Abstract
Low temperature causes a negative impact on plant growth and development, but plants evolve a series of mechanisms to respond to chilling stress, and one of them is CBF [C-repeat (CRT)/dehydration-responsive element (DRE) binding factor] gene family which has been well studied in different crops. In this paper, a new CBF1 gene, named as SpCBF1, was isolated from frost-tolerant Solanum pinnatisectum by PCR and analyzed for its function in cold-tolerance by over-expression technique. The ORF of SpCBF1 was 666 bp long and encoded a protein of 221 amino acids with a predicted molecular mass 24.5821 kDa and theoretically isoelectric point 5.0. SpCBF1 protein contained a highly conserved specific AP2/ERF domain. SpCBF1 was expressed in all tested tissues with the highest level in tuber and the lowest in root, and induced by chilling stress (0 °C). Under natural low temperature condition (1–10 °C), plants over-expressing SpCBF1 (OE) exhibited slighter necrotic lesion and lower necrotic injury, compared with untransformed Solanum tuberosum cv. Désirée (WT) and antisense-StCBF1 control lines. Over-expression of CBF1 increased the level of COR (cold-regulated) gene transcripts in OE lines, and the physiological indexes related to cold tolerance like the contents of SOD, soluble protein, MDA, proline and soluble sugar were higher in OE lines than in WT except RWC which was lower. All these results indicated that SpCBF1 gene plays a promoting role in potato responding to cold stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Low temperature occurring during the growth period often causes severe adverse effects on plant growth and development. Plants evolve a series of mechanisms to respond to chilling stress, so studies of these mechanisms can not only enrich our understanding of plant cold tolerance, but also provide a theoretical basis for cold-tolerance breeding.
Plants adapt to the chilling stress at molecular, cellular, physiological and biochemical levels by changing the expression of a variety of genes (Nakashima et al. 2009), which are thought to induce the accumulation of superoxide, hydrogen peroxide, and hydroxyl radicals to elevate stress tolerance (Xiong et al. 2002).
Among these genes responding to chilling stress, CBF family is of much interest because of the broad regulation functions. CBFs encode transcriptional activators which play a key role in cold-acclimation process during plant growth and development. The CBF protein contain an AP2/ERF domain (Stockinger et al. 1997), which can be distinguished from other AP2 domain containing proteins by amino acid signature sequences that flank the AP2 domain (Jaglo et al. 2001). When plants are exposed to low nonfreezing temperature, a kind of transcriptional activator ICE (inducer of CBF expression) is activated and then induces the expression of CBFs by combining with the ICE box in the promoter of CBF genes. Subsequently, transcription factors CBFs recognize the CRT/DRE sequences which are present in promoter regions of many COR genes and then induce a series of COR expressions (Thomashow 2001). Most of COR polypeptides have amphipathic α-helical regions which may have roles in stabilizing membranes against freezing damage and thus improving freezing tolerance of plants (Thomashow 1999). For now, many crops like rice, tobacco, strawberry, tomato and cucumber overexpressing Arabidopsis CBF1 showed strong cold tolerance (Lee et al. 2004; Wei et al. 2006; Jin et al. 2009; Hsieh et al. 2002; Singh et al. 2011; Gupta et al. 2012).
In Arabidopsis thaliana, six of CBF-encoding genes have been found, and CBF1, CBF2 and CBF3 form a head-to-tail, tandemly-linked gene cluster on chromosome 4 (Gilmour et al. 1998; Medina et al. 1999). Over-expression of CBF1 or CBF3 in transgenic Arabidopsis induced the expression of multiple cold-responsive CRT/DRE-containing genes and then enhanced cold tolerance of plants, whereas CBF2 mutants obviously showed stronger cold hardiness than wild-type whether cold acclimatized or not (Novillo et al. 2007), which demonstrate that CBF1 and CBF3 play positive regulation roles in inducing expression of CORs, but CBF2 plays a negative role. The other CBFs disperse across the genome and are not responsive to low temperature, but are responsive to other stresses like drought (Haake et al. 2002; Sakuma et al. 2002).
Up to now, in addition to Arabidopsis, CBFs or their analogue have been identified in Secale cereale (Jaglo et al. 2001), Brassica napus (Jaglo et al. 2001; Gao et al. 2002), Triticum aestivum (Jaglo et al. 2001; Shen et al. 2003), Hordeum vulgare (Choi et al. 2002; Xue 2002, 2003), Oryza sativa (Dobouzet et al. 2003), Solanum lycopersicum (Jaglo et al. 2001; Zhang et al. 2004), Zea mays (Qin et al. 2004), Capsicum annuum (Kim et al. 2004), Prunus avium (Kitashiba et al. 2004), Glycine max (Li et al. 2005), Avena sativa (Bräutigam et al. 2005), Populus (Benedict et al. 2006), Betula (Welling and Palva 2008), Hordeum spontaneum (James et al. 2008), Lolium perenne (Xiong and Fei, 2006; Zhao and Bughrara 2008), Solanum commersonii (Pennycooke et al. 2008), Eucalyptus gunnii (Navarro et al. 2009), Solanum habrochaites (Li et al. 2014) etc. In Solanaceae, the three CBF genes of many tomato cultivars, Solanum habrochaites, and two of the Solanum pimpinellifolium accessions are linked in series (Pennycooke et al. 2008), however, in contrast to Arabidopsis, not all three genes are responsive to cold. For example, only CBF1 is low temperature responsive in S. lycopersicum and S. habrochaites (Zhang et al. 2004; Pennycooke et al. 2008).
Potato, a crop that is not resistant to both high temperature and low temperature, likes cool growth conditions. The majority of the potato cultivars were not resistant to chilling stress, which has become the limiting factor in potato production, greatly affecting the scope of use of potato varieties and their planting time. Therefore, improving the cold tolerance of potato varieties will be able to achieve the potato early planting or prolonged cultivation, increasing the yield of potatoes, which will need available excellent cold tolerance genes.
In potato, previous studies showed that CBF1 and CBF4 are cold-responsive in both S. tuberosum and S. commersonii (Pennycooke et al. 2008). Therefore, to further explore the cold-tolerant genes, in this study, we cloned a SpCBF1 gene from Solanum pinnatisectum, a wild potato which shows strong tolerance to chilling stress and introduced it to cultivar Désirée. Further studies at the levels of phenotype, physiology, and biochemistry indicated that SpCBF1 over-expression enhances cold tolerance in potato plants by modulating the expression of COR genes.
Materials and methods
Plant materials and preparation
In this study, S. pinnatisectum was used as SpCBF1 gene donor and potato cultivar Désirée was employed as SpCBF1 gene receptor. The in vitro plantlets of both materials were cultured under a 12 h light: 12 h dark regime at 25 °C on MS medium (Murashige and Skoog 1962) containing 3% sucrose and 0.8% agar at pH 5.8. The 4-week-old plantlets were used for gene cloning or as explants source for transformation experiments, or transplanted to pots stuffed with an 1:1:1 mixture of loam, vermiculite and perlite and grew in growth chamber for cold treatment experiments and expression analysis.
SpCBF1 cloning
SpCBF1 gene was isolated from S. pinnatisectum plantlets with specific primers 5′- AGATCTTTAGCTCTCAACAACAATGAA-3′ and 5′-ACTAGTTGTACTATTTAGATAGAATAA-3′ designed based on potato CBF1 in the Solanaceae genomics resource by RT-PCR. Total RNA was extracted from leaves by TRIzol reagent (Invitrogen, USA) and then pre-treated by DNase I. Purified RNA (3 μl) was reversely transcribed to the first strand of cDNA using reverse transcriptase (TaKaRa) in a 20 μl reaction volume. The PCR thermal cycling parameters for amplifying SpCBF1 were 72 °C for 10 min followed by 95 °C for 4 min and 28 cycles of 95 °C for 45 s, 48.5 °C for 45 s, 72 °C for 45 s. PCR product was assessed by 1% agarose electrophoresis. Subsequently, the product of PCR was gel-purified with AxyPrep Gel DNA Extraction Kit (Axygen, China), and inserted into the pMD19-T vector (TaKaRa) for sequencing (Beijing Genomics Institute, China).
Construction of phylogenetic tree
The comparative sequence analysis was performed using ClustalX program. The phylogenetic tree of plant CBF proteins was constructed with the MEGA program (v5.1) by the neighbor-joining method with 1000 bootstraps. The tertiary structure of SpCBF1 was predicted by Swiss Model Server (http://swissmodel.expasy.org/).
Tissue-specific and time-course expression pattern of SpCBF1
Tissue expression analysis and cold-induced expression analysis were conducted by semi-quantitative RT-PCR. For SpCBF1 tissue expression analysis, total RNA was extracted from roots, stem, leaves, flowers, stolons and tubers of S. pinnatisectum plants using TRIzol reagent (Invitrogen, USA). For cold-induced expression analysis, total RNA was extracted from leaves of the plants exposed to 0 °C for 0, 1, 2, 4, 8, 16, 24 and 48 h, respectively, using TRIzol reagent. The RNAs were reversely transcribed to the first strand of cDNA using reverse transcriptase (TaKaRa) as described above. The conserved region of SpCBF1 was specifically amplified with the primers: 5′-AACCTATTATTCAGACCCAC-3′ and 5′-TGACTTCACAAACCCATT-3′ under the following reaction conditions: 94 °C for 5 min, followed by 29 cycles (25 cycles for cold-induced expression analysis) of 94 °C for 30 s, 53 °C for 30 s, 72 °C for 30 s and a final extension step of 72 °C for 10 min. SpEF-1α (Elongation Factors-1α) was used as an inner control standard and its 150 bp fragment was amplified with the primers 5′-TGAAAGCGAGGAAAGAACTA-3′ and 5′-TCCCATGAATGACCCGA-3′ under the following reaction conditions: 94 °C for 5 min, followed by 18 cycles of 94 °C for 20 s, 54 °C for 20 s, 72 °C for 20 s and a final extension step of 72 °C for 10 min. PCR products were separated on 1% agarose gel and stained with ethidium bromide for UV visualization. Quantity One software was used to analyze gray value of bands to help adjust the sample volume for the same brightness of the internal EF-1α bands from different samples, and the brightness of the target gene bands showed the difference of expression levels.
Potato transformation and identification
The ORF sequence of SpCBF1 was inserted into the position behind CaMV 35S promoter of the vector pCAMBIA1304 designating as pCAMBIA1304-SpCBF1 (Fig. 3a). The StCBF1 antisense sequence was cloned from potato cultivar Désirée using primers (primers 5′-AGATCTCTAAAGCTGCCACGTCAT-3′ and 5′-ACTAGTTTCACTATCATACTTTCCCACT-3′) and also inserted in pCAMBIA1304 as pCAMBIA1304-antisense-StCBF1. The vectors were transferred into Agrobacterium tumefaciens (strain GV3101) by the method of freeze–thaw (Hofgen and Willmitzer 1998). Stem internodes of Désirée plantlets were infected with vector-harbored strain and shoots regenerated as described by Liu et al. (2012).
To identify transgenic plants, two pairs of specific primers (primers 5′- AACCTCCTCGGATTCCATTG -3′ and 5′-GAAGCTAAAATAATTTCCTCGTCAG-3′ and primers 5′-AACCTCCTCGGATTCCATTG-3′ and 5′-CTGCACGTATCCCTCAGGC-3′) were designed for amplifying part of pCAMBIA1304-SpCBF1 and pCAMBIA1304-antisense-StCBF1. PCR was conducted with the following conditions: 94 °C for 5 min, followed by 32 cycles of 94 °C for 30 s, 54 °C for 30 s, 72 °C for 35 s or 94 °C for 5 min and 30 cycles of 94 °C for 50 s, 53 °C for 55 s, 72 °C for 60 s and a final extension step of 72 °C for 10 min, respectively. PCR products were separated on 1% agarose gel and stained with ethidium bromide for UV visualization.
Expression analysis of CBF1 in transgenic plants
To analyze the expression of CBF1 in transgenic plants, 3 plants of each OE line (A2, A3 and A6), each antisense line (B2, B9) and non-transgenic plants (WT) were used. Four-week-old in vitro transgenic lines and WT plantlets were transferred to pots and cultured under a 12 h light: 12 h dark regime at 25 °C for four weeks. Then, they were exposed to 0 °C for 24 h. Quantitative RT-PCR (qRT-PCR) were carried out with total RNA derived from leaves of transgenic lines and WT plants using the same primers (5′-AACCTATTATTCAGACCCAC-3′ and 5′-TGACTTCACAAACCCATT-3′) for amplifying conserved fragment of SpCBF1 and StCBF1 with the same condition described above. StEF-1α was used as an inner control standard and the primers and PCR reaction conditions were the same as described above. qRT-PCR was accomplished in ABI7500 Fast Real-Time PCR Systems machine (ABI, USA) according to the following SYBR Premix Ex Taq (TaKaRa) reaction system and steps: 94 °C for 1 min, (95 °C 15 s, 54 °C 20 s) × 40. The corresponding expression level(2−ΔΔCT) of CBF1, PI and DHN10 mRNA were calculated as follows: ΔΔCT = (CT.Target − CT.EF-1a) × X − (CT.Target − CT.EF-1α) × T. X stands for different lines and T stands for one time expression of target gene calibrated by StEF-1α.
Expression analysis of COR genes
COR genes StPI (Genbank: EU849681.1) and StDHN10 (Genbank: EU849680.1) were examined for their expression level using the same cDNAs for analysis of CBF1 in OE lines by qRT-PCR. Primers (5′-GACATTGCGAGTCGTTGA-3′ and 5′-CTCATTTAGCATGGCTTAGTGC-3′) were designed for amplifying the specific fragments of StPI and primers (5′-CATCACAAAGATGGAAAA-3′ and 5′-GTTAGGTAAATAGGAAATCA-3′) were utilized for amplifying the specific fragments of StDHN10. PCR conditions: 94 °C for 4 min followed by 28 cycles of 94 °C for 25 s, 54 °C for 25 s, 72 °C for 25 s and a final extension step of 72 °C for 10 min. qRT-PCR was performed as described in expression analysis of CBF1 above.
Measure of physiological and biochemical indexes related to cold-tolerance
Twelve plants of OE lines (A2, A3 and A6) and WT were moved to growth chambers and exposed to 0 °C for 48 h. After the treatment, these plants were dedicated for the assay of superoxide dismutase (SOD), soluble proteins, malonaldehyde (MDA), relative water content (RWC), proline and soluble sugars contents.
RWC assay
RWC was measured by the method of Jiang and Zhang (2001)
MDA content
About 1 g fresh tissue of the plantlets above was grinded in 4 ml 0.6% 2-thiobarbituric acid (TBA) using a mortar and pestle. The homogenate was centrifuged at 4000 r/min for 10 min, and the 2 ml supernatant was then mixed with 2 ml 10% trichloroacetic acid (TCA). MDA was determined by Jin’s method (Jin et al. 2013).
SOD enzyme activity and soluble protein content
Fresh leaves (0.5 g) were grinded in a mortar and pestle with 8 ml of 50 mM cool phosphate buffer (pH 7.8) containing 1% (w/v) polyvinylpolypyrrolidone (PVP) for the detection of superoxide dismutase (SOD, EC 1.15.1.1). The homogenate was centrifuged at 10,000 g for 25 min at 4 °C, and supernatant was used for the assay of enzyme-activity detection and protein-content determination. Total SOD activity was assayed according to the method described before (Yang et al. 2013). One unit of SOD (U) was defined as the amount of crude enzyme extract required to inhibit the reduction rate of NBT by 50%. Soluble proteins were determined by Bradford’s method (Bradford 1976) using bovine serum albumin (BSA) as a standard.
Contents of proline and soluble sugars
Fresh leaves (0.5 g) were cut into chips and extracted in 5 mL of 1% boiled sulfosalicylic acid for proline assay. Freed proline was determined by acid ninhydrin method (Bates et al. 1973) using a spectrophotometer in a wavelength of 520 nm, using ninhydrin as specific reagent and proline as standard. Soluble sugars were determined by anthrone method (Li et al. 2000) using sucrose as standard.
Cold treatment assay
To implement cold treatment, 15 plants of A3, antisense-StCBF1 (a StCBF1-downregulated transgenic line constructed and preserved by our laboratory: B9, Fig. S1) and WT lines were placed in greenhouse with natural light and temperatures from Dec. 03, 2013. to Jan. 03, 2013 (the average daily minimum temperature is 1 °C and the average daily maximum temperature is 10 °C. Nanjing, 32°02′38″ of North latitude). The number percentage of necrotic injuries (W2/W1 × 100%, W1 means the gross weight of 20 leaves from 5 plants which contained every four leaves from bottom to top; W2 means the weight of necrotic parts of blade including necrotic spots and water stained parts cut by scissors) was used as an index of the ability to resist low temperature and the estimation of necrotic injury was conducted by the method of Janowiak et al. (2003). The experiment was repeated three times.
Statistical analysis
Data were means ± standard errors of three independent experiments. Student’s t test(two-tailed) was conducted where appropriate. The asterisk indicates a significant difference (* means P < 0.05, ** means P < 0.01) in two comparisons.
Results
SpCBF1 cloning and expression pattern
Using the known related species tomato CBF1 sequence as reference, we performed alignment using BLAST, the potato CBF1 gene from the Solanaceae genomics resource (http://solanaceae.plantbiology.msu.edu/), and one S. tuberosum fragment (PGSC0003DMT400037121) having high homology with that in tomato was obtained, which was first named as StCBF1 in our work. Then, SpCBF1 was amplified from S. pinnatisectum with the sequence-specific primers designed according to StCBF1 sequence by RT-PCR. The cDNA of SpCBF1 consisted of an open reading fragment of 666 bp, encoding a protein of 221 amino acid residues with a predicted molecular mass 24.5821 kDa and theoretically isoelectric point 5.0. The SpCBF1 had no intron when compared with the DNA sequence given in the Solanaceae genomics resource. SpCBF1 protein contained a highly conserved specific AP2/ERF domain like other CBF proteins and alignment analysis showed that the amino acid sequence of SpCBF1 was highly similar to its homologues derived from other 10 plant species (Fig. 1a). Three-dimensional structure analysis showed that the polypeptide contained one α-helix and three β-pleated sheets (Fig. 1b). Phylogenetic analysis demonstrated that SpCBF1 was clustered in a clade with other Solanum plants and was most close with the CBF1 of S. commersonii and S. tuberosum (Fig. 1c).
Sequence alignment, tertiary structure model and phylogenetic analysis of SpCBF1. a Amino acid sequences alignment. Sequences used for alignment are derived from Solanum tuberosum (StCBF1, ACB45096.1), Solanum commersonii (ScCBF1, ACJ26751.1), Solanum habrochaites (ShCBF1, ACB45087.1), Solanum lycopersicum (SlCBF1, AAS77820.1) Lycopersicon hirsutum (LhCBF1, BAE17131.1), Capsicum annuum (CaCBF1, AAR88363.1), Populus trichocarpa (PtCBF1, ABO48363.1), Arabidopsis thaliana (AtCBF3, ABV27138.1), Arabidopsis thaliana (AtCBF1, AAV80413.1), Arabidopsis thaliana (AtCBF2, AAV80415.1). Boxes indicate conserved regions of CBFs. b Tertiary structure model of SpCBF1. c Phylogenetic analysis. Sequences were aligned by CLUSTALX and analyzed using the MEGA 4.1. Construction was carried out by the parsimony method with 1000 bootstrap replicates and a consensus tree was generated. Database accession numbers are as follows: Solanum pinnatisectum (SpCBF1), Solanum tuberosum (StCBF1, ACB45096.1), Solanum lycopersicum (SlCBF1, AAS77820.1; SlCBF2, AAS77821.1), Solanum commersonii (ScCBF1, ACB45093.1; ScCBF2, ACB45094.1; ScCBF3, ACB45092.1), Solanum habrochaites (ShCBF1, ACB45087.1; ShCBF2, ACB45088.1; ShCBF3, ACB45078.1), Capsicum annuum (CaCBF1, AAR88363.1), Populus trichocarpa (PtCBF1, ABO48363.1), Manihot esculenta (MeCBF1, AFB83707.1), Morus alba var. multicaulis (MamCBF1, AFQ59977.1), Arabidopsis thaliana (AtCBF1, AAV80413.1; AtCBF2, AAV80415.1; AtCBF3, ABV27149.1), Nicotiana tabacum (NtCBF2, ACE73694.1; NtCBF3, ACE73695.1), Betula pendula (BpeCBF1, ADZ23479.1), Betula platyphylla (BplCBF1, ADZ23479.1), Malus domestica (MdCBF1, AAZ20446.1), Camellia sinensis (CsCBF1, AFN93974.1), Gossypium hirsutum (GhCBF1, ABD65473.1), Bruguiera gymnorhiza (BgCBF1, AFU81298.1), Oryza sativa Japonica Grou (OsCBF, NP_001056909.1), Lolium perenne (LpCBF, BAF36838.1), Avena sativa (AsCBF, CAJ21276.1), Aegilops biuncialis (AbCBF2, CBX87016.1)
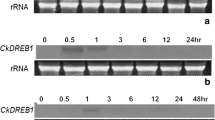
Tissue-specific and chilling-treated expression pattern of SpCBF1
To study the expression pattern of the SpCBF1 gene, the level of this gene mRNA was measured in various tissues of S. pinnatisectum by semi-quantitative RT-PCR. The result showed that SpCBF1 was expressed in all tested tissues and the highest level was observed in tuber then in stems and leaves, and the lowest level in roots (Fig. 2a). To know the response of SpCBF1 to cold stress, S. pinnatisectum plants were subjected to 0 °C treatment at different times. The results showed that SpCBF1 was induced by cold treatment and the highest level of the transcript appeared at 2 h treatment, then the content decreased gradually (Fig. 2b).
Production and identification of potato transgenic lines
SpCBF1-overexpressing transgenic potato lines were constructed using potato cultivar Désirée as receptor (Fig. 3a). In total, 8 independent over-expression (OE) lines were obtained by screening of hygromycin B resistance and termed as A1-A8. Electrophoretic analysis identified an expected band in 5 OE transgenic lines (A2, A3, A4, A6, A8) (Fig. 3b). Further, the expression level of CBF1 in three OE lines (A2, A3, A6) was detected by qRT-PCR. The results showed that the level of CBF1 transcript was higher in the OE lines, compared with WT control (Fig. 3c), indicating that the transgenes had been integrated into potato genome and expressed normally.
The construction and PCR identifications of SpCBF1 transgenic potato lines. a schematic representations of pCAMBIA1304-SpCBF1 vector with site of restriction enzymes. b PCR-based identifications of over-expression transgenic lines. The DNA template was extracted from bacteria (lane +), non-transformed (lane WT) and transformed (lane A1-8) lines. * means the positive line. M, marker DL2000. c Quantitative RT-PCR analyses of CBF1 transcripts in over-expression transgenic lines. Total RNAs were extracted from leaves of transgenic lines (A2, 3 and 6) and non-transgenic lines (WT). EF-1α expression was used as the loading control. Error bars indicate SE. The asterisk indicates a significant difference (*P < 0.05; **P < 0.01) between transgenic lines and WT at 25 °C. Student’s t test (two-tailed) was conducted
CBF1 regulates COR expression in transgenic lines
COR gene is a class of genes that are located downstream of the CBF gene, which respond to low temperature. To understand how SpCBF1 regulates plant cold tolerance, the expression of CBF1, StPI (Genbank: EU849681.1) and StDHN10 (Genbank: EU849680.1) in OE lines (A2, A3, A6) exposed to 0 °C condition were analyzed (Fig. 4). The result showed that the expression of CBF1, StPI and StDHN10 in OE lines increased compared with WT and they had the same expression pattern, suggesting that CBF1 in potato under cold stress regulates the expression of PI and DHN10.
Quantitative RT-PCR analyses of CBF1, StDHN10 and StPI transcripts in transgenic lines and WT under 0 °C after 24 h treatment. Total RNAs were extracted from leaves of transgenic lines (A2, 3 and 6) and WT. EF-1α expression was used as the loading control. Error bars indicate SE. The asterisk indicates a significant difference (*P < 0.05; **P < 0.01) between the transgenic lines and WT. Student’s t test (two-tailed) was conducted
Change of physiological and biochemical indexes in transgenic lines
Under low temperature, some physiological and biochemical indexes of plants, such as RWC, MDA, ROS, soluble protein, proline and soluble sugar, will be changed to the corresponding adversity. In this study, the six indexes were checked in A2, A3, A6 and WT under 0 °C condition. The result showed that the contents of RWC, SOD, soluble proteins, proline and soluble sugars in OE lines were higher, in contrast with the WT (Fig. 5a, c-f) except MDA which was lower (Fig. 5b). These results showed that overexpression of CBF1 could make the physiological indexes in favor of adaptation to cold stress, which enhanced the cold resistance of plants.
Physiological and biochemical index of transgenic lines (A2, 3 and 6) and WT under 0 °C for 48 h. Determination of RWC (a), MDA (b), SOD (c), soluble proteins (d), Proline (e) and soluble sugars (f). Error bars indicate SE. The asterisk indicates a significant difference (*P < 0.05; **P < 0.01) between transgenic lines and WT. Student’s t test (two-tailed) was conducted
Analysis of cold-tolerance abilities of potato transgenic lines
Plants that grow at chilling temperature often exhibit symptoms of damage on the phenotype, such as water-soaking, wilting, and necrotic lesions. In order to get closer insights, three potato lines (A3, WT and antisense line) were placed under natural conditions in winter for cold-tolerance assay. Phenotypic observation showed that water-soaking, wilting, and necrotic lesions occur on both of OE and the control plants after 2 weeks of growth at low temperature (1–10 °C), but the degrees of injuries varied among the different lines. There were less necrotic lesions on the leaves of A3 line and more serious water-soaking, wilting and necrotic lesions on the leaves of antisense-CBF1 line and WT (Fig. 6a).
Leaves phenotypes of transgenic line A3, WT and antisense-StCBF1 control (a) and percent of necrotic injury (b) under natural light and temperature in winter. Error bars indicate SE. The asterisk indicates a significant difference (**P < 0.01) among A3, WT and antisense-StCBF1 control. Student’s t test (two-tailed) was conducted
Necrotic lesion has been used in earlier studies as reliable parameter to evaluate the degree of chilling injury (Janowiak and Markowski 1987; Janowiak et al. 2002). Damage analysis showed that after growth at low temperature for one month, the percentage of necrotic lesions on leaves was evidently lower in A3 line (6.25%) and higher (11.67 and 15.08%) in WT and antisense-CBF1 line (Fig. 6b). The results demonstrated that over-expression of SpCBF1 enhanced potato plant tolerance to cold stress, which were consistent with the results of physiological and biochemical indexes analyzed above.
Discussion
CBF1 gene, an important member of CBF family, has been isolated from different plants which can be mentioned as Arabidopsis thaliana (Stockinger et al. 1997), Brassica campestris (Gao et al. 2002), Hordeum vulgare (Xue 2003), Solanum lycopersicum (Hsieh et al. 2002) etc. In potato crop, CBF genes are only reported in Solanum commersonii and Solanum tuberosum, and the expression of CBF1 and CBF4 is induced by low temperature (Pennycooke et al. 2008). In this study, we cloned a CBF1 gene from S. pinnatisectum, a potato wild relative with strong cold resistance. The SpCBF1 shared 95% homology with S. commersonii CBF1 (Fig. 1a) and its encoding protein was highly similar to CBF1 from Solanum plants (Fig. 1c) and had an AP2 domain, demonstrating that SpCBF1 belonged to CBF gene family.
In Arabidopsis, tomato and some potatoes, CBF1 is a low temperature inducible gene (Novillo et al. 2007; Pennycooke et al. 2008). In this study, we examined the expression of SpCBF1 under 0 °C and the result showed that it was also induced by chilling temperature, whose value reached the highest level after 2 h (Fig. 2b), suggesting that SpCBF1 is a cold-sensitive gene like that in Arabidopsis and tomato.
It has been reported that CBF1 is involved in the process of drought resistance and cold resistance in plants (Stockinger et al. 1997; Xu et al. 2014). Over-expression of AtCBF1 could obviously improve cold tolerance of plants such as potato, strawberry, tomato and cucumber (Pino 2006; Jin et al. 2009; Singh et al. 2011; Gupta et al. 2012). In order to study the function of SpCBF1, we constructed SpCBF1 overexpressing transgenic lines. Then, we detected the cold resistance index and performance of the transgenic lines in molecular, physiological and phenotypic aspects (Figs. 4, 5, 6). Cold resistance analysis showed that cold injury in the transgenic line overexpressing SpCBF1 was only 1/2 of WT and 1/3 of StCBF1 antisense line. The COR genes are the targets of transcription factors CBFs (Jaglo et al. 2001), so we detected the transcriptional levels of two COR genes StDHN10 and StPI, and the expression of StDHN10 and StPI was induced by constitutively expressing SpCBF1 (Fig. 4). In Arabidopsis, ectopic constitutive over-expression of CBF genes can induce the expression of COR genes in whole plant cold-resistance in the absence of chilling stress (Van Buskirk and Thomashow 2006). Similar result is also observed in tomato where a set of COR genes are responsive to CBF over-expression (Zhang et al. 2004). Low temperature stress may cause a series of physiological changes in plants. Physiological analysis revealed that in transgenic lines RWC, ROS, the contents of soluble protein, proline and soluble sugar were increased and MDA was declined, compared with WT. RWC is an important index that reflects the growth status of plant under cold stress. MDA is a kind of product of membrane-lipid peroxidation whose content reflects the level of membrane injury under chilling stress. When facing chilling stress, the plant can increase the level of MDA and reduce ROS (reactive oxygen species) accumulation and subsequent oxidative damage to increase its tolerance. Soluble proteins, proline and soluble sugars, as osmolytes in plants, can prevent cell from cold-induced water loss which can cause many dysfunctions. Our results suggest that SpCBF1, by inducing the expression of COR genes and modulating the stability of the cell membrane, positively regulates the cold tolerance of potato.
Different Solanum species evolve different cold-resistant mechanisms. Solanum tuberosum cv. Désirée could not acclimatize well when exposed at 4 °C for 3 weeks while another two species Solanum phureja CHS and Solanum tuberosum PS3 are more cold-resistant (Oufir et al. 2008). Analysis of transcriptome data and contents of soluble carbohydrates, polyols and free polyamines in these three Solanum plants showed their different responses to the cold stress were caused by many affected genes involved in carbohydrate and polyamine metabolism (Oufir et al. 2008). This result implied that there is a differential response to cold stress among different species due to the transcript expression. In our work, overexpressing SpCBF1 in cultivar Désirée enhanced its cold tolerance (Fig. 6), indicating that the different cold tolerance of Solanum tuberosum and Solanum pinnatisectum may due to the different expression of CBF1 gene or the different sequence between these two potatoes, which needs to be further studied.
CBF1 not only conferred tolerance to low temperatures (Lee et al. 2004; Wei et al. 2006; Jin et al. 2009; Hsieh et al. 2002; Singh et al. 2011; Gupta et al. 2012), but also confers tolerance to other stresses. Overexpressing ScCBF1 in Solanum tuberosum and Solanum commersonii plants enhanced their drought tolerance (Pino et al. 2013). AtCBF1 overexpression in potato plants enhanced salt tolerance (Kwon et al. 2013) and confers tolerance to high light conditions at 22 °C by enhancing the ability to cope with an excess of radiant energy (Storani et al. 2015). In our work, we only detected the chilling tolerance of overexpressing SpCBF1 in cultivar Désirée, further tolerance assays for other stresses like drought, salinity and high-light condition should be studied for its further function analysis.
Our work provides significant insights in the function of the gene SpCBF1 in regulating cold tolerance in Solanum and SpCBF1 may be utilized for modifying potato cultivar cold tolerance. Our data obtained show only that this protein is involved in chilling tolerance but not in freezing tolerance. Therefore, further studies in freezing tolerance are needed to elucidate its cold-resistant mechanism.
Abbreviations
- OE:
-
Over-expression
- WT:
-
Untransformed control
- COR:
-
Cold-regulated
- Sp :
-
Solanum pinnatisectum
- CBF :
-
C-repeat (CRT)/dehydration-responsive element (DRE) binding factor
- SOD:
-
Superoxide dismutase
- MDA:
-
Malonaldehyde
- RWC:
-
Relative water content
References
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Benedict C, Skinner JS, Meng R, Chang Y, Bhalerao R, Huner NPA, Finn CE, Chen THH, Hurry V (2006) The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell Environ 29(7):1259–1272
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Bräutigam M, Lindlöf A, Zakhrabekova S, Gharti-Chhetri G, Olsson B, Olsson O (2005) Generation and analysis of 9792 EST sequences from cold acclimated oat, Avena sativa. BMC Plant Biol 5(1):18
Choi DW, Rodriguez EM, Close TJ (2002) Barley Cbf3 gene identification, expression pattern, and map location. Plant Physiol 129(4):1781–1787
Gao MJ, Allard G, Byass L, Flanagan AM, Singh J (2002) Regulation and characterization of four CBF transcription factors from Brassica napus. Plant Mol Biol 49(5):459–471
Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16(4):433–442
Gupta N, Rathore M, Goyary D, Khare N, Anandhan S, Pande V, Ahmed Z (2012) Marker-free transgenic cucumber expressing Arabidopsis cbf1 gene confers chilling stress tolerance. Biol Plant 56(1):57–63
Haake V, Cook D, Riechmann J, Pineda O, Thomashow MF, Zhang JZ (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130(2):639–648
Hsieh TH, Lee JT, Yang PT, Chiu LH, Charng YY, Wang YC, Chan MT (2002) Heterology expression of the Arabidopsis C-repeat/dehydration response element binding Factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol 129(3):1086–1094
Jaglo KR, Kleff S, Amundsen KL, Zhang X, Haake V, Zhang JZ, Delts T, Thomashow MF (2001) Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol 127(3):910–917
James VA, Neibaur I, Altpeter F (2008) Stress inducible expression of the DREB1A transcription factor from xeric, Hordeum spontaneum L. in turf and forage grass (Paspalum notatum Flugge) enhances abiotic stress tolerance. Transgenic Res 17(1):93–104
Janowiak F, Markowski A (1987) Effect of chilling on germination, growth, survival and membrance permeability in seedlings of different breeding forms of maize (Zea mays L.). Acta Physiol Plant 2:77–87
Janowiak F, Maas B, Dörffling K (2002) Importance of abscisic acid for chilling tolerance of maize seedlings. J Plant Physiol 159(6):635–643
Janowiak F, Luck E, Dörffling K (2003) Chilling tolerance of maize seedlings in the field during cold periods in spring is related to chilling-induced increase in abscisic acid level. J Agron Crop Sci 189(3):156–161
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42(11):1265–1273
Jin WM, Dong J, Liu Y, Zhang YP, Pan QH (2009) Genetically transformed strawberry (Fragaria × ananassa Duch.) with cold-inducible transcription factor CBF1. In: LopezMedina J (ed) Vi international strawberry symposium, pp 529–532
Jin Q, Zhu K, Cui W, Xie Y, Han B, Shen W (2013) Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant, Cell Environ 36(5):956–969
Kim S, An CS, Hong YN, Lee KW (2004) Cold-inducible transcription factor, CaCBF, is associated with a homeodomain leucine zipper protein in hot pepper (Capsicum annuum L.). Mol Cells 18(3):300–308
Kitashiba H, Ishizaka T, Isuzugawa K, Nishimura K, Suzuki T (2004) Expression of a sweet cherry DREB1/CBF ortholog in Arabidopsis confers salt and freezing tolerance. J Plant Physiol 161(10):1171–1176
Kwon TR, Lee SK, Park SR, Siddiqui ZS, Moon SJ, Park SC, Byun MO (2013) Atcbf1 gene enhances salt tolerance in potato (Solanum tuberosum L.). Plant. Stress 7(1):34–38
Lee SC, Huh KW, An K, An G, Kim SR (2004) Ectopic expression of a cold-inducible transcription factor, CBF1/DREB1b, in transgenic rice (Oryza sativa L.). Mol Cells 18:107–114
Li H, Sun Q, Zhao S (2000) Principles and techniques of plant physiological biochemical experiment. Higher Education, Beijing, pp 195–197
Li XP, Tian AG, Luo GZ, Gong ZZ, Zhang JS, Chen SY (2005) Soybean DRE-binding transcription factors that are responsive to abiotic stresses. Theor Appl Genet 110(8):1355–1362
Li ZJ, Zhang LL, Li JF, Xu XY, Yao QH, Wang AX (2014) Isolation and functional characterization of the ShCBF1 gene encoding a CRT/DRE-binding factor from the wild tomato species Solanum habrochaites. Plant Physiol Biochem 74:294–303
Liu SP, Zhu YP, Xie C, Jue DW, Hong YB, Chen M, Hubdar AK, Yang Q (2012) Transgenic potato plants expressing StoVe1 exhibit enhanced tolerance to Verticillium dahliae. Plant Mol Biol Rep 30(4):1032–1039
Medina JN, Bargues M, Terol J, Pérez-Alonso M, Salinas J (1999) The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol 119(2):463–470
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Nakashima K, Ito Y, Yamaguchi-Shinozaki K (2009) Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol 149(1):88–95
Navarro M, Marque G, Ayax C, Keller G, Borges JP, Marque C, Teulieres C (2009) Complementary regulation of four Eucalyptus CBF genes under various cold conditions. J Exp Bot 60:2713–2724
Novillo F, Medina J, Salinas J (2007) Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. P Natl Acad Sci USA 104(52):21002–21007
Oufir M, Legay S, Nicot N, Moer KV, Hoffmann L, Renaut J, Hausman JF, Evers D (2008) Gene expression in potato during cold exposure: Changdes in carbohydrate and polyamine metabolisms. Plant Sci 175:839–852
Pennycooke JC, Cheng H, Roberts SM, Yang Q, Rhee SY, Stockinger EJ (2008) The low temperature-responsive, Solanum CBF1 genes maintain high identity in their upstream regions in a genomic environment undergoing gene duplications, deletions, and rearrangements. Plant Mol Biol 67(5):483–497
Pino MT (2006) Ectopic overexpression of Arabidopsis CBF genes enhances freezing tolerance of two potato species. Dissertation, Oregon State University
Pino MT, Ávila A, Molina A, Jeknic Z, Chen THH (2013) Enhanced in vitro drought tolerance of Solanum tuberosum and Solanum commersonii plants overexpressing the ScCBF1 gene. Cien Inv Agr 40(1):171–184
Qin F, Sakuma Y, Li J, Liu Q, Li Q, Shinozaki K, Yamaguchi-Shinozaki K (2004) Cloning and functional analysis of a novel DREB1/CBF transcription factor involved in cold-responsive gene expression in Zea mays L. Plant Cell Physiol 45(8):1042–1052
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-Binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in Dehydration-and Cold-inducible gene expression. Biochem Bioph Res Co 290(3):998–1009
Shen YG, Zhang WK, He SJ, Zhang JS, Liu Q, Chen SY (2003) An EREBP/AP2-type protein in Triticum aestivum was a DRE-binding transcription factor induced by cold, dehydration and ABA stress. Theor Appl Genet 106(5):923–930
Singh S, Rathore M, Goyary D, Singh RK, Anandhan S, Sharma DK, Ahmed Z (2011) Induced ectopic expression of At-CBF1 in marker-free transgenic tomatoes confers enhanced chilling tolerance. Plant Cell Rep 30:1019–1028
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94(3):1035–1040
Storani L, Hernando CE, Staneloni RJ, Ploschuk E, Rugnone ML, Striker GG, Casal JJ, Chernomoretz A, Yanovsky MJ (2015) AtCBF1 overexpression confers tolerance to high light conditions at warm temperatures in potato plants. Am J Potato Res 92:619–635
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Biol 50(1):571–599
Thomashow MF (2001) So what’s new in the field of plant cold acclimation? Lots! Plant Physiol 125(1):89–93
Van Buskirk HA, Thomashow MF (2006) Arabidopsis transcription factors regulating cold acclimation. Physiol Plant 126(1):72–80
Wei S, Sun Z, Han L, Yu L (2006) Transgenic tobacco plants over-expressing Arabidopsis transcriptional factor CBF1 show morphological and biochemical characteristics associated with cold tolerance. Asian J Plant Sci 5:932–939
Welling A, Palva ET (2008) Involvement of CBF transcription factors in winter hardiness in birch. Plant Physiol 147:1199–1211
Xiong Y, Fei SZ (2006) Functional and phylogenetic analysis of a DREB/CBF-like gene in perennial ryegrass (Lolium perenne L.). Planta 224(4):878–888
Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14(suppl 1):S165–S183
Xu FH, Liu ZX, Xie HY, Zhu J, Zhang JR, Kraus J, Blaschnig T, Nehls R, Wang H (2014) Increased drought tolerance through the suppression of ESKMO1 gene and overexpression of CBF-Related genes in Arabidopsis. PLoS ONE 9(9):e106509
Xue GP (2002) Characterisation of the DNA-binding profile of barley HvCBF1 using an enzymatic method for rapid, quantitative and high-throughput analysis of the DNA-binding activity. Nucl Acids Res 30(15):e77–e77
Xue GP (2003) The DNA-binding activity of an AP2 transcriptional activator HvCBF2 involved in regulation of low-temperature responsive genes in barley is modulated by temperature. Plant J 33(2):373–383
Yang L, Xie C, Li W, Zhang R, Jue D, Yang Q (2013) Expression of a wild eggplant ribosomal protein L13a in potato enhances tolerance to Verticillium dahliae. Plant Cell Tiss Org 115(3):329–340
Zhang X, Fowler SG, Cheng H, Lou Y, Rhee SY, Stockinger EJ, Thomashow MF (2004) Freezing-sensitive tomato has a functional CBF cold response pathway, but a CBF regulon that differs from that of freezing-tolerant Arabidopsis. Plant J 39(6):905–919
Zhao H, Bughrara SS (2008) Isolation and characterization of cold-regulated transcriptional activator LpCBF3 gene from perennial ryegrass (Lolium perenne L.). Mol Genet Genom 279(6):585–594
Acknowledgements
This study was supported financially by grants from the National Natural Science Foundation of China (11171155), the National Pear Industry Technology System (NO. CARS-29) and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions: Modern horticultural science (PAPD).
Author information
Authors and Affiliations
Contributions
Z.W., S.K., Y.X. and Y.Q. designed research; S.K., T.R., M.X., C.J. and M.C. performed research; S.K., Z.W., M.X., C.J. and Y.Q. analyzed data; and Z.W., S.K. and Y.Q. wrote the paper.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
PCR identifications of antisense-StCBF1 potato lines. a, PCR-based identifications of antisense-StCBF1 potato lines. The DNA template was extracted from bacteria (lane +), non-transformed (lane WT) and transformed (lane B1-14) lines. * means the positive line. M, marker DL2000. b, Quantitative RT-PCR analyses of StCBF1 transcripts in antisense lines. Total RNAs were extracted from leaves of transgenic lines (B2 and B9) and non-transgenic lines (WT). EF-1α expression was used as the loading control. Error bars indicate SE. The asterisk indicates a significant difference (* P < 0.05; ** P < 0.01) between transgenic lines and WT at 25 °C. Student’s t test (two-tailed) was conducted (PDF 181 kb)
Rights and permissions
About this article
Cite this article
Zhu, W., Shi, K., Tang, R. et al. Isolation and functional characterization of the SpCBF1 gene from Solanum pinnatisectum. Physiol Mol Biol Plants 24, 605–616 (2018). https://doi.org/10.1007/s12298-018-0536-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-018-0536-1