANOVA was performed using GenStat (GenStat Release 12.1, VSN International, Oxford, UK). The multivariate analysis was performed using three packages, including the FactoMineR, the ‘corrplot and Factoextra in the R software (Kassambara 2017; R Core Team 2013; Wei and Simko 2017). The RDPIs were calculated using the Plasticity function (Ameztegui 2017) implemented in the R software (R Core Team 2013).
Results
Descriptive data and the effect of genotype
Plants grew vigorously for up to the 45 days in the soil-filled pots and showed no symptoms of mineral deficiencies or stress. Descriptive statistics including range and coefficient of variation on traits measured are presented in Table 2. The CoV for the traits examined were generally less than 50% (Table 2). There was a significant effect (p < 0.05 or p < 0.001) of genotype on over 70% (18 out of 25) of the traits (Table 2). Generally, diameter- and inter-branch-related traits did not exhibit significant differences between genotypes (Table 2). Examinations on the relationship between drought/well-watered (D/W) ratios suggest that drought generally reduced measured values of shoot or root-related traits by approximately, 2–22% (Table 2). However, drought enhanced some traits, including DtS, IBUNR and TRL, by approximately 2–23%, except RDW which was enhanced by 267% (Table 2). For NL and NS, neither the water supply nor genotype x water supply interaction was significant (p < 0.05) (Table 2).
Phenotypic variation among traits of cassava genotypes
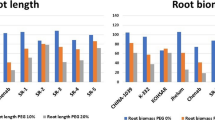
There was considerable variation for many root traits among the different cassava genotypes. Phenotypic variation in root traits mostly ranged from 1.0-fold in the inter-branch distance of basal roots to 26.7-fold and 30.3-fold in root fresh weight under drought and well-watered conditions, respectively (Supplementary Table S1). The phenotypic variations in SFW, LA, SRL and TRL under both drought and well-watered conditions were relatively higher (Supplementary Table S1). Significant genotypic variation (p < 0.05; Table 2) was observed in several traits (18 out of 25 traits), including plant biomass (Fig. 1a), number and area of leaves (Fig. 1b), total length and number of roots (Fig. 1c). There was also signification variation (p < 0.05) among the genotypes in the branching density of basal and upper nodal roots but not in lower nodal roots (p = 109; Fig. 1d). Among genotypes, H8 obtained the highest values for most of the roots traits, including the number of roots, shoot biomass, total root length, branching densities, diameters, the inter-branch distance of various root categories under both drought and well-watered conditions (Supplementary Table S1). Among the seven yet-to-be-released lines (i.e.: genotype A1-G7), genotype A1 appeared superior in most traits and C3 mostly obtained lesser mean values for most traits under drought and well-watered conditions (Supplementary Table S1; Fig. 1a–c).
Effect of water supply on the shoot and root traits
There was a significant effect (p < 0.05 or p < 0.001) of water supply on most (19 out of 25) of the traits examined (Table 2). While plants which were supplied with less water took an additional day to sprout (Fig. 2a), generally, plants exposed to drought recorded significantly lower values for various traits compared to those exposed to non-drought conditions (Figs. 2b–f). After 45 days of growth, drought stress significantly decreased the fresh weight of shoots and roots by 24.8 and 26.2% and dry weight by 26.1 and 24.7%, respectively (Fig. 2b). Drought reduced the proliferation of categories of roots, such that the branching density of basal roots, lower and upper nodal roots declined by 10.0, 11.6 and 19.7%, respectively under limited water supply (Fig. 2c). The trend was similar to the diameters and numbers of various roots (Fig. 1d, e). Plants which received more water developed relatively broader and additional leaves, as well as longer roots. Drought stress decreased leaf area by 15.4% and plants subjected to 80% FC generally obtained 19.1% longer roots (Fig. 2f).
Effect of water supply on root shoot and root systems traits of pot-grown cassava plants. a Days to stem sprouting; b fresh and oven-dried biomass of roots and shoots; c branching density of various root classes; d diameter of various root classes; e number of various root classes; f total root length and leaf area
Multivariate analysis of shoot and root traits
The first five principal components (PCs) had an eigenvalue greater than one and explained 87.3% of the total variation among the cassava genotypes studied for the twenty-five shoot and root system traits included in the PCA. The eigenvectors indicated that 80% of the traits measured had positive weight for PC 1, which accounted for 59.2%, of the total variations among the eight cassava genotypes (Fig. 3a, b). The variation in PC1 was explained mostly by the shoot biomass and all root-related traits except derivative traits such as root-to-shoot ratio and specific root length (Fig. 3a). The relative magnitude of an eigenvector for the second PC was 9.7%, explained mostly by the number of leaves and shoots (stems) (Fig. 3a, b). Important traits on the third dimension included days-to-sprouting and accounted for approximately 7.3% of the variation (Fig. 3a). Figure 3b shows a biplot of traits vectors and the location of the genotypes according to their PC scores. The largest variation explained by the biplot came from shoot number-, basal- and nodal root-related traits, including leaf numbers, shoot numbers, the inter-branch distance of basal and upper nodal roots, and branching density of lower nodal roots, as indicated by the relative length of their vectors (Fig. 3b). The next largest variation explained by the biplot came from biomass-, root length- and root number-related traits (Fig. 3b).
Results of PCA on 25 traits measured from 8 genotypes of cassava assayed at 30 and 45 days after planting. a Loading scores of traits on each component of the first five significant components. Components with eigenvalues greater than one were considered significant and the scale is indicated in the bar by the matrix; b Genotype by trait PCA biplot showing trait vectors and location of the single objects. Arrow lines represent vectors that quantify the magnitude and direction of a trait’s contribution to that axis; c Plot of quality of representation of the variables (cos2 of variables) on the factor map for all dimensions considered significant following the PCA; d Plot showing the total contribution of variables in accounting for the variability in the first two components. The red dashed line on the graph indicates the expected average contribution. The variables with a contribution greater than this expected average are considered important. Contribution of the first twenty variables is shown here. Trait names are shown in acronyms previously defined in this paper, and also in Table 2
In the upper right quadrant of the biplot, a strong relationship between leaf-, biomass-, root length- and number-related traits are shown. In another quadrant, a strong relationship is shown between diameter-branching density and inter-branch distance-related traits (Fig. 3b). Regardless of the level of water supply, all plants of genotypes G7 resolved in the upper right quadrant of the biplot in the direction of biomass-, root length- and number-related traits. All plants of genotypes B2 and C3 were found in the lower left quadrant of the biplot in the direction of root-to-shoot ratio and specific root length, while all plants of genotype H8 resolved in the lower right quadrant of the biplot in the direction of root branching densities, root diameters, and inter-branch distances of second-order roots (Fig. 3b). Droughted and well-watered plants of the remaining genotypes resolved in different quadrants of the biplot, and the direction of different RSA traits (Fig. 3b). Analysis of quality of representation of the variables on the factor map suggested that leaf area, all biomass- and root number-related traits and total root length were highly represented on PC1 with cos2 between 0.40 and 0.91 (Fig. 3c). Seventeen traits contributed above the average cut-off point to the variability in the first two dimensions (PC1 and PC2). In ranking order, the first 10 of these were NUNR, BdUNR, TNR, TNNR, TRL, SFW, IntDistUNR, BdLNR, NL and SDW (Fig. 3d).
Multivariate analysis performed independently for drought-stressed plants and well-watered plants showed that although the number of roots was consistently a major contributing trait to variation under both drought and well-watered conditions, shoot-related traits including shoot biomass, number of shoots, leaves and leaf area were dominant traits under drought conditions (Fig. 4a, b). On the other hand, root-related traits including root biomass, length and root proliferation-related traits were the major contributors of variation in RSA, having contributed above the average cut-off point to the variability in the first two dimensions (Fig. 4b). Results from the PCA of D/W ratios for each trait revealed that the first six PCs were significant and explained 97.7% of the total variations. Among the traits examined, the R: S ratio, NUNR, DUNR, BdUNR, NLNR, DLNR, BdLNR, IntDistLNR, NBR, DBR, BDBR, IntDistBR, TNNR and TNR showed the highest eigenvector in PC 1 (Fig. 4c). In PC 2, the LA, SFW, SDW, RFW, RDW and SRL were the important traits (Fig. 4c). Twelve shoot and root-related traits contributed above the average cut-off point to the variability of D/W ratios in the first two dimensions. In ranking order, these included LA, TNR, RFW, BdUNR, NLNR, BDBR, RDW, TNNR, SDW, SFW, BdLNR and NUNR (Fig. 4d).
Plot of the contribution of variables to the first two principal components for droughted to a well-watered ratio (D/W) for traits measured from cassava plants grown in soil-filled pots for up to 45 days after planting. a Plot of contributions from PCA of well-watered plants only; b plot of contributions from PCA of droughted plants only; c loading scores of D/W ratios for traits on each component of the first six significant components. Components with eigenvalues greater than one were considered significant and the scale is indicated in the bar by the matrix; d plot of the contribution of variables to the first two principal components from the PCA of droughted to a well-watered ratio (D/W) for all measured traits. The red dashed line on the graph indicates the expected average contribution. The variables with a contribution greater than this expected average are considered important. Contribution of the first twenty variables is shown here. Trait names are shown in acronyms previously defined in this paper
Relative Distance Plasticity Index
Relative Distance Plasticity Indices (RDPI) ranged from 0.022 to 0.704 with a mean of 0.204. The RDPI calculations revealed that root and shoot characteristics of the young cassava plants were significantly altered by the soil moisture conditions, and that plasticity was dependent on the genotype and measured trait (Fig. 5). Thus, genotype was was significant (p < 0.001), and the largest RDPI was recorded by the F6 genotype and the least by the H8 genotype (Fig. 5a). Also, plasticity varied significant (p < 0.001) across the traits measured (Fig. 5b). Upper nodal roots-related traits showed the most plastic responses to reduced water supply (Fig. 5b). Specifically, the number of upper nodal roots was primarily affected by the watering regime and obtained the highest RDPI (Fig. 5b; p < 0.001) followed by branching density and then the diameter of the same root category. Generally, biomass-related traits such as RDW, SFW and SDW and count-related traits such as the number of shoots, the total number of nodal roots and number of lower nodal roots obtained intermediate RDPIs (Fig. 5b). Basal roots-related traits including diameter, number and inter-branch distance of basal roots were among the traits that obtained the least RDPIs (Fig. 5b).
Relative Distance Plasticity Indices (RDPI) of cassava genotypes (a) and shoot or root systems traits (b) measured in juvenile cassava plants subjected to varying soil water availability. Trait names are shown in acronyms previously defined in this paper, and also in Table 2
Discussion
Eight genotypes of cassava were grown in soil-filled pots under varying soil water levels to study RSA development based essentially on the traits related to production (root numbers and branching densities), primary growth or elongation (total root length), and secondary growth or thickening (root diameters) of various root classes. Growing cassava in pots which could force downwards growth of roots, potentially changing the configuration of the RSA and imposing water stress by supplying different amounts of irrigation water under excluded rainfall conditions, is an artificial situation. This experiment, however, is important to study some of the physiological mechanisms underlying cassava tolerance to drought and response of cassava to water shortage at the early stage of growth. Lowe et al. (1982) effectively grew cassava from stem cuttings in sand-filled 30-cm fibre pots in controlled-environment chambers and noted that basal roots were more numerous than nodal roots and produced a proportionately greater number of tubers. The results here corroborated earlier reports, where cassava was grown for relatively extended periods in pots or under field conditions, that the root systems of cassava are adventitious in origin, consisting of the main root axis and orders of lateral roots (Adu et al. 2018a; Subere et al. 2009). The eight cassava genotypes used here might not necessarily reflect the total variation of Manihot exculenta, but genotypic variation in traits related to root production, primary and secondary root growth in the same panel of cassava cultivars has been reported (Adu et al. 2018a).
Effects of genotype and soil moisture on root system architecture
The physiological responses of cassava to water stress and possible mechanisms underlying its tolerance to drought have been extensively reviewed by Okogbenin et al. (2013). These responses include modifications in stomatal conductance, formation, life, scars and photosynthesis of leaves, in addition to other growth parameters. It also includes changes in water-use efficiency, osmoregulation, abscisic acid accumulation, storage and fibrous roots and shoots (Okogbenin et al. 2013). Root systems play important roles in crop growth and stress responses but traits of the RSA of cassava have rarely been utilized in breeding programmes so of particular importance to the present study is the adaptation of RSA to drought. Plants under drought stress, for example, develop long deep roots as an escape mechanism to evade water stress (Okogbenin et al. 2013). Studies on 10 IITA genotypes revealed large genotypic differences in fibrous root weight and root length was measured 2–5 weeks after planting (Okogbenin et al. 2013). Genotypic variation under water deficit conditions has been identified in cassava (El-Sharkawy 2007; El-Sharkawy et al. 1998; Okogbenin et al. 2003; Subere et al. 2009). The previously reported variations are exemplified in Tables 2 and S1, which show that significant variations were found in 72 per cent of measured traits among the cassava cultivars when grown in two contrasting soil moisture levels, either at low (30% FC) or high (80% FC). This finding is pertinent and can be exploited to develop water-use-efficient genotypes, given the fact that genetic differences in root traits within germplasm at the juvenile growth stage could persist throughout the growth cycle of cassava (Adu et al. 2018a; El-Sharkawy 2003). In this study, water supply generally inhibited root production and development, but the degree of inhibition differed among the genotypes. If the degree of root growth inhibition is computed as the ratio of the drought to well-watered treatments, the genotype that showed higher ratio (less inhibition) might better adapt to water-deficit conditions (Subere et al. 2009). For the traits which were suppressed by drought, the ratios suggested that, to a large extent, genotypes A1, G7 and H8 might be the top three drought-adaptable genotypes.
In the present study, the production (root numbers and branching densities) and growth (root length, diameters, and biomass) of the roots were affected by soil moisture level, a deficit of which generally suppressed either production and/or growth. Similar suppression of various cassava root traits under drought has been earlier reported (Kengkanna et al. 2019; Duque and Setter 2013; Pardales and Yamauchi 2003; Connor et al. 1981). Duque and Setter (2013) reported that when cassava plants were grown in pots over 30 days, growth of all plant parts of water-stressed decreased substantially. The present degree of inhibition in various traits due to water stress is consistent with that of Connor and Palta (1981) who noted that root yield of 1 to 5-months cassava under moisture stress was reduced by 32 to 60%. Pardales and Yamauchi (2003), for example, reported that drought reduced the number of lateral roots between 44 and 82 DAP and Oliveira et al. (2015) observed a reduction of up to 42% for some traits when cassava accessions were subjected to water deficit. Aina et al. (2007) also observed that height and stem girth of 16 weeks-old screen house-grown cassava plants irrigated to 25% FC was reduced by 12.6 and 16.3%, respectively, compared to plants irrigated to 75% FC. Equally, Okogbenin et al. (2003) reported a 37% and 22% decline in shoot growth and root yield due to moisture stress. The results of Okogbenin et al. (2003) exemplify a more pronounced suppression of shoot-related traits than root-related traits under moisture stress. Others, including Aina et al. (2007) have also reported a more pronounced reduction of root traits than shoot traits under drought. Here, the level of suppression was nearly the same for the shoot and root biomass. Aina et al. (2007) suggested that the duration of water stress and the stage of its application are critical determinants of the gravity of the stress on cassava plants.
Traits of the root system architecture and key traits that determined variability
To identify root traits that determined variability among the cassava genotypes regardless of soil moisture condition, a PCA was performed using the values of the entire dataset. The results suggest that the root traits which determined variability among the cassava germplasm at the juvenile stage of growth include root numbers, root branching density, and total root length. This was corroborated by the results of the analysis of quality of representation of the variables on the factor map which suggested that root numbers, branching density and total root length, were largely the principal traits which contributed to the variability in the first two dimensions. Traits that are correlated with the first two dimensions in a PCA are the most important in explaining the variability in a given dataset (Adu et al. 2018a; 2019). Variables which are uncorrelated with any PC have low contribution and are reductant in explaining variability. It must be noted, however, those important contributors to variability might not be necessarily be explaining the mechanisms underlying genetic variation or presenting distinguishable important plant functions. Similarly, uncorrelated traits to principal components could have important plant functions. The traits identified here significantly contribute to genetic variability in the panel of cassava genotypes examined, and could be exploited in the breeding of genotypes adapted to soil moisture and soil mineral nutrient stress conditions. Moreover, droughted and well-watered plants of the majority of the genotypes resolved in different quadrants of the biplot in the direction of different traits (Fig. 3B). This exemplifies different RSA configuration for the genotypes under the two water supply conditions and provides an opportunity for insightful parental selection in breeding programmes.
To identify root traits that determined variability either at optimal or reduced soil water conditions, PCA was performed independently for the values of the well-watered and reduced water datasets. Important root traits under well-watered conditions include biomass, number and length of roots, suggesting that root production or proliferation was enhanced under well-watered conditions. Important root traits under water deficit conditions were nodal root number-related traits, root biomass and root branching density and diameter-related traits. It is interesting to note the lack of contribution of TRL under drought in this study. Possibly, root confinement within the pots resulted in reduced root growth. In an attempt to relieve stress at the zone of root elongation, impedance to root growth may have led to increased proliferation of roots and an increase in root diameter. This phenomenon could explain why root numbers, branching and diameter and not root length were important under drought in this study. It is also interesting to note that shoot-related traits such as shoot biomass and leaf area were among the influential traits to variability under drought. Here, the leaf area was the foremost contributing trait to variability or contributor to the principal component under drought (Fig. 4d). Further, the PCA of the D/W ratios showed that leaf area, root number factors (TNR, TNNR, and NLNR), root biomass, upper nodal root factors (BDUNR and IBUNR) and IBLNR, were the important traits responsible for the variation in RSA among the cassava genotypes under reduced soil moisture. Even so, the D/W ratio of 1.003 for leaf area could suggest that the trait was of limited biological significance in the present study. The present results show that the production of roots (root numbers and biomass) and laterals (the branching ability of upper nodal roots) and inter-branch distance of lower nodal roots were responsible for variability under reduced soil moisture level.
Traits of the root system architecture and key traits that determined the response to reduced soil moisture
Phenotypic plasticity is important for plants’ response to stress conditions. To identify traits that were responsible for plastic responses and to obtain an integrative index related to the sensitivity to drought stress of the various genotypes, the RDPI was computed. The RDPI has been used to study plant adaptation to varying environments and to evaluate growth responses under stressful conditions (Fry et al. 2018; Marchiori et al. 2017; Valladares et al. 2006). The present results indicated that all traits studied showed some level of plasticity in response to soil moisture level, even though the plasticity presented here were relatively low (RDPI < 0.5) (Fig. 5), compared to the RDPIs reported by Fry et al. (2018) for grasses and species of Asteraceae in response to drought. There was some evidence that traits that contribute more to variability also have greater plasticity in response to the soil moisture level, indicating that these traits correspond with traits with the largest amount of variation. Albeit presenting relatively low RDPI, the root system traits contributing superiorly to variation (NUNR and BdUNR; Fig. 3d), also showed the highest plasticity values while one of the lowest contributors to variability (DtS) presented low plasticity index (Figs. 3d, 5b). Thus, the three traits that showed the most plastic response to drought (i.e.: NUNR, BdUNR and DUNR), related to upper nodal roots (Fig. 5b), where significantly more, denser and thicker roots were recorded under high moisture conditions (Fig. 2). It has been reported that root production and branching might be critical to the plastic responses of cassava roots and may be related to the crop’s adaptability to soil moisture stress conditions (Duque and Villordon 2019; Subere et al. 2009; Suralta et al. 2008; Wang and Yamauchi 2006; Banoc et al. 2000). The present results suggest that the secondary growth and ability to maintain or increase root number or density of upper nodal roots under limited soil moisture may be related to good growth and yield performance of cassava under drought conditions. Under field conditions, the upper nodal roots might be important for a more horizontal spread of the root system and may be contributing to drought adaptation by lateral root branching.
There was also some evidence that not all traits that contributed significantly and highly to variation present higher plasticity indices in response to soil moisture conditions (Figs. 3d and 5b). The number of all root categories (TNR) and the number of all nodal roots (TNNR) which were among the high contributing traits to variability (Fig. 3d) showed relatively moderate plasticity or RDPIs (Fig. 5b). Biomass related traits, including SFW and RDW, seem to, largely, conserve their rank in variability and plasticity contributions. However, the extent and importance of plasticity in traits varied by genotypes (Fig. 5a). Phenotypic plasticity and trait variability among genotypes could be subject to different constraints because trait variability is attributed to continuing evolutionary processes that generate or constrain variation (Henn et al. 2018). Whereas not all the traits measured are likely to respond to the soil moisture stress imposed in this study, the measure of phenotypic plasticity was also a response to rapid and shorter duration of the stress where only the genotypes which could respond quickly would show plastic responses.
It is important to note that leaf growth might be critical to the plastic responses and may be related to the crop’s adaptability to soil moisture stress conditions (Wang and Yamauchi 2006). For the growth duration employed in this study, leaf growth parameters, including leaf number and area, were not among the traits with the highest plasticity indices, albeit contributing strongly to variation under drought conditions (Fig. 4b, d). Indeed, as pointed out previously, the D/W ratio for the leaf area suggested that it is unlikely for the trait to be of biological significance at this stage of growth. The trend was similar for other shoot-related traits such as the number of leaves and shoots, for which neither the water supply nor the interaction of genotype x water supply, was significant. Perhaps, for longer growth periods or late-maturing cultivars, modifications in leaf production and growth could be a crucial adaptation strategy under water stress conditions. This adaptation could be crucial given that roots represent a large carbon cost for plants and so when carbon costs are considered along with water acquisition, increased root biomass as a result of root proliferation, may be unbeneficial, particularly for a short growth period. For shorter growth periods or early maturing cultivars, a plant may employ a drought escape strategy, where greater root biomass would likely not be beneficial. Greater root biomass is likely to be more advantageous for extended growth periods, as a larger root system may provide a cost-effective advantage later in the growth of plants (Adu et al. 2019). Also, the results possibly corroborate the suggestion by El-Sharkawy (2007) that under water deficit conditions, there is increased suppression of shoot than root growth, and one adaptive trait of cassava to drought is the reduction of its canopy by reducing the number of leaves through the shedding of older leaves and reducing leaf area by forming smaller new leaves.
El-sharkawy and Cock (1987) noted that genotypes with high yield potential under drought are characterized by having more intensive and extensive fine root system which enables the acquisition of more water from larger and deeper volumes of soil. Moreover, the intensive and extensive fine root system is positively related to higher leaf area and the greater biomass production under severe and prolonged soil–water stress conditions (El-sharkawy and Cock 1987). In cereals, including maize, increased production of adventitious roots may however lead to less carbon and energy partitioning for deeper rooting, shoot growth and yield (Saengwilai et al. 2014). In cassava, different strategies, including reduced shoot growth, speedy closure of stomata, and rapid shedding of old leaves, which possibly balance the metabolic costs of increased adventitious root production, might be operational under drought (Kengkanna et al. 2019; Zhao et al. 2015). While the present results suggest that upper nodal root numbers branching density and diameter could be used to screen for and select cassava genotypes adapted to drought conditions at the juvenile stage of growth, the persistence of these traits and their relationship with yield and yield components under drought conditions in the field must be confirmed.
Conclusions
Cassava is an important source of carbohydrate, providing both food security and income for many people in the tropics. Generally, there has been lesser investments into cassava research compared to cereals and legumes, but for root system studies of cassava, the situation is even more ominous. This has contributed to the dearth of data on cassava root system architecture and the plastic responses of cassava root systems to various edaphic stresses, including the response of cassava cultivars to water deficits. Cassava cultivars that can tolerate water deficit conditions are needed to minimize yield loss in the tropics, but conventionally breeding cassava ideotypes with traits conferring tolerance to drought could take a long time. Phenotyping cassava root systems at the juvenile stage provides a time-saving and less laborious option for a multi-trait selection in breeding for improved genotypes in cassava. This study examined various measures of shoot and root growth of a panel cassava genotypes after growing them in well-watered or soil moisture deficit conditions. A substantial variation for the various traits evaluated was observed among the cultivars, indicating a considerable amount of genotypic variability. Both above-ground canopy and root development were inhibited by drought, but the degree of inhibition which was between 2 and 22% for various traits, differed among the genotypes. Multivariate analyses indicated that root traits which differentiate the RSA of cassava at the juvenile stage of growth, regardless of soil moisture level, include root biomass, root numbers, root branching density, and total root length. Important traits which differentiate the germplasm under well-watered conditions include root number, shoot, and root biomass, length and branching density factors. Important traits which differentiate the germplasm under water deficit conditions were mainly shoot-related traits including shoot biomass and root number-related traits. Phenotypic plasticity was found in most traits, where count, branching density and diameter of upper nodal roots showed the greatest plasticity. These traits corresponded with the traits contributing greatly to variation. Even so, not all influential traits to variability showed plastic responses of cassava growth under water deficit. The data revealed that plastic responses of cassava to drought are dependent on trait and genotype. In general, the results suggest that upper nodal roots-related traits could have importance in breeding cassava to better tolerate water deficit conditions because these contributed highly to variability and also showed the greatest plastic responses to drought. Thus, under water deficit conditions, cassava may adapt by altering the rate of upper nodal root production and growth. The secondary growth and ability to maintain or increase the root number or density of upper nodal roots under limited soil moisture may be related to good growth and yield performance of cassava under drought conditions. Under field conditions, the upper nodal roots might be important for a more horizontal spread of the root system and may be contributing to drought adaptation by lateral root branching. Although upper nodal root production and branching might be critical to the plastic responses of cassava roots and may be related to the crop’s adaptability to soil moisture stress conditions, this has to be verified under field conditions.
Data availability
All data have been presented in the paper.
References
Adenle AA, Aworh OC, Akromah R, Parayil G (2012) Developing GM super cassava for improved health and food security: future challenges in Africa. Agric Food Secur 1:11. https://doi.org/10.1186/2048-7010-1-11
Adu MO, Asare PA, Yawson DO, Ackah FK, Amoah KK, Nyarko MA, Andoh DA (2017) Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere 3:29–39. https://doi.org/10.1016/j.rhisph.2016.12.004
Adu MO, Asare PA, Asare-Bediako E, Amenorpe G, Ackah FK, Afutu E, Amoah MN, Yawson DO (2018a) Characterising shoot and root system trait variability and contribution to genotypic variability in juvenile cassava (Manihot esculenta Crantz) plants. Heliyon 4(6):e00665. https://doi.org/10.1016/j.heliyon.2018.e00665
Adu MO, Yawson DO, Armah FA, Abano EE, Quansah R (2018b) Systematic review of the effects of agricultural interventions on food security in northern Ghana. PLoS ONE 13(9):e0203605. https://doi.org/10.1371/journal.pone.0203605
Adu MO, Asare PA, Yawson DO, Dzidzienyo DK, Nyadanu D, Asare-Bediako E, Afutu E, Tachie-Menson JW, Amoah MN (2019) Identifying key contributing root system traits to genetic diversity in field-grown cowpea (Vigna unguiculata L. Walp.) genotypes. Field Crops Res 232:106–118. https://doi.org/10.1016/j.fcr.2018.12.015
Adu MO, Asare PA, Yawson DO, Nyarko MA, Abdul Razak A, Kusi AK, Tachie-Menson JW, Afutu E, Andoh DA, Ackah FK, Vanderpuije GC (2020) The search for yield predictors for mature field-grown plants from juvenile pot-grown cassava (Manihot esculenta Crantz). PLoS ONE 15(5):e0232595
Aina OO, Dixon AG, Akinrinde EA (2007) Effect of soil moisture stress on growth and yield of cassava in Nigeria. Pak J Biol Sci 10:3085–9090. https://doi.org/10.3923/pjbs.2007.3085.3090
Ameztegui A (2017) Plasticity: an R package to determine several plasticity indices. GitHub repository. https://github.com/ameztegui/Plasticity
Banoc DM, Yamauchi A, Kamoshita A, Wade LJ, Pardales JR (2000) Genotypic variations in the response of lateral root development to fluctuating soil moisture in rice. Plant Prod Sci 3:335–343. https://doi.org/10.1626/pps.3.335
Bayitse R, Tornyie F, Bjerre AB (2017) Cassava cultivation, processing and potential uses in Ghana. Handbook on Cassava. Nova Science Publishers, pp 313–333
Catalogue of Crop Varieties Released and Registered in Ghana (2015), vol. 1
Connor DJ, Palta J (1981) Response of cassava to water shortage III. Stomatal control of plant water status. Field Crops Res 4:297–311. https://doi.org/10.1016/0378-4290(81)90080-0
Connor DJ, Cock JH, Parra GE (1981) Response of cassava to water shortage I. Growth and yield. Field Crops Res 4:181–200. https://doi.org/10.1016/0378-4290(81)90071-X
Daryanto S, Wang L, Jacinthe PA (2016) Drought effects on root and tuber production: a meta-analysis. Agric Water Manag 176:122–131. https://doi.org/10.1016/j.agwat.2016.05.019
de Oliveira EJ, Morgante C, de Tarso Aidar S, de Melo Chaves AR, Antonio RP, Cruz JL, Coelho Filho MA (2017) Evaluation of cassava germplasm for drought tolerance under field conditions. Euphytica 213:188. https://doi.org/10.1007/s10681-017-1972-7
dos Santos Silva PP, eSousa MB, de Oliveira EJ (2019) Prediction models and selection of agronomic and physiological traits for tolerance to water deficit in cassava. Euphytica 215:73. https://doi.org/10.1007/s10681-019-2399-0
Duque LO, Setter TL (2013) Cassava response to water deficit in deep pots: root and shoot growth, ABA, and carbohydrate reserves in stems, leaves and storage roots. Trop Plant Biol 6:199–209. https://doi.org/10.1007/s12042-013-9131-3
Duque LO, Villordon A (2019) Root branching and nutrient efficiency: status and way forward in root and tuber crops. Frontiers Plant Sci. https://doi.org/10.3389/fpls.2019.00237
El-Sharkawy MA (2003) Cassava biology and physiology. Plant Mol Biol 53:621–641. https://doi.org/10.1007/s11103-005-2270-7
El-Sharkawy MA (2005) How can calibrated research-based models be improved for use as a tool in identifying genes controlling crop tolerance to environmental stresses in the era of genomics—from an experimentalist’s perspective? Photosynthetica 43:161–176. https://doi.org/10.1007/s11099-005-0030-1
El-Sharkawy MA (2007) Physiological characteristics of cassava tolerance to prolonged drought in the tropics: implications for breeding cultivars adapted to seasonally dry and semiarid environments. Braz J Plant Physiol 19:257–286. https://doi.org/10.1590/S1677-04202007000400003
El-Sharkawy MA, Cock JH (1987) Response of cassava to water stress. Plant Soil 100:345–360. https://doi.org/10.1007/BF02370950
El-Sharkawy MA, López LFC, de Tafur MSM, Caicedo J (1998) Genotipic differences in productivity and nutrient uptake and use efficiency of cassava as influenced by prolonged water stress. Acta Agron 48:9–22
FAO (2002) The state of food and agriculture. Food and Agriculture Organization of the United Nations, Rome
FAO (2013) Save and grow: cassava. A guide to sustainable production intensification. Food and Agriculture Organization of the United Nations, Rome
Fry EL, Evans AL, Sturrock CJ, Bullock JM, Bardgett RD (2018) Root architecture governs plasticity in response to drought. Plant Soil 433:189–200. https://doi.org/10.1007/s11104-018-3824-1
Henn JJ, Buzzard V, Enquist BJ, Halbritter AH, Klanderud K, Maitner BS, Michaletz ST, Pötsch C, Seltzer L, Telford RJ, Yang Y (2018) Intraspecific Trait Variation and Phenotypic Plasticity Mediate Alpine Plant Species Response to Climate Change. Front Plant Sci 9:1548. https://doi.org/10.3389/fpls.2018.01548
Izumi Y, Yuliadi E, Iijima M (1999) Root system development including root branching in cuttings of cassava with reference to shoot growth and tuber bulking. Plant Prod Sci 2:267–272. https://doi.org/10.1626/pps.2.267
Kassambara A (2017) Practical guide to principal component methods in R: PCA, M (CA), FAMD, MFA, HCPC, Factoextra vol 2. STHDA
Kengkanna J, Jakaew P, Amawan S, Busener N, Bucksch A, Saengwilai P (2019) Phenotypic variation of cassava root traits and their responses to drought. Appl Plant Sci 7(4):e01238. https://doi.org/10.1002/aps3.1238
Lowe SB, Mahon JD, Hunt LA (1982) Early development of cassava (Manihot esculenta). Can J Bot 60:3040–3048. https://doi.org/10.1139/b82-359
Marchiori PE, Machado EC, Sales CR, Espinoza-Núñez E, Magalhães Filho JR, Souza GM, Pires R, Ribeiro RV (2017) Physiological plasticity is important for maintaining sugarcane growth under water deficit. Frontiers Plant Sci 9:1548. https://doi.org/10.3389/fpls.2018.01548
MoFA (2017) Agricultural sector progress report 2017. Ministry of Food and Agriculture (MoFA), Ghana. Retrieved 01 Aug 2019 from http://mofa.gov.gh/site/wp-content/uploads/2018/09/MoFA%202017%20AGRICULTURAL%20PROGRESS%20REPORT_Final.PPMED.MoFA.pdf
Okogbenin E, Ekanayake IJ, Porto MCM (2003) Genotypic variability in adaptation responses of selected clones of cassava to drought stress in the Sudan savanna zone of Nigeria. J Agron Crop Sci 189:376–389. https://doi.org/10.1046/j.1439-037X.2003.00050.x
Okogbenin E, Setter TL, Ferguson M, Mutegi R, Ceballos H, Olasanmi B, Fregene M (2013) Phenotypic approaches to drought in cassava. Frontiers Physiol 4:93. https://doi.org/10.3389/fphys.2013.00093
Oliveira EJD, Aidar SDT, Morgante CV, Chaves ARDM, Cruz JL, Coelho Filho MA (2015) Genetic parameters for drought-tolerance in cassava. Pesquisa Agropecuária Brasileira 50:233–241. https://doi.org/10.1590/S0100-204X2015000300007
Pardales JR Jr, Esquisel CB (1996) Effect of drought during the establishment period on the root system development of cassava. Jpn J Crop Sci 65:93–97. https://doi.org/10.1023/A:1017511806128
Pardales JR, Yamauchi A (2003) Regulation of root development in sweet potato and cassava by soil moisture during their establishment period. Plant Soil 255:201–208. https://doi.org/10.1023/A:1026160309816
Rogers ED, Benfey PN (2015) Regulation of plant root system architecture: implications for crop advancement. Curr Opin Biotechnol 32:93–98. https://doi.org/10.1016/j.copbio.2014.11.015
Saengwilai P, Tian X, Lynch JP (2014) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166:581–589. https://doi.org/10.1104/pp.113.232603
Shan Z, Luo X, Wei M, Huang T, Khan A, Zhu Y (2018) Physiological and proteomic analysis on long-term drought resistance of cassava (Manihot esculenta Crantz). Sci Rep 8:17982. https://doi.org/10.1038/s41598-018-35711-x
SRID-MoFA (2011) Agriculture in Ghana Facts and Fig. (2010) Ministry of Food and Agriculture, Statistics, Research and Information Directorate (SRID). Retrieved on 01 Aug 2019 from http://mofa.gov.gh/site/wp-content/uploads/2011/10/AGRICULTURE-IN-GHANA-FF-2010.pdf
Subere QJO, Bolatete D, Bergantin R, Pardales A, Belmonte JJ, Mariscal A, Sebidos R, Yamauchi A (2009) Genotypic variation in responses of cassava (Manihot esculenta Crantz) to drought and rewatering: root system development. Plant Prod Sci 12:462–474. https://doi.org/10.1626/pps.12.462
Suralta RR, Inukai Y, Yamauchi A (2008) Genotypic variations in responses of lateral root development to transient moisture stresses in rice cultivars. Plant Prod Sci 11:324–335. https://doi.org/10.1626/pps.11.324
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Thorup-Kristensen K, Kirkegaard J (2016) Root system-based limits to agricultural productivity and efficiency: the farming systems context. Ann Bot 118:573–592. https://doi.org/10.1093/aob/mcw122
Valladares F, Sanchez-Gomez DA, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116. https://doi.org/10.1111/j.1365-2745.2006.01176.x
Wang H, Yamauchi A (2006) Growth and function of roots under abiotic stress in soils. Plant-environment interactions. In: Huang B (ed) Growth and function of roots under abiotic stress in soils, 3rd edn. CRC Press, Boca Raton, pp 271–320
Wei T, Simko V (2017) R Package “corrplot”: visualization of a Correlation Matrix (Version 0.84). https://github.com/taiyun/corrplot
Zhao P, Liu P, Shao J, Li C, Wang B, Guo X, Yan B, Xia Y, Peng M (2015) Analysis of different strategies adapted by two cassava cultivars in response to drought stress: ensuring survival or continuing growth. J Exp Bot 66:1477–1488. https://doi.org/10.1093/jxb/eru507
Acknowledgements
I am grateful to Dr Godwin Amenorpe of the Nuclear Agricultural Research, Biotechnology and Nuclear Agriculture Research Institute, Atomic Energy Commission, Legon, Accra, Ghana, for providing the genetic materials used in this study. I would also like to thank Drs David Oscar Yawson and Paul Agu Asare of the College of Agriculture and Natural Sciences, University of Cape Coast, Cape Coast, Ghana, for the internal review and advice on the manuscript. I am grateful to Dr Josiah Wilson Tachie-Menson for his assistance in the analysis and to Ahmed Abdul Razak for his assistance in sampling for this work.
Funding
This work did not receive any specific sponsorship.
Author information
Authors and Affiliations
Contributions
MOA conceived the idea for this work and designed this experiment. He acquired the data with help from technicians. He analysed and interpreted the data. MOA drafted the original manuscripts and revised it for important intellectual content after an internal review.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Adu, M.O. Causal shoot and root system traits to variability and plasticity in juvenile cassava (Manihot esculenta Crantz) plants in response to reduced soil moisture. Physiol Mol Biol Plants 26, 1799–1814 (2020). https://doi.org/10.1007/s12298-020-00865-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-020-00865-4