Abstract
The North Western Himalayan region, particularly the Kashmir valley, harbors a rich diversity of rice landraces, yet systematic studies on climate resilience are lacking. The present study was aimed at investigating the root traits and their interplay with shoot morphological and physiological traits under drought stress using seven indica and three japonica rice varieties released by SKUAST-Kashmir and their possible role in adaptive capacity to different environments. Significant variability was observed in root and shoot traits across the varieties. Root traits exhibited a wide phenotypic range, including rooting depth, fresh and dry weight, diameter, length, volume, surface area, and length density. Associations between root and shoot traits were identified, with notable correlations such as shoot weight with root shoot ratio, shoot length with root dry weight, diameter, and surface area, and tiller number with various root traits. Additionally, physiological traits like canopy temperature depression showed significant correlations with root depth and surface area. Principal component analysis (PCA) analysis grouped japonica type varieties together with a notable outlier, Chenab, due to its superior root traits among indica varieties. These findings emphasize the substantial contribution of root traits to productivity and advocate for their integration into varietal development processes. In view of the increasing evidences in crop plants about their role of root traits in defining plant productivity and reproductive fitness, study of root traits could provide valuable insights into the patterns of crop adaptation to diverse areas and growing environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Rice is the major food crop of the world, grown in more than 100 countries over an area of 164 Mha and accounts for nearly 50% of the calories to half of global population in Asia, Latin America, and Africa. The annual rice consumption is highest in China (154.89 MT) followed by India (103.5 MT) and Vietnam (73.34 MT) [1]. In India, rice is the staple food for more than 70% of population where around 4000 varieties and hybrids are grown to satiate hunger and fulfil diverse food preferences of a heterogenous consumer base [2]. The Western Himalayan tract, including Kashmir valley represents a potential hot spot of rice diversity and tens of thousands of landraces were grown and many of them still persist on account of their food value, adaptability and cultural integration. In India, temperate rice is grown in the North-Western Himalayan (NWH) region (comprising Jammu and Kashmir, Himachal, and Uttrakhand) and North-Eastern hill (NEH) states [3,4,5]. In the recent past, there has been considerable genetic erosion and consequent decline in area covered under local landraces (both scented and non-scented) in the North-Western Himalayan due to the inroads made by popularisation of high yielding varieties and the low yield and disease susceptibility of landraces [6]. More than 100 landraces of rice have been documented from in Kashmir valley (Mushk Budji, Zag, Til Zag, Quadir Beigh, Kamad, Nun-beoul, Lar Beoul, Zaged etc.),, SKUAST-Kashmir has released more than a dozen varieties belonging to typical indica (Shalimar Rice-1, -2, -3 and -4) and japonica (K-332, Kohsar, Shalimar Rice-5) types.
A large number of studies have shown the detrimental effect of drought on vegetative growth and yield of many food crops including rice [7, 8]. Especially during flowering, water stress causes significant yield loss due to impaired reproductive fitness and grain filling primarily caused by bottlenecks imposed on resource remobilization including limited carbohydrate supply to sink [9, 10]. Root traits are important determinants of plant productivity under stress. Root system architecture (RSA) refers to the spatial structure and distribution of root system including the placement of root axes [11] and is crucial for plant growth and development as well as agricultural productivity [8].
The root system of rice represents a typical monocot root system, comprising of fibrous roots, primarily consisting of post-embryonic crown roots emerging from the stem nodes. Several beneficial features of rice root system have been functionally characterised with potential functional benefits to plants and as such have been used as selection criteria for improving drought-resilience in rice for sustainable rice production. Fortunately, for plant breeders, substantial genetic variation has been observed in rice germplasm (both cultivated and wild) for different root morphological traits [11,12,13]. Obvious variation in root system of indica and japonica rice have been reported, with indica types having fine superficial roots, that are highly branched with narrow xylem vessels and lower root/shoot ratio, and, the japonica types having longer, more coarse, less branched roots with wider vessels and a higher root/shoot ratio [14, 15].
In the field conditions, the large-scale studies on root traits in most of the crops including rice have been precluded by the obvious challenges in creating managed stress conditions and as such very limited information is available. Moreso, in case of rice germplasm from Western Himalayan region, very limited attempts have been made to elucidate the genetic architecture of the root traits in relation to drought tolerance which necessitates the study of the diversity of such traits for rice improvement in stressful environments. The present work was the first comprehensive study on root traits and their relationship with shoot morphological and physiological traits under drought stress for the varieties released by SKUAST-Kashmir for the North Western Himalayan Kashmir region.
2 Materials & methods
2.1 Plant material
The material comprised 10 varieties released by SKUAST-Kashmir for temperate regions of Kashmir valley during the period 1956–2017. Seven out of 10 varieties have been released for low to mid-altitude (1600–1800 m above mean sea level; MASL) and three are recommended for higher altitudes above 2200 MASL. The seven low-altitude varieties belong to the Oryza subspecies indica, whereas the three high-altitude varieties belong to the Oryza subspecies japonica. Among these varieties, K-332 is used as a national check for cold tolerance whereas Shalimar Rice-5 is used as a national check for japonica rice. The details of the pedigree, year of release, and adaptability ranges of these varieties are presented in Table S1.
2.2 Phenotyping system
In vitro stress The polyethylene glycol, PEG-6000 (HIMEDIA) was used create an in vitro screening system at three concentrations viz., control (0%), 10% and 20%. Four seeds of each genotype were surface sterilized with 0.5% NaOCl for one minute, rinsed thoroughly with distilled water and were put in petri plates containing moist filter paper with different concentrations of PEG-6000 and allowed to germinate in a germinator at 25 °C and 75% humidity in darkness. Primary root length and root biomass was measured after seven days. The design used was factorial CRD with three replications [16]. All the images were taken on a black background paper of 15 cm height from similar height under unform lighting.
Column culture experiment A controlled environment greenhouse experiment was conducted in the greenhouse facility at the Department of Genetics & Plant Breeding, SKUAST-Kashmir using the standard procedure given by [17]. All 10 varieties were characterized for their root traits in a greenhouse using a column culture phenotyping system comprising of PVC columns of 1.2 m height and 12 cm internal diameter. The rice seeds were surface sterilized for 1 min with 70% alcohol and 2% sodium hypochlorite (NaClO) and rinsed with deionized water in 10 × 10 cm Petri plates. The sterilized seeds were allowed to pre-germinate. After germination, when the radicle was about 5 mm long, the seeds were transplanted into the PVC columns [16]. The growing medium comprised of a 1:1 soil and sand mixture fertilized with a slow-release fertilizer Osmocote (19:6:12 N:P2O5:K2O) @ 2 g per column before sowing and evenly mixed with the top 2 cm growing medium. Initially, four seeds were planted per column, but once the plants were established only two plants per column were kept. Plants were maintained under optimum temperature. For imposing the drought treatment, plants were maintained under 100% field capacity from sowing to the four-leaf stage; then stress was imposed by withholding water for four weeks development stage (48 days after sowing; DAS). The duration of the drought stress was 28 days. In the drought stress treatment, the soil moisture content at the end was 30% [18].
2.3 Phenotyping root and shoot traits under drought stress
After 48 days, the roots were carefully harvested by gently inverting the columns at about 140° to allow the contents (growing medium and plants with entire root system) to slip out. The intact roots were carefully separated from the column. The shoots were separated from the roots by cutting at the base of the stem. After removing the shoots, the roots were spread on a flat surface to measure their length (from the base of the stem to the tip of the root system) as an estimate of rooting depth. Similarly, root and shoot biomass were measured using an electronic balance, while as shoot length was measured from base of stem to leaf tip. The root system was then washed with water to remove any adhering soil or sand particles, placed between moist paper towels, sealed in Ziploc bags, transported to the laboratory, and stored at 4 °C. For root analysis, the root system of each genotype was stretched and sliced into 30 cm long portions and each portion was submerged in water and carefully spread to maximize root separation and minimize overlap for scanning with an Epson photo scanner (Epson Perfection V700 with 6400 dpi resolution, Epson, Long Beach, CA) [13]. The scanned root image for each genotype was analyzed using Rhizo-Vision Explorer Version 2.03 [19] to estimate total root length (TRL), root perimeter (PRM), average root diameter (ARD), root volume (VOL), root surface area (SA), and root length density (RLD). The root images were acquired on a Grayscale to a resolution of 700 dots per inch (dpi). The analysis was done on the root morphology by setting the rough edge and noise removal to a higher level. The image acquisition took on average 40 s per sample. The roots of all the genotypes were dried in an oven at 60 °C for 7 days to determine the dry weight (g plant−1) [13].
2.4 Effect of drought stress on physiological parameters
Canopy Temperature Deficit (CTD) was measured 3 weeks after the imposition of drought stress. CTD was measured by a thermal imaging camera (FLIR Systems Inc., USA) on top fully expanded leaf at an angle of 45° between 11 a.m.to1 p.m. [16]. Relative Water Content (RWC) was taken as an average of four leaves per replication. The sampled leaves were immediately weighed to obtain their fresh weight (FW). Then, the leaves were floated in distilled water in 50 ml falcon tubes till saturation (6 h), and the turgid weight (TW) was measured. Subsequently, the water saturated leaves were subjected to drying at 60 °C for 72 h to estimate their dry weights (DW). RWC, expressed as percentage, was calculated from FW, TW and DW using following formula [20]:
Membrane stability index (MSI) was estimated using the electrical conductivity protocol of Sairam et al. [21] using leaf discs 20 mm diameter, thoroughly washed in tap water and transferred to falcon tubes containing 10 mL of deionized water and incubated for 5 h. After five hours, initial electrical conductivity (C1) was estimated using an electrical conductivity meter (HM COM-80 Digital EC meter). These samples were again transferred to a water bath and kept at 100 °C for 10–15 min and again cooled down for estimation of electrical conductivity (C2) and membrane stability index as follows:
2.5 Statistical analysis
The data pertaining to various root and shoot traits were analyzed using JASP software developed by CIMMYT [22] for graphical analysis as well as estimation of descriptive statistics as well as Standard deviation, including correlation heat map. PCA was done using R software using the factoMineR package (R Core Team).
3 Results and discussion
3.1 Response to PEG-6000
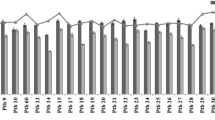
There was significant decrease in both root length and root biomass under PEG-6000 induces stress (Table S2, Figure S1, Fig. 1). At 20% PEG-6000 concentration, root length was highest in SR-2 (7.87 cm), followed by SR-5 (7.17 cm), China-1039 (6.67 cm), Jhelum (5.97 cm) and K-332 (5.83 cm), while as lowest was observed in case of Chenab (2.51 cm). Largest decrease in root length was observed in case of Chenab and SR-3, while as lowest decrease was recorded in SR-2 and SR-5. In case of root biomass, highest value was recorded in SR-5 (65.00 g), followed by China-1039 (62.00 g), and Kohsar (61.67 g). while as lowest root biomass was observed in case of Chenab (18.67 g). Largest decrease in root biomas was observed in case of Jehlum, SR-3 and Chenab, while as lowest decrease was recorded in Kohsar and SR-5. Genotypes showing better parametrs also showed better performance in column culture experiment, indicating the effectiveness of the approach. Similar results in Bangladeshi rice cultivars have been reported by Sagar et al. [23]. PEG-6000 offers an effective screening technique under controlled conditions, using standardized PEG solution, and creates consistent environments for inducing water stress [24] and has been effectively demonstrated in terms of its success in identifying genotypes with improved drought tolerance [25].
3.2 Adaptation niche and variability for root and shoot traits
The graphical representation of the variation of root and shoot traits and descriptive statistics are presented in Figure S2, S3 and Figs. 1 and 2 and Tables 1 and 2 respectively. Significant variability was observed for root and shoot traits among rice varieties released by SKUAST-Kashmir. The phenotypic range of major root traits was recorded at 63.3–93.67 cm (rooting depth), 2.52–9.69 g (root fresh weight), 0.51–1.59 g (root dry weight), 0.24–1.11 (root shoot ratio), 449.94–2409.56 cm (total root length), 0.25–2.38 mm (average diameter), 265.51–3321.09 cm (perimeter), 12.19–1989.28 cm3 (root volume), 110.82–3588.09 cm2 (root surface area), and 0.06–0.31 cm/cm3 (root length density). The varieties with the highest values for various root traits were Chenab (Root depth), SR2 (root fresh weight), K332 (root dry weight and perimeter), China 1039 (root shoot ratio), SR-5 (root length and root length density), Jhelum (root diameter), and SR-4 (volume and surface area). In the case of the root traits, japonica varieties (K-332, Kohsar and Shalimar Rice-5) had better root traits such as root depth, root dry weight, root length, perimeter, surface area and root length density compared to indica varieties. Gong et al. [15] evaluated four rice genotypes (two indica super rice hybrids and two japonica varieties) and found that most of the root traits such as root-shoot ratio, root length, root number, root volume, and root dry weight of japonica rice were higher than those of indica rice varieties, while root diameter was higher in case of indica varieties. Similarly, Uga et al. [26] evaluated 59 indica and japonica rice accessions and found that japonica upland rice was characterized by a larger stele and xylem structures, while as the indica group had wide variation in root morphology compared to the japonica group, similar to the finding in our study. Root traits are the key traits for enhancing rice production under drought stress [27] and rice plant function under water stress is determined by the spatial and temporal distribution of the root system. Rice production under water stress can be forecasted by taking root dry weight and root length into account [28]. Manivannan et al. [29] observed an increase in the rice root length under drought stress because of rise in abscisic acid concentration in the roots and rice varieties with profound and prolific root systems show better adaptability under limited moisture supply [26, 30]. In rice, the genotypes having larger root systems, with higher root diameters, many lateral roots and higher root-shoot ratio are important for improving drought tolerance [30]. In a recent study Chu et al. [12] analysed the root traits in relation to N-stress in japonica-indica hybrids and found that such hybrids had better root traits compared to japonica-japonica hybrids, resulted in better nitrogen use efficiency and grain yield under nitrogen stress. Wang et al. [31] evaluated a japonica variety DN-425 for various root traits under various irrigation and nitrogen regimes and reported that grain yield and nitrogen use efficiency were positively correlated with the root length density, root surface area density, root volume density.
Among shoot traits, a broad range was recorded, 7.13–12.28 g (shoot weight), 29–64 cm (shoot length), and 5.33–8.33 (number of tillers). Similarly, among physiological traits recorded, broad range was observed as in the case of CTD (− 3.10–2.0 °C), RWC (49.75–81.45%), and MSI (37.44–43.79%). The highest value of shoot and physiological traits were recorded in case of China-1039 (shoot weight), Shalimar Rice-4 (shoot length), Jhelum (number of tillers and relative water content), Shalimar Rice-3 (canopy temperature depression) and Shalimar Rice-1 (membrane stability index). Saha et al. [32] also reported wide variation in rice varieties from Bangladesh. Physiological traits such as CTD, RWC and MSI are essential determinants of crop response under water stress. The values recorded for shoot morphological and physiological traits under drought stress were significantly lower than under irrigated conditions (data not shown).
Selection for a deep and prolific root system on the basis of parameters such as root length, surface area, volume, and average diameter is a destructive process and is not easy because of the obvious difficulty to measure roots in situ [33]. Therefore, non-destructive selection criteria for improved root traits based on their correlation with above-ground morphological and physiological traits are important. Pinto and Reynolds [34] measured root growth in subsets of iso-QTL lines of wheat and provided definitive evidence for the involvement of roots in drought response, not necessarily through deeper or more extensive roots, but through root plasticity to adapt to the specific needs of the environment, such as drought. They found that roots of the lines with higher CTD showed a greater distribution at depth. In fact, water availability determines root development, influencing the position of lateral branches and root hairs, a response called “hydropatterning” reported in crops like Arabidopsis, maize and rice [35, 36].
3.3 Relationship between root and shoot traits
Among root traits, a significant positive relationship (p < 0.05) was observed (Fig. 3) between root length and root length density (r = 0.999) and perimeter (r = 0.909), followed by a relationship between root surface area and root diameter (r = 0.817). Similarly, root fresh weight was positively correlated with root volume (r = 0.739), root surface area (r = 0.703), and root diameter (r = 0.507), and root dry weight was positively correlated with perimeter (r = 0.471), root shoot ratio (r = 0.394) and root length density (r = 0.245), so was root volume and root surface area (r = 0.779). Several negative correlations were also found, such as between root dry weight with root diameter, root depth, surface area and volume (r = − 0.565, − 0.483, − 0.471, and − 0.379 respectively). Similarly, root fresh weight was negatively correlated with root dry weight and root shoot ratio (r = − 0.547 and − 0.333). Among shoot traits, a significant positive relationship (p < 0.05) was observed (Fig. 4) between shoot length and canopy temperature depression (r = 0.606) followed by number of tillers with relative water content and membrane stability index (r = 0.333 and 0.255 respectively). Shoot weight was negatively correlated with relative water content (r = − 0.303).
Correlation heat map for 16 root and shoot traits in the rice genotypes under consideration under drought stress. RD Rooting depth, RFW Root fresh weight, RDW Root dry weight, RSR root shoot ratio, RL Root length, RDia Root diameter, PERI Root perimeter, VOL Root volume, SA Root surface area, RLD Root length density, SW Shoot weight, SL Shoot length, NT Number of tillers, CTD Canopy temperature depression, RWC Relative water content, MSI Membrane stability index
GT biplot for 16 root and shoot traits in the rice genotypes under consideration under drought stress. RD Rooting depth, RFW Root fresh weight, RDW Root dry weight, RSR root shoot ratio, RL Root length, RDia Root diameter, PERI Root perimeter, VOL Root volume, SA Root surface area, RLD Root length density, SW Shoot weight, SL Shoot length, NT Number of tillers, CTD Canopy temperature depression, RWC Relative water content, MSI Membrane stability index
In terms of the association between root and shoot traits, our results (Fig. 3) indicated that shoot weight was significantly correlated with root shoot ratio (r = 0.915). Shoot length was significantly correlated with root dry weight, root diameter, and surface area (r = 0.311, 0.302 and 0.281 respectively). Similarly, the number of tillers was significantly correlated with root fresh weight, root diameter, root volume, and surface area (r = 0.329, 0.720, 0.452 and 0.395 respectively). Among physiological traits, canopy temperature depression was significantly correlated with root depth and surface area (r = 0.285 and 0.309 respectively). Relative water content was significantly correlated with root fresh weight, root diameter, perimeter and volume (r = 0.611, 0.513, 0.334 and 0.401 respectively). Similarly, the membrane stability index was significantly correlated with root fresh weight, root depth and surface area (r = 0.453, 0.513 and 0.318 respectively). Among notable negative correlations were the ones between the number of tillers and root dry weight, root length, perimeter and root length density (r = − 0.724, − 0.517, − 0.668 and − 0.516 respectively). The increased resource uptake through higher root surface area might have helped the plants to produce more tillers, leading to increased shoot biomass production. The positive correlation between root dry weight and shoot dry weight may be attributed to the increased resource capture through increased root mass might have contributed to the increased shoot dry weight. In turn, the additional photo-assimilates might be allocated to roots thereby increasing root dry weight [33].
The PCA revealed that the first five components (eigenvalue > 1) accounted for about 88% of the variation. To gain a deeper understanding of the trait relationships depicted in the GT biplot using the first two principal components, we conducted a PCA analysis to assess the extent of variation among the genotypes and their relationships with the observed traits (Fig. 4) that accounted for 52.5% of variation based on all 16 root and shoot traits and found almost similar relationships as depicted by correlation heatmap. The japonica type varieties (K-332, Kohsar and Shalimar Rice-5) were grouped almost together along with Chenab (indica type), as it had overall better root traits among all indica varieties, whereas all other indica varieties were grouped together. Similar results have been reported in rice by several workers. Verma et al. [37] evaluated North-East Indian rice varieties for root and shoot traits using multivariate analysis and reported that the first two major PC’s contributed a total of 55.51% to the overall variability in the population with shoot weight, root volume, dry shoot weight, and fresh root weight as main component traits. Guimaraes et al. [38] observed that first three PC’s based on root traits account for 90% of variation. Similarly, Kaysar et al. [39], who reported that PC1 comprised principally of root dry weight, and root fresh weight, while the PC2 was mainly associated with the traits root volume and the clusters of cultivars were clearly separated based on these traits.
4 Conclusion
The study revealed wide variation in root and shoot parameters of released varieties and japonica types grown at higher altitudes had better root systems as compared to indica types grown at lower altitudes. In view of the observation that recent high-yielding varieties of both indica and japonica type varieties viz., Shalimar Rice-4 and Shalimar Rice-5 had better root system, it can be safely concluded that root traits significantly contribute towards better productivity and should be considered in the varietal development process. Similarly, varieties like K-332 that has persisted for the last 50 years in the farmers’ fields are attributed to its better performance, adaptability and resilience driven by improved root architectural traits. This variety has marvellous cold tolerance and is adapted to high altitude areas of Kashmir valley. The persistence of K-332 under such conditions highlights the importance of root architectural traits in diverse adaptive capacities to different sub-optimal situations. Since there are increasing evidences in crop plants including rice about the definite role of root traits in defining plant productivity and reproductive fitness under both ambient and stressed conditions, there is a need to study root traits in crop plants vis-a-vis their adaptive niches. This could provide valuable insights into the patterns of crop adaptation to diverse areas and growing environments. In the present study japonica varieties K332, Kohsar and Shalimar Rice-5 and indica varieties Shalimar Rice-4 were identified as having better root and shoot traits under stress and as such can be used as parents in developing climate resilient rice varieties for western Himalayan Kashmir.
Data availability
Data is provided within the manuscript or supplementary information files.
References
FAO. FAOSTAT. 2022. www.faostat.com
Singh S, Mustard A. India grain and feed annual. Global agricultural information network (GAIN) report no IN2026. New Delhi: USDA-Foreign Agricultural Services; 2012.
Roy S, Banerjee A, Mawkhlieng B, Misra AK, Pattanayak A, Harish GD, Bansal KC. Genetic diversity and population structure in aromatic and quality rice (Oryza sativa L.) landraces from north-eastern India. PLoS ONE. 2015;10(6):e0129607.
Salgotra RK, Gupta BB, Bhat JA, Sharma S. Genetic diversity and population structure of basmati rice (Oryza sativa L.) germplasm collected from north western Himalayas using trait linked SSR markers. PLoS ONE. 2015;10(7):e0131858.
Umakanth B, Vishalakshi B, Sathish Kumar P, Rama Devi SJS, Bhadana VP, Senguttuvel P, Madhav MS. Diverse rice landraces of north-east India enables the identification of novel genetic resources for Magnaporthe resistance. Front Plant Sci. 2017;8:1500.
Parray GA, Shikari AB. Conservation and characterization of indigenous rice germplasm adapted to temperate/cooler environments of Kashmir valley. ORYZA-An Int J Rice. 2008;45(3):198–201.
Singh VK, Singh BD, Kumar A, Maurya S, Krishnan SG, Vinod KK, Singh AK. Marker-assisted introgression of Saltol QTL enhances seedling stage salt tolerance in the rice variety “Pusa Basmati 1.” Int J Genomics. 2018. https://doi.org/10.1155/2018/8319879.
Zhang J, Zhang N, Liu YX, Zhang X, Bin H, Qin Y, Haoran X. Root microbiota shift in rice correlates with resident time in the field and developmental stage. Sci China Life Sci. 2018;61(6):613–21.
Panda D, Swati SM, Behera PK. Drought tolerance in rice: focus on recent mechanisms and approaches. Rice Sci. 2021;28(2):119–32.
Kamoshita A, Babu RC, Boopathi NM, Fukai S. Phenotypic and genotypic analysis of drought-resistance traits for development of rice cultivars adapted to rainfed environments. F Crop Res. 2008;109(1–3):1–23.
Lynch J. Root architecture and plant productivity. Plant Physiol. 1995;109(1):7.
Chu G, Xu R, Chen S, Xu C, Liu Y, Abliz B, Zhang X, Wang D. Root morphological-physiological traits for japonica/indica hybrid rice with better yield performance under low N conditions. Food Energ Security. 2020;11(2): e355.
Shafi S, Zaffar A, Riyaz I, Shikari AB, Najeeb S, Zargar SM, Djanaguiraman M, Gurumurthy S, Sofi PA. Differential drought responses in deep and shallow-rooted rice genotypes: enzymatic and non-enzymatic insights. Plant Physiol Rep. 2024. https://doi.org/10.1007/s40502-024-00788-2.
Lafitte HR, Price AH, Courtois B. Yield response to water deficit in an upland rice mapping population: associations among traits and genetic markers. Theor Appl Genet. 2004;109(6):1237–46.
Gong J, Xing Z, Hu Y, Zhang H, Huo Z, Xu K, Guo B. Difference of root morphological and several physiological characteristics between indica and japonica super rice varieties. Acta Agron Sin. 2014;40(6):1066–80.
Sofi PA, Djanaguiraman M, Siddique KHM, Prasad PVV. Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Indian J Plant Physiol. 2018;23:796–809.
Shashidhar HE, Henry A, Hardy B. Methodologies for root drought studies in rice. Los Banos: International Rice Research Institute; 2012.
Black CA. Method of soil analysis part 2. Chem Microbiological Prop. 1965;9:1387–8.
Seethepalli A, Dhakal K, Griffiths M, Guo H, Freschet GT, York LM. Rhizo vision explorer: open-source software for root image analysis and measurement standardization. AoB Plant. 2021;13(6):plab056.
Turner, NC. and Jones, MM. (1980) Turgor maintenance by osmotic adjustment: a review and evaluati on In: Turner, NC. and Kramer, PJ. (Eds.) Adaptati on of plants to water and high temperature stress, pp: 87–103
Sairam RK, Deshmukh PS, Shukla DS. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 1997;178:171–8.
JASP Team (2020). JASP software version 0.18. Computer software, CIMMYT (downloaded from https://jasp-stats.org/).
Sagar A, Rauf F, Mia M, Shabi T, Rahman T, Hossain AKMZ. Polyethylene Glycol (PEG) induced drought stress on five rice genotypes at early seedling stage. J Bangladesh Agric Univ. 2020;18(3):606–14.
Swain H, Valarmathi M, Balasubramani V, Boopathi NM, Raveendran M. Evaluation of backcross inbred lines (BILs) introgressed with drought tolerant QTLs using polyethylene glycol (PEG) induced water stress in rice. Electron J Plant Breed. 2020;11(03):887–95.
Anupriya, R., Thankappan, S., Senthil, A., Rajakumar, D., Geetha, S., and Binodh, A. K. (2020). Phytohormone cross-talk and antioxidant gadgets tussles osmotic stress in indigenous rice landraces during seed germination.
Uga Y, Ebana K, Abe J, Morita S, Okuno K, Yano M. Variation in root morphology and anatomy among accessions of cultivated rice (Oryza sativa L.) with different genetic backgrounds. Breed Sci. 2009;59(1):87–93.
Panda D, Mishra SS, Behera PK. Drought tolerance in rice: focus on recent mechanisms and approaches. Rice Sci. 2021;28(2):119–32.
Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA. Root traits contributing to plant productivity under drought. Front Plant Sci. 2013;4:442.
Manivannan P, Jaleel CA, Sankar B, Kishorekumar A, Somasundaram R, Lakshmanan GA, Panneerselvam R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloid Surf B Biointerfaces. 2007;59(2):141–9.
Mishra SS, Behera PK, Panda D. Genotypic variability for drought tolerance-related morpho-physiological traits among indigenous rice landraces of Jeypore tract of Odisha. India J Crop Improvement. 2019;33(2):254–78.
Wang Z, Jia Y, Fu J, Qu Z, Wang X, Zou D, Wang J, Liu H, Zheng H, Wang J, Yang L, Zhu H, Zhao H. An analysis based on japonica rice root characteristics and crop growth under the interaction of irrigation and nitrogen methods. Front Plant Sci. 2022;13: 890983.
Saha SR, Hassan L, Haque MA, Islam MM, Rasel M. Genetic variability, heritability, correlation and path analyses of yield components in traditional rice (Oryza sativa L.) landraces: Variability and traits association in rice. J Bangladesh Agric Univ. 2019;17(1):26–32.
Narayanan S, Mohan A, Gill KS, Prasad PV. Variability of root traits in spring wheat germplasm. PLoS ONE. 2014;9(6): e100317.
Pinto RS, Reynolds MP. Common genetic basis for canopy temperature depression under heat and drought stress associated with optimized root distribution in bread wheat. Theor Appl Genet. 2015;128(4):575–85.
Bao Y, Aggarwal P, Robbins NE, Sturrock CJ, Thompson MC, Tan HQ, Tham C. Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc Natl Acad Sci. 2014;111(25):9319–24.
Shafi S, Zaffar A, Riyaz I, Zargar SM, Najeeb S, Sofi PA. A non-destructive, low cost and high throughput colorimetric method for chlorophyll estimation in rice (Oryza sativa L.). Discov Plant. 2024;1(1):2.
Verma H, Borah JL, Sarma RN. Variability assessment for root and drought tolerance traits and genetic diversity analysis of rice germplasm using SSR markers. Sci Rep. 2019;9:16513.
Guimaraes PHR, de Lima IP, de Castro AP, Lanna AC, Melo PGS, de Raïssac M. Phenotyping root systems in a set of japonica rice accessions: can structural traits predict the response to drought? Rice. 2020;13:67.
Kaysar MS, Sarker UK, Monira S, Hossain MA, Haque MS, Somaddar U, Uddin MR. Dissecting the relationship between root morphological traits and yield attributes in diverse rice cultivars under subtropical condition. Life. 2022;12(10):1519.
Acknowledgements
The first author acknowledges the greenhouse facilities provided by Division of Genetics & Plant Breeding, SKUAST-Kashmir.
Author information
Authors and Affiliations
Contributions
PAS, SMZ, AS conceived the idea and wrote the manuscript, SS, AZ, IR, MR- Executed the experiment, PAS, SN, PVVP reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
SKUAST-Kashmir is the custodian of the crop genetic resources of Kashmir valley. The material used in the present study comprised of varieties released by the university and notified by the appropriate authorities. The authors are fully authorised to use the material for research as per the existing guidelines of the institution.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shafi, S., Zaffar, A., Riyaz, I. et al. Comprehensive phenotyping of SKUAST-K released rice varieties reveals significant role of root traits in drought resilience. Discov Agric 2, 49 (2024). https://doi.org/10.1007/s44279-024-00068-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44279-024-00068-5