Abstract
Sugarcane is an important crop producing around 75 % of sugar in world and used as first generation biofuel. In present study, the genomic and gene based microsatellite markers were analyzed by low cost Single Strand Confirmation Polymorphism technique for genetic evaluation of 22 selected sugarcane genotypes. Total 16 genomic and 12 Expression Sequence Tag derived markers were able to amplify the selected sugarcane genotypes. Total 138 alleles were amplified of which 99 alleles (72 %) found polymorphic with an average of 4.9 alleles per locus. Microsatellite marker, VCSSR7 and VCSSR 12 showed monomorphic alleles with frequency 7.1 % over the average of 3.5 obtained for polymorphic locus. The level of Polymorphic Information Content (PIC) varied from 0.09 in VCSSR 6 to 0.88 in VCSSR 11 marker respectively with a mean of 0.49. Genomic SSRs showed more polymorphism than EST-SSRs markers on selected sugarcane genotypes whereas, the genetic similarity indices calculated by Jaccard’s similarity coefficient varied from 0.55 to 0.81 indicate a high level of genetic similarity among the genotypes that was mainly attributed to intra specific diversity. Hence, the SSR-SSCP technique helped to identify the genetically diverse clones which could be used in crossing program for introgression of sugar and stress related traits in hybrid sugarcane.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sugarcane (Saccharum spp.) is a C4 polyploid grass belonging to genus Saccharum of the family Poaceae, tribe Andropogoneae. It contributes as economically important crop through its high value products, sugar and ethanol. About 75 % of the global need of sugar has been achieved by the improved sugarcane varieties grown in tropical and subtropical regions of world (Singh et al. 2006). Present day sugarcane cultivars are inter specific hybrids (2n = 100–300) and are believed to have originated mainly from complex hybridization events of Saccharum officinarum (2n = 80, x = 10) and Saccharum spontaneum (2n = 40–128, x = 10) (Sreenivasan et al. 1987) and back crossed to S. officinarum. They have 100 to 130 chromosomes from which 80 to 90 % of the genome from S. officinarum and 10–20 % of the genome from S. spontaneum and 8–13 % by inter specific exchange (D’hont et al. 1996; Piperidis et al. 2010).
Plant breeding has significantly improved the yield of sugarcane by introgression of novel genes that contribute for higher yield, biotic and abiotic stress resistance into the desirable cultivars. The successive backcrossing events are being performed between Saccharum hybrids and Saccharum officinarum for introgression of these traits (Pinto et al. 2006). Moreover, the S. officinarum contributes to the high sucrose content and S. spontaneum contributes to resistance against pathogen and other stress trait which improve the genetic gain in hybrid vigor.
However, only few clones of these species are being commercially exploited than those of modern hybrid varieties and are one of the principal causes of present slow rate of introgression to broaden the genetic base of sugarcane (Besse et al. 1998). Although conventional breeding has contributed to the development of agronomically improved cultivars, limitations such as narrow gene pool, complex genome, poor fertility and the long breeding/selection cycle make it difficult to undertake further improvement.
For achieving improved productivity in a crop, it is essential to maintain a high degree of genetic diversity among the commercial varieties and breeding population. The cultivated sugarcanes clones are predominantly out crossing and are maintained by vegetative propagation which shows low genetic variability among the clones. Deciphering phylogenetic relationships between the selected sugarcane genotypes could assist in understanding the genome organization in modern sugarcane and its relative species. Identifying diverse genotypes through the molecular markers has great potential to contribute to the genetic improvement of sugarcane by identifying novel genetic variation, improving the heritability and expending the production of elite genetic material, as well as providing an improved understanding of sugarcane genome. Since, last two decades there are numerous PCR based advanced molecular marker techniques such as AFLP (Amplified Fragment Length Polymorphism) (Selvi et al. 2005), STMS (Sequence Tag Micro Satellite) (Singh et al. 2005), ISSR (Inter Simple Sequence Repeat) (Kalwade et al. 2012), TRAP (Target Region Amplified Polymorphism) (Devarumath et al. 2013), 5S rRNA intergenic spacer sequences (Pan et al. 2000) have been used in explaining genetic diversity among different accessions of sugarcane. Microsatellites or simple sequence repeat (SSR) marker amplifies tandemely di to hexa and even hepta nucleotide repeats which are often widely distributed on eukaryotic genome. SSRs are ideal markers that exhibit great precision in polymorphism, reproducibility, multi-allelic nature and their transferability across genera (Parida et al. 2010; Devarumath et al. 2012). It also helps in breeding process including germplasm fingerprinting, genetic mapping and marker-assisted selection (Aitken et al. 2005). Recently, microsatellites have been used to set up the genetic map in Saccharum spp. (Aitken et al. 2005) as well as other poacea crops. Microsatellites or SSRs are categorized into the genomic (gSSR) and gene based Expressed Sequence Tags (EST-SSRs). These SSRs are identified and isolated by different techniques such as shotgun and the enriched cDNA library respectively (Jarne and Lagoda 1996). gSSR (Cordeiro et al. 2000) and EST-SSRs (Pinto et al. 2004, 2006; Oliveira et al. 2009) have been recently developed and have proved its integrity in sugarcane genome.
Presently, several sugarcane databases have been generated (Vettore et al. 2003) to access the information. The International Sugarcane Microsatellite Consortium funded by the International Consortium for Sugarcane Biotechnology (ICSB) and independent institutes promotes the sugarcane research world widely. These have resulted in deposition of large data of sugarcane genome and it can be freely accessed online from various databases like National Centre for Biotechnology Information (NCBI), Sugarcane Gene Index database (SoGI) and Sugarcane EST (SUCEST) database. By using the information from these databases, informative microsatellite primers were designed and applied for DNA fingerprinting on selected sugarcane Germplasm (Devarumath et al. 2013). SUCEST database developed by Brazilian sugarcane consortium (http://sucest.lad.ic.unicamp.br/en/), alone has 237,954 ESTs, isolated from 26 sugarcane tissue libraries and deposited for public accesses.
In the present study, single strand conformation polymorphism (SSCP) is based on electrophoretic detection of conformational changes in single stranded DNA molecules resulting from point mutations or other forms of small nucleotide changes. Therefore, any change in the sequence theoretically causes a shift in the mobility of the analyzed molecule upon electrophoresis in a neutral polyacrylamide gel. This technique was originally developed for rapid analysis of mutations (Gasser et al. 2006; Orita et al. 1989; Spinardi et al. 1991) and has been used extensively in human genetics to detect single-point mutations (Hayashi 1992). The application of SSCP to SSR amplified products helps to detect conformational changes in single stranded DNA molecules and hence enhances polymorphism of microsatellite repeats to facilitate molecular genetic diversity analysis (Srivastava et al. 2005, 2011; Swapna et al. 2011; Swapna and Srivastava 2012).
Materials and methods
Plant material and DNA isolation
Young leaves from twenty two sugarcane genotypes on the basis of brix % were collected for the genomic DNA extraction (Table 1). All sugarcane genotypes were grown in the Vasantada Sugarcane Institute (VSI), sugarcane research station, Pune India situated at 18° 31′ N latitude and 73° 55′ E longitude. Collected leaves were frozen in liquid nitrogen and stored in −80 °C for preservation last to the DNA isolation.
Genomic DNA was isolated from the harvested stored leaves of each sugarcane genotypes by using modified CTAB method (Aljanabi et al. 1999). Quality and quantity of DNA were estimated by UV-visible spectrophotometer and also visually by ethidium bromide staining on 0.8 % (w/v) agarose gel. DNA was diluted to uniform concentration 10 ng/ul for microsatellite marker analysis.
Primer selection and PCR amplification
Seventeen primer sequence information was received from the CIRAD, France as given in Table 2 (VCSSR 1 to 17) which was designed to target genomic region (gSSR) of Saccharum hybrid cultivar R570 microsatellite DNA except for one pair (VCSSR5) an EST-SSR from the SUCEST database. Another 11 primers were designed from the flanking region of cultivated sugarcane ESTs deposited in NCBI database as given in Table 2 (http://www.ncbi.nlm.nih.gov) and genomic and EST-SSRs motifs were identified by WEBSAT software and the primers flanking the SSR repeat motifs were designed according to the primer 3. The primers were synthesized from the Sigma (Sigma-Aldrich, USA).
The primers were initially screened on the genomic DNA for optimization of annealing temperature. PCR reaction was performed in 96 well plate thermal cycler (Eppendorf AG 22331, Mastercycler, Germany) with reaction volume of 20 μl. PCR reaction containing 1× PCR buffer with 10 mM Tris-Cl (pH 8.3), 50 mM KCl, 2.0 mM MgCl2, 100 μM each dNTP, 0.2 μM primer forward and reverse primer, 10 ng of template DNA, and 0.5U of Taq polymerase (Sigma-Aldrich, USA) was used. PCR amplification conditions were set as initial denaturation at 94 °C for 6 min followed by 30 cycles of denaturation at 94 °C for 1 min, annealing at 50–62 °C for 1 min, extension at 72 °C for 2 min, and final extension at 72 °C for 7 min. The amplified products 2 μl were initially resolved on 1.5 % agarose gels in 1× Tris Borate EDTA (TBE) buffer under room temp at constant voltage 100 V to determine DNA fragment products. The gels were documented after the ethidium bromide staining under ultraviolet light using gel documentation unit (UVITech). The confirmed amplified primers were used further for the SSCP analysis.
SSCP analysis
SSCP protocol used as described by Swapna et al. (2011) was performed in either 6 % or 8 % denaturating polyacrylamide gel (29:1) depending on the molecular weight of the amplified fragments. SSCP technique was used to separate the different conformers of amplified product of primer pairs. We used urea (10 %) in the gels to sharpen the bands of sDNA fragments and clear the background that might otherwise obscure bands that are diagnostic of a polymorphism (Yip et al. 1999). The PCR products were diluted 1:3 ratios with solution containing 10 mM NaOH, 95 % formamide, 0.05 % bromophenol blue and 0.05 % Xylene cynol FF and were denatured at 95 °C for 5 min. The denatured PCR product was cooled in ice and loaded on the gel. Different voltage and temperature conditions were tested. Best results were obtained when gels were pre-run at 120 V for 30 min, followed by a run at 120 V for 4 h at room temperature without glycerol with 1x TBE use as running buffer. Since the conditions of SSCP may influence the separation of different conformers (Spinardi et al. 1991). After electrophoresis gels were silver stained described by Beidler et al. (1982).
Data analysis
Amplified products with reproducible and distinct bands were scored as ‘1’ or ‘0’ depending on its presence or absence. The binary matrix was developed from the silver stained bands from all 22 sugarcane genotypes. gSSRs and EST-SSRs were treated as dominant markers as sugarcane is hybrid species possess multiple incorporation of chromosome sets that pairs and recombine freely. Allelic diversity at a given locus was measured by the Polymorphic Information Content (PIC) of polymorphic primer and it calculated as.
Where, pi 2 is the frequency of the ith allele and the summation extends over n alleles (Mateescu et al. 2005). PIC was used as a tool to measure the information that a given marker locus could provide for the pool of genotypes however, the ability of primer to distinguish between genotypes was assessed by calculating their resolving power (Rp) as Rp = ∑l b . Where, l b is band in formativeness, l b = 1- (2 × │0.5-p i │), where p i is the proportion of genotype containing the band i th (Prevost and Wilkinson 1999). The genetic association among genotypes was evaluated using Jaccard’s similarity coefficient. Similarity matrices were generated using ‘SIMQUAL’ program of NTSYS-pc software 2.02 version (Rohlf 2000). Similarity coefficients produced by binary data matrix were used for cluster analysis of sugarcane genotypes using ‘SHAN’ program and dendrogram was obtained using un-weighted pair-group method with arithmetic average (UPGMA) and test for association was calculated by matrix correlation cophenetic value by MxComp program.
Results
SSCP polymorphism in sugarcane genotypes
Twenty two sugarcane genotypes were analyzed by using microsatellite markers (Table 1 and 2). Electrophoretic analysis of SSR-SSCP markers showed the multiple band profiles for sugarcane genotypes which were determined on denaturating PAGE (Fig. 1). The amplified bands were detected by SSCP procedure was considered as a dominant marker for the genetic evaluation study in elite sugarcane genotypes. The number of total allele scored (nTA), number of polymorphic alleles (nPA), polymorphic information content (PIC) and resolving power (Rp) obtained per SSR markers are shown in Table 2. A total of 138 conformers were analyzed in these genotypes by using 28 SSRs primers of which 99 alleles were found to be polymorphic (72 %) with an average 4.9 allele per locus. However, the average of polymorphic alleles was found to be 3.5 (Table 2). The polymorphic allele range for the amplified locus varied from 1 (VCSSR 6, VNSSR 3, 6,10) to 8 (VCSSR 2) with an average of 4.5, however two gSSR markers VCSSR7, VCSSR12 did not showed any polymorphism among the alleles amplified. Hence, these primers were counted as mono morphic markers with frequency 7.1 % over the average of 3.5 obtained for polymorphic locus. The content of polymorphism (PIC), which provides an estimate of the discriminatory power of marker (0.42) ranged from 0.09 (VCSSR 6) to 0.88 (VCSSR11) with a mean value 0.49. The potential of marker in terms of the resolving power (Rp) to distinguish the morphologically similar selected elite sugarcane genotypes in polymorphism was found to be 1.82. Among the set of polymorphic markers the range of Rp value was found 0.27 and 7.09 for the VCSSR6 and VCSSR8 markers respectively.
Some of the primers produced polymorphic bands that were specific to genotypes with common parentage background and not for the individual genotypes and specific trait, thus they may not have any diagnostic value in varietal identification. Pearson correlation between the observed PIC and Rp was found 0.71 considered to be significant for the present marker system (Fig. 2) analyzed through SSCP methodology.
Genetic similarity and clustering between genotypes
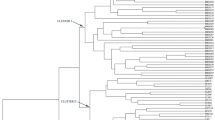
Amplified DNA profiles generated by genomic and EST-SSRs were scored from independent silver stained gels for making identical data sets. The binary matrix data were analyzed for the determination of genetic similarity (GS) and cluster analysis within 22 sugarcane genotypes. Genetic similarity indices were estimated by Jaccards similarity coefficient. The similarity values ranged from 55 to 81 % with mean value of 68 %. The close proximity found in the genetic similarity explained the genetic association among the selected genotypes. The differentiation of sugarcane genotypes were presented in the phenogram according to Jaccard’s similarity coefficient and UPGMA analysis module (Fig. 3). A phenogram were clearly resolved into 3 main clusters contributed high and significant cophenetic value r = 0.91, P = 0.99 and mental t = 4.9 indicating good fit with GS values. However, the principle coordinate analysis (PCA) calculated from the similarity matrix was found 30.42 % of cumulative variation among the genotypes illustrated from the eigen module used for 3D plot (Fig. 4). The total cumulative variation of three axes was distributed as 12.77 %, 9.79 and 7.86 % on X, Y and Z axis respectively.
The number of genotypes in a group were varied from 3 (cluster I) to 13 (cluster II). Cluster I formed by sugarcane genotypes showed 73 % GS in between the CoVSI 5–86 and CoVSI 48–188 which were generated by the VSI institute while, the third MS 68/47 showed 72 % GS with mean of 72.5 %. These genotypes in cluster I pertaining to the low sucrose content but showed resistance to the diseases. Cluster II was largest cluster found in the mentioned phenogram with more or less genetically similar genotypes were grouped, which are preferably used in parentage selection for various breeding program to develop high vigor and stress resistance hybrid varieties. Some of the pair of genotypes showed the strongest genetic similarity with their pedigree and hence it could not be differentiated by the microsatellite SSCP study. Sugarcane genotypes Co 775, Co 80020, Co 97015, Co 92008 and CoC 90063, Co 6806 were more genetically similar in compared with the rest of cultivars. The close association between CoC 90063, Co 6806 genotypes was due to the involvement of the common parentage CoC 671. However, the Co 6806 and Co 62198 found 77 % GS with common female and male parentage as Co 775. However, Co 6907 and Co7704 showed 75 % GS which had a common female parentage Co 740 in their crosses. In cluster III, out of four genotypes three Co 94012, CoC 671 and Co 86032 were raised from major involvement of CoC 671 in their parentage background with 76 % GS. As CoC 671 sugarcane genotype has predominantly use in several Indian sugarcane breeding programs to introgression the high sucrose trait into the hybrid vigor. The CoVSI 9805 genotype was found genetically diverse of 64 % GS compared with the clusters formed by other sugarcane genotypes; moreover it had a poly cross parentage background with involvement of different male parents crossed with common female parent Co 8371. Hence, it was found more diverse among the selected sugarcane genotypes.
Discussion
Single strand conformational polymorphism technique was used to identify the different conformers of amplified DNA fragments by gSSRs and EST-SSRs primers. Polyploidy and aneuploid genome of the sugarcane hybrids is higher as compared to the other crops of Poacae members and it is difficult to dissect the genome and carry out genetic evaluation in present laboratory system at lowest cost. The SSRs markers are ideal molecular marker tool use for the DNA fingerprinting of complex genome of plants like sugarcane and sorghum (Cordeiro et al. 2001). The multi alleles determined through SSCP denaturating PAGE and improved silver staining methodology able to distinguish bands with less than 4 bp difference. By this procedure, sequence characterized amplified region (SCAR) marker can be develop for the specific trait of sugarcane. In account of the sugarcane, there has been less report on the SSCP procedures used for the genetic evaluation of sugarcane DNA by Srivastava et al. (2005, 2011) and Swapna et al. (2011). It also overcomes the problem of agarose based genetic analysis which shows limited polymorphism. Srivastava et al. (2005) analyzed genetic fidelity of micro propagated plants developed from CoLk 9617 to Co 1148 sugarcane varieties by using 5 SSRs. Moreover, the SSR-SSCP determined the genetic diversity among the 14 S. spontaneum germplasm which showed 59.53 % of polymorphism by using 9 sets of SSRs which are lesser than the 72 % polymorphism estimated by the 28 SSRs. The higher rate of polymorphism may due to the presence of saccahrum hybrid cultivars developed as inter specific and inter generic hybrids. The Indian sugarcane clone and the genotypes with the ‘Co’ origin have shown the significant distribution at 50–80 % GS through EST-SSR SSCP technique (Swapna et al. 2011) which shows more similar results with the obtained 55–81 % GS in present study. This could reinforce the utility of SSR-SSCP approach for genetic evaluation of more number of tropical and subtropical sugarcane cultivars of Indian origin.
The results demonstrate that genomic and EST-SSR loci are able to generate sufficient level of polymorphism to ensure their use as molecular markers in sugarcane genetic evaluation. Optimized 28 sets of microsatellite primers amplified 22 sugarcane genotypes that provided significant information about the elite sugarcane cultivars in terms of the number of alleles, PIC and Rp values. The allelic range obtained in several studies of sugarcane had a considerable similarity with the present alleles found (Swapna et al. 2011; Devarumath et al. 2012). The Pearson correlation coefficient between the PIC and Rp values considerably showed the reproducibility of selected marker in SSCP procedure. The higher PIC and Rp value obtained by the primer indicates more in formativeness towards the genotype discrimination and genetic diversity studies. Subsequently, it would be preferred to use such microsatellite markers for DNA fingerprinting of other sugarcane genotypes that has important trait of interest in account of higher sugar, fiber content and resistance to several stresses. Genetic relationship shown by the cluster analysis also reflects similar results obtained by the PCA distribution, which represent the total variation among the sugarcane genotypes. This helps to understand the genetic relatedness between and among the group of genotypes selected for the genetic evaluation study.
The independent genetic evaluation studies conducted on sugarcane found the average range of PIC 0.69 generated by 365 EST-SSRs (Marconi et al. 2011), 0.73 by 342 EST-SSRs (Oliveira et al. 2009) and 0.66 by 30 EST-SSRs (Pinto et al. 2004) on similar 15 varieties and 3 species of sugarcane but there was lack of ‘VSI’ or ‘Co’ varieties involvement in their parentage background. These mentioned PIC found relatively greater than the observed PIC 0.42 it may be due to the less number of EST-SSRs and gSSRs used in genetic evaluation of sugarcane. This is in support of the PIC value 0.23 generated by 30 EST-SSRs used for deciphering the 5 sugarcane cultivars (Cordeiro et al. 2001) which was found nearly similar to the PIC 0.38 value obtained by the 12 EST-SSR markers. This signifies the utility and robust nature of SSRs in the sugarcane genetic evaluation study. While, PIC across the ancestral species and relative genera of sugarcane found to be more than that for the cultivar obtained through successive backcrossing events (Pinto et al. 2006). However, the SSRs data form desirable groups in the phenogram that would shows greater potentiality towards the selection of diverse clone for breeding purpose by virtue of their desirable characters. Some of sugarcane genotypes such as CoC 671, Co 86032, Co 85004, Co 740 are being repeatedly used for the crossing program in India on account of its high sucrose content and the higher yield per hectare (Nair et al. 2002). Cluster I genotypes may be used for the introgression on of resistance traits in spite of the low sugar content. The sucrose accumulation associated enzymes activities in these low sucrose sugarcane genotypes and other high sucrose genotypes showed better findings about the selection of high and low sugarcane representative which used in gene expression analysis (Kalwade et al. 2014). However, POJ 2878 use as progenitor parents in crop improvement program in several commercial breeding programs (Saccharum spp.) in most of the international sugarcane research station (Lu et al. 1994).
The polymorphic variation in amplification determined by SSR markers changes the genetic pattern among the selected genotypes. However, the mono morphic banding pattern revealed the conserved coding nature of genome among the sugarcane. The binding efficiency of gSSRs to the genomic DNA is readily achieved during amplification than the ESTs-SSRs (Cordeiro et al. 2001). Similar type of finding has been noted in present genetic evaluation study through SSCP procedures. Binding efficiency and amplification by the EST-SSR primers are found less to genomic region this may due to a) the intervening intron in between the binding sites of exon, b) lengthening of targeted DNA on genome or c) the alteration of binding site by insertion mutation. This may be the reason of failure of amplification by 17 SSR primers from the set of 45 primers. This may affect the robustness of SSRs in genetic evaluation studies of sugarcane.
Our finding determined the PIC and RP values estimated by the gSSRs are greater than the EST-SSRs. Thus it can be concluded that the rate of finding of polymorphism in genomic region are more as it posses the introns intervening the exons. Moreover, the gSSRs targeted more number of dinucleotide than trinucleotide repeat motifs and the EST-SSRs targeted tri and tetra nucleotide motif repeats among the amplified primers. Beside the use of EST-SSRs in genetic evaluation, it can target the some important quantitative traits like sucrose accumulation and fiber content in sugarcane which revealed the functional co-linearity towards the selection of desirable parentage. However, the PIC obtained in different accession of sugarcane using SSR markers indicates the consistency of SSRs amplification with present study (Pinto et al. 2004, 2006).
The use of SSRs-SSCP was found to be more informative in the complex sugarcane genome analysis, where the classical tools are of limited advantages. However, most of the similar genotypes used in the ISSR marker (Kalwade et al. 2012; Devarumath et al. 2012) and the recent SNP and TRAP marker (Devarumath et al. 2013) found closed genetic similarity and grouping among the genotypes which explained about the integrity and transferability of the polyploid sugarcane genome. The allelic variation reported through the SSCP-SSRs was found more in sugarcane than the high throughput fragment analyzer (Cordeiro et al. 2001; Swapna et al. 2011) which shows significance of SSCP over costlier technique. Multi alleles determined through SSCP showed insertion-deletion changes that are well reported in human (Orita et al. 1989) and other organisms (Orti et al. 1997) genome studies while only few reports are available in case of plants. This technique also found informative in DNA inheritance study of the tree like oak (Bodenes et al. 1996) and extinct species. As a much cost effective alternative, SSCP analysis can thus be routinely used in laboratories with minimum facilities. As the modern hybrid sugarcane are having complex genome which arise due to the involvement of common ancestral parentage and successive hybridization events (Nair et al. 2002; Kalwade et al. 2012; Devarumath et al. 2012) where, SNP detection and novel alleles can be achieved through the SSCP technique.
Thus, the SSRs-SSCP was found to be more useful molecular marker tool for sugarcane genotypes differentiation and genetic evaluation study. Through such low cost molecular marker technique diverse parentage can be selected from the mixed population of sugarcane cultivars. It can also be use as a potential molecular tool for the novel gene findings which would overcome the predominating challenges in sugarcane encompassing to sucrose accumulation, fiber content and resistant trait through the genetic transformations of novel genes to the desirable varieties.
References
Aitken KS, Jackson PA, McIntyre CL (2005) A combination of AFLP and SSR markers provides extensive map coverage and identification of homo (eo) logous linkage groups in a sugarcane cultivar. Theor Appl Genet 110:789–801
Aljanabi SM, Froget L, Dookun A (1999) An improved and rapid protocol for the isolation of polysaccharide and polyphenol free sugarcane DNA. Plant Mol Biol Report 17:1–8
Beidler JR, Hilliard PR, Rill RL (1982) Ultrasensitive staining of nucleic acids with silver. Anal Biochem 126:374–380
Besse P, Taylor G, Carroll B, Berding N, burner D, McIbtyre (1998) Assessing genetic diversity in a sugarcane germplasm collection using an automated AFLP analysis. Genetica 104:143–153
Bodenes C, Laigret F, Kremer A (1996) Inheritance and molecular variations of PCR-SSCP fragments in pedunculate oak (Quercus robur L.). Theor Appl Genet 93:348–354
Cordeiro GM, Taylor GO, Henry RJ (2000) Characterization of microsatellite markers from sugarcane (Saccharum spp.) a highly polyploidy species. Plant Sci 155:161–168
Cordeiro GM, Casu R, McIntyre CL, Manners JM, Henry RJ (2001) Microsatellite markers from sugarcane (Saccharum spp.): ESTs cross-transferable to Erianthus and Sorghum. Plant Sci 160:1115–1123
D’hont A, Grivet L, Feldmann P, Glaszmann JC, Rao S, Berrding N (1996) Characterization of the double genome structure of modern sugarcane cultivars (Saccharum spp.) by molecular cytogenetics. Mol Gen Genet 250:405–413
Devarumath RM, Kalwade SB, Kawar PG, Sushir KV (2012) Assessment of genetic diversity in sugarcane germplasm using ISSR and SSR markers. Sugar Tech 14:334–344
Devarumath RM, Kalwade SB, Bundock P, Eliott FG, Henry R (2013) Independent target region amplification polymorphism and single-nucleotide polymorphism marker utility in genetic evaluation of sugarcane genotypes. Plant Breed 132:736–747
Devarumath RM, Kalwade SB, Kulkarni PA, Devarumath SS, Suprasanna P (2013) OMICS application in crop science. In: Barth D (ed) Integrating Omics approaches in sugarcane improvement. Chapter 7. Taylor & Francis Group, FL: CRC press
Gasser RB, Hu M, Chilton NB, Campbell BE, Jex AJ, Otranto D, Cafarchia C, Beveridge I, Zhu X (2006) Single-strand conformation polymorphism (SSCP) for the analysis of genetic variation. Nat Protoc 1(6):3121–3128
Hayashi K (1992) PCR-SSCP: a method for detection of mutations. Genet Anal Tech Appl 3:73–79
Jarne P, Lagoda PJL (1996) Microsatellites, from molecules to populations and back. Trends Mol Ecol 11:424–429
Kalwade SB, Devarumath RM (2014) Functional analysis of the potential enzymes involved in sugar modulation in high and low sugarcane cultivars. Appl Biochem Biotechnol 172:1982–1998
Kalwade SB, Kawar PG, Devarumath RM, Sushir KV (2012) Genetic profiling of sugarcane by using ISSR molecular marker. Electronic J of Plant Breeding 3:621–628
Lu YH, D’Hont A, Walker DJT, Rao PS, Feldmann P, Glaszmann JC (1994) Relationship among ancestral species of sugarcane revealed with RFLP using single copy maize nuclear probes. Euphytica 78:7–18
Marconi TG, Costa EA, Miranda H, Mancini MC, Cardoso-Silva CB, Oliveira KM, Pinto LR, Mollinari M, Garcia A, Sousa AP (2011) Functional markers for gene mapping and genetic diversity studies in sugarcane. BMC Research Notes 4:264
Mateescu RG, Zhang Z, Tgai K, Phavaphutanon J, Wursten NI, Lust G, Quaas R, Murphy K, Acland GM, Todhunter RJ (2005) Analysis of allele fidelity, polymorphic information content, and density of microsatellites in a genome-wide screening for Hip Dysplasia in crossbreed pedigree. J Hered 96:847–853
Nair NV, Selvi A, Sreenivasan TV, Pushpalatha (2002) Molecular diversity in Indian sugarcane cultivars as revealed by randomly amplified DNA polymorphisms. Euphytica 127:219–255
Oliveira KM, Pinto LR, Marconi TG, Mollinari M, Ulian EC, Chabregas SM, Falco MC, Burniquist W, Garcia AAF, Souza AP (2009) Characterization of new polymorphic functional markers for sugarcane. Genome 52:191–209
Orita M, Iwahana H, Kanzawa H, Hayashi K, Sekiya T (1989) Detection of human DNA by gel electrophoresis as single strand conformation polymorphisms. Proc Natl Acad Sci U S A 86:2766–2770
Orti G, Pearse DE, Avise JC (1997) Phylogenetic assessment of length variation at a microsatellite locus. Proc Natl Acad Sci U S A 94:10745–10749
Pan YB, Burner DM, Legendre BL (2000) An assessment of the phylogenetic relationship among sugarcane and related taxa based on the nucleotide sequence of 5S rRNA intergenic spacers. Genetica 108:285–295
Parida SK, Pandit A, Gaikwad K, Sharma TR, Srivastava PS, Singh NK, Mohapatra T (2010) Functionally relevant microsatellite in sugarcane unigenes. BMC Plant Biology 10:251
Pinto LR, Oliveira KM, Ulian EC, Garcia AAF, de Souza AP (2004) Survey in the sugarcane expressed sequence tag database (SUCEST) for simple sequence repeats. Genome 47:795–804
Pinto LR, Oliveira KM, Marconi T, Garcia AAF, de Souza AP (2006) Characterization of novel sugarcane expressed sequence tag microsatellites and their comparison with genomic SSRs. Plant Breed 125:378–384
Piperidis G, Piperidis N, D’Hont A (2010) Molecular cytogenetics investigation of chromosome composition and transmission in sugarcane. Mol Genet Genomics 284:65–73
Prevost A, Wilkinson MJ (1999) A new system of comparing primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98:107–11
Rohlf FJ (2000) NTSYSpc numerical taxonomy and multivariate analysis system. Exeter Software, New York
Selvi A, Nair NV, Noyer JL (2005) Genomic constitution and genetic relationship among the tropical and subtropical Indian sugarcane cultivars revealed by AFLP. Crop Sci 45:1750–1757
Singh RK, Singh P, Misra P, Singh SP, Singh SB (2005) STMS marker for tagging high sugar genes in sugarcane. Sugar Tech 7:74–76
Singh RK, Singh P, Singh SP, Mohapatra T, Singh SB (2006) Mapping QTLs for sugar content and segregation analysis in sugarcane. Sugar Cane International 24:7–13
Spinardi L, Mazars R, Theillet C (1991) Protocols for an improved detection of point mutation by SSCP. Nucleic Acid Res 19:4009
Sreenivasan TV, Ahloowalia BS, Heinz DJ (1987) Cytogenetics. In: Heinz DJ (ed) Sugarcane improvement through breeding. Elsevier, New York, pp 211–253
Srivastava S, Jain R, Gupta PS, Singh J (2005) Analysis of genetic fidelity in micro propagated plants of sugarcane using SSR-SSCP assay. Indian J Genet 65:327–328
Srivastava S, Gupta PS, Singh PK, Singh J, Jain R (2011) Genetic diversity analysis of Saccharum spontaneum germplasm using SSR-SSCP and RAPD markers. Indian J Agri Sci 81:914–920
Swapna M, Srivastava S (2012) Molecular marker applications for improving sugar content in sugarcane. Springer Briefs Plant Sci. doi:10.1007/978-1-4614-2257-0_1
Swapna M, Sivaraju K, Sharma RK, Singh NK, Mohapatra T (2011) Single-strand conformational polymorphism of EST-SSRs: a potential tool for diversity analysis and varietal identification in sugarcane. Plant Mol Biol Rep 29:505–513
Vettore AL, da Silva FR, Kemper EL, Souza GM, da Silva AM (2003) Analysis and functional annotation of an expressed sequence tag collection for tropical crop sugarcane. Genome Res 13:2725–2735
Yip SP, Hopkinson DA, Whitehouse DB (1999) Improvement of resolution of asymmetric-PCR SSCP products. Biotechniques 22:606–608
Acknowledgments
Authors gratefully acknowledge Dr. A. D’Hont CIRAD, France CIRAD France for providing the microsatellite primers sequence information, thankful to the Shri. Shivajirao Deshmukh, Director General, Vasantdada Sugar Institute (VSI), Pune for their constant support during research work and Plant Breeding section for providing sugarcane genotypes leaf samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalwade, S.B., Devarumath, R.M. Single strand conformation polymorphism of genomic and EST-SSRs marker and its utility in genetic evaluation of sugarcane. Physiol Mol Biol Plants 20, 313–321 (2014). https://doi.org/10.1007/s12298-014-0231-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-014-0231-9