Abstract
Assessment of the extent of genetic variability within chickpea is fundamental for chickpea breeding and conservation of genetic resources and is particularly useful as a general guide in the choice of parents for breeding hybrids. To establish genetic diversity among 60 accessions of chickpea comprising landraces, internationally developed improved lines, and cultivars, genetic distances were evaluated using 14 simple sequence repeat markers. These markers showed a high level of polymorphism; a total of 59 different alleles were detected, with a mean of 4.2 alleles per locus. The polymorphic information content (PIC) value ranged from 0.31 to 0.89. All the markers, with the exception of TAA170, TA110, GA34, and Ts35, were considered to be informative (PIC > 0.5), indicating their potential usefulness for cultivar identification. Based on the UNJ clustering method, all accessions were clustered in five groups, which indicated the probable origin and region similarity of Iranian landraces over the other cultivars. It also represents a wide diversity among available germplasm. The result has firmly established that introduction of genetic materials from exotic sources has broadened the genetic base of the national chickpea breeding program. As further implications of the findings, this study can be useful for selective breeding for specific traits and in enhancing the genetic base of breeding programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chickpea is an important self-pollinated grain legume crop, grown mainly in West Asia, North Africa, and the Indian subcontinent, where it is a basic component of the human diet. Vavilov (1926) was the first to identify areas with similar physiographic characteristics with maximum variability for the major cultivated species. Chickpea is a highly valued commodity and an important source of proteins for human consumption in several developing countries; it is classified among the high-protein seeds with a mean protein content of 22 %. The region in the present day southeastern Turkey and the adjoining areas of Syria and northeast of Iran has been proposed to be the Vavilovian center of origin for the cultivated chickpea (Cicer arietinum L.; van der Maesen 1987; Talebi et al. 2008b). Iran, as the secondary center of genetic diversity for some food legumes in general (Mekibeb et al. 1991) and chickpea in particular (Talebi et al. 2008b), possesses a large number of chickpea germplasm collections from different geographical regions (Naghavi and Jahansouz 2005). For effective utilization of these germplasm collections in breeding programs, genetic characterization in terms of measure of the extent and pattern of genetic diversity within and between populations is essential (Carvalho 2004; Rubenstein et al. 2005) not only to unveil the magnitude of genetic diversity available in the germplasm for conservation purposes but also to determine genes useful for possible progress in future breeding programs. Nowadays in Iran, chickpea cultivation is mainly based on registered varieties or genetic materials selected by some constitutors (such as universities and national plant research stations), for improving agronomic and nutritional traits. Nevertheless, in some areas, landraces, which usually take their name from the location where they are traditionally cultivated, are still used. Some of these landraces are much appreciated but, they generally survive on farms in marginal areas, being therefore exposed to a strong risk of genetic erosion or extinction (Jannatabadi et al. 2013). Landraces or traditional cultivars are locally adapted diverse populations, which are the results of natural selection and farmers cultivation methods. They have contributed with genetic material to many breeding programs and constitute important plant genetic resources. Genetic characterization can be made by different methods ranging from the conventional methods like the use of descriptor lists of morphological characters and biochemical and molecular methods (Carvalho 2004; de Vicente et al. 2005), all with their own comparative advantages and disadvantages. However, it is generally believed that the use of molecular markers is more reliable and repeatable as compared to characterization based on morphological characters (Carvalho 2004). For chickpea, various marker systems such as amplified fragment length polymorphism (AFLP), internal transcribed spacer (ITS), restriction fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), and microsatellite markers such as simple-sequenced repeats (SSR) or sequenced tagged microsatellite sites (STMS) have been used for diversity analysis (Iruela et al. 2002; Nguyen et al. 2004; Talebi et al. 2008a, b; Singh et al. 2008; Jannatabadi et al. 2013). Despite the large number of chickpea germplasm collections held in Iran, most of them have not been characterized at either morphological or molecular levels (Naghavi and Jahansouz 2005; Saeed et al. 2011). From a few morphological, AFLP, and RAPD marker-based studies in Iran, it has been reported that there exists high morphological but low biochemical and molecular diversity (Talebi et al. 2008a, b; Saeed et al. 2011). Another study was also conducted based on SSR markers but was limited to a few Iranian landrace accessions and additional varieties and wild spp., from other countries (Saeed et al. 2011). However, the results could hardly be inferred for Iranian landrace and exotic germplasm accessions because of less representation in terms of both number and geographical coverage of accessions. This study was, therefore, designed to assess genetic diversity of Iranian chickpea germplasm accessions at both morphological and molecular level using SSR markers and, thereby, establish if there is any definite relationship between genetic diversity and geographical sources of origin.
Materials and methods
Sixty chickpea (C. arietinum L.) accessions comprised of 29 landrace accessions from different geographical location of Iran and 31 improved cultivars (Table 1) provided by Seed and Plant Improvement Institute, Karaj, Iran, were considered for the study of genetic variation both at morphological and molecular level. Field experiments were laid out in randomized complete block design (RCBD) with three replications in 2011. Seeds were hand-drilled and each plot consisted of a three rows of 3 m with spacing of 0.3 m between rows and 10 cm between plants within a row. Six plants were randomly chosen from each plot to measure the number of seeds per plant (SP), plant height (PH), 1,000-seed weight (TSW), plant biomass (BIO), seed yield (g/plant), and consequently harvest index (HI). Principal component analysis (PCA) was performed for seven traits. Subtracting from each observation the mean value of the trait, and subsequently dividing by its respective standard deviation, gave standardized observations of traits with an average of 0 and standard deviation of 1 or less. These standardized values were used to perform the PCA using GENSTAT 6.1. Genotype scores (eigenvectors) of the three principle components (PCs) (data not shown) that had eigenvalues greater than unity were subjected to cluster analysis using DARwin version 5.0 and unrooted neighbor-joining (NJ) method as the clustering algorithm (Perrier et al. 2003).
Total genomic DNA was extracted from a pool of six plants of each accession following a CTAB extraction protocol (Lassner et al. 1989). A total of 20 microsatellite markers were screened in the accessions of which 14 were polymorphic (Table 2). The SSR markers were synthesized as per the sequences of Winter et al. (2000) from Cinagene, Iran. PCR reactions were composed as described previously by Jannatabadi et al. (2013) and PCR reactions were performed in a volume of 20 μl in Eppendorf thermocyclers. PCR products were analyzed using 3 % MethaPhor agarose electrophoresis gels stained with ethidium bromide. Frequencies of incidence of all polymorphic alleles for each SSR marker were calculated and used for determining statistical parameters. Confusion probability (Cj) and discriminating power (Dj) of each SSR were estimated according to Tessier et al. (1999) and polymorphic information content (PIC) following Botstein et al. (1980). Alleles were numbered as “a1,” “a2,” etc., sequentially from the largest to the smallest sized band. No distinction was made between amplified products of varied intensity, when the amplified products were within the expected size range. Reconfirmation of null allele was done and the bands which were appearing as artifact or bands which were either diffused or highly faint and null alleles even after reconfirmation were not considered for analysis. Tree construction following unrooted NJ tree using similarity matrix was carried through DARwin 5.0.128 (Perrier et al. 2003) analysis. Bootstrap analysis using 1,000 bootstrap values was performed for the node construction.
In both populations (landrace and improved cultivars), number of allele (Na), effective number of alleles (Ne), gene diversity (He), and PIC were calculated by GENALEX 6.1 software (Peakall and Smouse 2006).
Results
Diversity pattern of morphological traits
In this study, results of analysis of variance and descriptive statistics indicated that the accessions differed significantly for all the traits revealing the presence of a considerable diversity, which could be utilized in developing high-yielding cultivars through selection breeding (Tables 3 and 4). The results of variance analysis of seven morphological traits showed significant differences among the examined accessions indicating the presence of variability which can be exploited through selection (Table 3). According to ANOVA (Table 3), the effect of “replication” was significant for all measured morphological traits. The variability of accessions in different replications in field experimental design may be reflected to the nature of landrace accessions that were affected by various factors in their locations. Selections made by farmers according to aims of use may be the reason for the genetic variability between the landraces across locations. This might also be the main reason for genetic differences between the replications among collected genetic materials. For each of the traits evaluated, the descriptive statistics including the extreme accession mean values along with the corresponding accessions, the means, median, and variance with their coefficient of variation (CV) obtained on the basis of the averages of data are summarized in Table 4. Among traits, grain yield (g/plant) ranged from 4.12 to 22 with a mean value of 10.11 g/plant. High differences between the maximum and minimum mean values were found for all other traits. The heritability estimates were high (>60 %) for character plant height, number of seeds per plant, number of primary branches, and 100-seed weight (Table 4). A dendrogram was constructed from the standardized value of morphological traits. The accessions were grouped into five clusters at an average Euclidian distance value of 4.89 (Fig. 1). The first cluster contains accessions from different geographical regions; these accessions were late maturity and higher grain yield compared to others. The second cluster contains ten accessions with moderate yield and early flowering date. The third cluster contains ten accessions originated from distant regions and compared to clusters I and II, these accessions showed significant lower yield and biomass production. The fourth cluster contains five accessions and same to other clusters; these accessions originated from different parts of Iran. The fifth cluster contained eight accessions that most of them (except two) originated from International Center for Agricultural Research in the Dry Areas (ICARDA) (Fig 1). We found that there was no relationship between morphological divergence and geographical origins because accessions from one place entered into more than one cluster. Conversely, accessions from different geographical origins were relatively unique and tend to be clustered in one part of the dendrogram, which information suggests that the morphological variation analyzed is determined not only by environmental differences but also by genetic factors.
Allelic variation of SSRs and diversity pattern
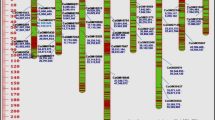
In the present study, a total of 25 SSR loci were analyzed, covering various bin locations on different linkage group. Fourteen SSR loci in the genetic material under study were found to be highly polymorphic (Table 5). The 14 STMS loci analyzed produced 59 alleles with an average 4.2 alleles per marker. The number of alleles ranged from 3 to 6, whereas the maximum was observed in TA96. Of the 59 alleles detected in these accessions, 10 were classified as “rare” because of their low frequency (<0.03), 26 as “common” (0.03–0.20), and 23 were classified the “most frequent” alleles (>0.20) (Table 5). Rare alleles per locus was one at all SSR loci analyzed except at TA159, TA89, and GA34, which showed non rare alleles. High values of discriminating power (Dj ≥ 0.66) and low values of confusion probability (Cj ≤ 0.30) were obtained for all SSR markers except for GA20 and TA110 (Table 5). Most of the polymorphic marker in the present study was TA96 with a PIC value of 0.89 and six alleles identified. The high Dj values indicate the lack of close genetic relatedness in the accessions analyzed, and the greater PIC values indicate the suitability of the microsatellites used for diversity analysis (Fig 2). The genetic similarity matrix was further analyzed using unweighted neighbor-joining (UNJ) clustering algorithm by software program DARwin 5.0. The radial branching clearly delineated the accessions in five major clusters (Fig 3). Cluster I contained 18 accessions, which six of them are Iranian landrace accessions and were collected from central and south regions of Iran. Cluster II contained six accessions which four of them were landrace accessions that originated from central and south region of Iran. Cluster III contained 15 accessions, which mostly are landrace accessions that originated from north and northwest of Iran. Clusters IV and V contained 13 and five accessions, respectively (Fig 3). All the genotypes that grouped into these clusters were cultivated accessions that mainly originated from ICARDA. To explore genetic diversity among the accessions within each of the groups of landrace and cultivated chickpea, four parameters of genetic diversity were also calculated separately for each group (Table 6). A total of 52 alleles were observed in the landrace group, ranging from two to six, with an average of 3.71 alleles per locus, and 43 alleles were observed in the cultivated group, which ranged from two to five, with an average of 3.07. The mean values of number of effective alleles, gene diversity, and PIC for landrace accessions were 2.21, 0.37, and 0.54, respectively, which were higher than the group of cultivated germplasm (1.68, 0.29, and 0.45, respectively).
Discussion
Evaluation of genetic diversity and an understanding of the genetic relationships in the germplasm collection are needed to be able to adopt effective conservation and management strategies and also facilitate the thorough utility of these genetic resources in crop improvement programs. World chickpea germplam has a narrow genetic base (Nguyen et al. 2004) and lacks the desirable traits needed for ready utilization in varietal improvement programs. We found relatively acceptable genetic diversity within available Iranian landrace chickpea accessions which is contrary to the findings of Iruela et al. (2002), who reported low level of genetic diversity within C. arietinum compared to the wild species. The selection of accessions for this study is primarily based on different geographical origins. Therefore, we believe that there is need of molecular marker studies as a complementary studies for the morphological traits in the field. It will reduce the amount of materials for study as well as the costs of experiments. The relationship observed using molecular markers may provide information on the history and biology of genotypes, but it does not necessarily reflect what may be observed with respect to agronomic traits (Métais et al. 2000). In this study, we evaluated 14 SSR markers in 60 chickpea accessions representing popular cultivated chickpea cultivars and landrace accessions from different geographical zones of Iran. The SSR analysis showed considerable genetic diversity, detecting a total of 59 alleles with an average of 4.2 alleles per locus and an average PIC of 0.57, which ranged from 0.31 to 0.89. Among the two groups included in this study, higher genetic variability was found in the group of landrace accessions with higher values for the diversity parameters (allele number, PIC, and gene diversity) than cultivated chickpea. These results are in accordance with other studies, which concluded that a very low level of genetic variation exists in cultivated chickpea (Choudhary et al. 2012; Keneni et al. 2011). The magnitude and pattern of genetic variation detected in this study can be useful for more systematic germplasm management and utilization in breeding programs (Tanya et al. 2011). The exploitation of crosses between genetically distant parents (e.g., recombinants parents from the landrace accessions and the introduced cultivated accessions) and those from diverse local sources may produce higher heterosis, better genetic recombination, and segregation in their progenies and result in varieties with broad genetic base (Chahal and Gosal 2002). Future germplasm collection and utilization strategies should take into consideration the magnitude and pattern of genetic diversity established by the present investigation.
The lower genetic diversity in cultivar chickpea in comparison to landrace accessions reported in this study supports the conclusion that chickpea has a narrow genetic base (Nguyen et al. 2004). These results indicate that despite extensive breeding efforts, the varieties under current cultivation are closely related among themselves. This is probably due to the use of few key varieties for hybridization. This explains why yield improvement and increased tolerance to various biotic and abiotic stresses have been slow in chickpea. Hence, it is imperative to broaden the genetic base of the cultivated chickpea to increase the yield and reduce its vulnerability to diseases and insect pests by introducing traits from across the ecologically adapted landrace genotypes of the primary gene pool. Landrace chickpea germplasm represent a potential source of new alleles for improving yield, quality, and stress resistance in cultivated chickpea (Singh et al. 2008; Jannatabadi et al. 2013). In conclusion, the present study revealed significant diversity and relationships and provided information on landrace and cultivar chickpea populations. The genetic diversity evaluated can provide the basis for future chickpea crop variety identification, conservation, and management. This investigation also proved the efficiency and effectiveness of SSR markers to unravel methodological limitation in DNA fingerprinting and study of genetic diversity in chickpea as denoted by others (Sefera et al. 2011).
References
Botstein D, With RL, Skolnick M, Davis RW (1980) Construction of genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32:314–331
Carvalho MA (2004) Germplasm characterization of Arachis pintoi Krap. and Greg. (Leguminosae). PhD Thesis, University of Florida, USA
Chahal GS, Gosal SS (2002) Principles and procedures of plant breeding: biotechnological and conventional approaches. Narosa, New Delhi
Choudhary P, Khanna SM, Jain PK, Bharadwaj C, Kumar J, Lakhera PC, Srinivasan R (2012) Genetic structure and diversity analysis of the primary gene pool of chickpea using SSR markers. Genet Mol Res 11(2):891–905
de Vicente MC, Guzmán FA, Engels J, Rao VR (2005) Genetic characterization and its use in decision making for the conservation of crop germplasm. The role of biotechnology. In: International workshop the role of biotechnology for the characterization and conservation of crop, forestry, animal and fishery genetic resources. FAO, Fobiotech, Ecogene, Siga, Turin, pp 121–128, 5–7 March 2005
Iruela M, Rubio J, Cubero JI, Gil J, Milan T (2002) Phylogenetic analysis in the genus Cicer and cultivated chickpea using RAPD and ISSR markers. Theor Appl Genet 104:643–651
Jannatabadi AA, Talebi R, Armin M, Jamalabadi J, Baghebani N (2013) Genetic diversity of Iranian landrace chickpea (Cicer arietinum L.) accessions from different geographical origins as revealed by morphological and sequence tagged microsatellite markers. J Plant Biochem Biotechnol. doi:10.1007/s13562-013-0206-x
Keneni G, Bekele E, Imtiaz M, Dagne K, Getu E, Assefa F (2011) Genetic diversity and population structure of Ethiopian chickpea (Cicer arietinum L.) germplasm accessions from different geographical origins as revealed by microsatellite markers. Plant Mol Biol Report 30:654–665
Lassner MW, Peterson P, Yoder JI (1989) Simultaneous amplification of multiple DNA fragments by polymerase chain reaction in the analysis of transgenic plants and their progeny. Plant Mol Biol Report 7:116–128
Mekibeb H, Demissie A, Tullu A (1991) Pulse crops of Ethiopia. In: Engels JMM, Hawkes JG, Worede M (eds) Plant genetic resources of Ethiopia. Cambridge University Press, UK, pp 328–343
Métais I, Aubry C, Hamon B, Jalouzot R (2000) Description and analysis of genetic diversity between commercial bean lines (Phaseolus vulgaris L.). Theor Appl Genet 101:1207–1214
Naghavi MR, Jahansouz MR (2005) Variation in the agronomic and morphological traits of Iranian chickpea accessions. J Integ Plant Biol 47:375–379
Nguyen TT, Taylor PW, Redden RJ, Ford R (2004) Genetic diversity estimates in Cicer using AFLP analysis. Plant Breed 123:173–179
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Perrier X, Flori A, Bonnot F (2003) Data analysis methods. In: Hamon P, Seguin M, Perrier X, Glaszmann JC (eds) Genetic diversity of cultivated tropical plants. Science Publishers, Enfield, Montpellier, pp 43–76
Rubenstein DK, Heisey P, Shoemaker R, Sullivan J, Frisvold G (2005) Crop genetic resources: an economic appraisal. United States Department of Agriculture (USDA). Economic Information Bulletin No. 2. (www.ers.usda.gov)
Saeed A, Hovsepyan H, Darvishzadeh R, Imtiaz M, Panguluri SK, Nazaryan R (2011) Genetic diversity of Iranian accessions, improved lines of chickpea (Cicer arietinum L.) and their wild relatives by using simple sequence repeats. Plant Mol Biol Report 29:848–858
Sefera T, Abebie B, Gaur PM, Assefa K, Varshney RK (2011) Characterisation and genetic diversity analysis of selected chickpea cultivars of nine countries using simple sequence repeat (SSR) markers. Crop Pasture Sci 62:177–187
Singh R, Singhal V, Randhawa GJ (2008) Molecular analysis of chickpea (Cicer arietinum L.) cultivars using AFLP and STMS markers. J Plant Biochem Biotechnol 17:167–171
Talebi R, Fayaz R, Mardi M, Pirsyedi SM, Naji AM (2008a) Genetic relationships among chickpea (Cicer arietinum) elite lines based on RAPD and agronomic markers. Int J Agric Biol 8:1560–8530
Talebi R, Naji AM, Fayaz F (2008b) Geographical patterns of genetic diversity in cultivated chickpea (Cicer arietinum L.) characterized by amplified fragment length polymorphism. Plant Soil Environ 54:447–452
Tanya P, Taeprayoon P, Hadkam Y, Srinives P (2011) Genetic diversity among Jatropha and Jatropha-related species based on ISSR markers. Plant Mol Biol Report 29:252–264
Tessier C, David J, This P, Boursiquot JM, Charrier A (1999) Optimization of the choice of molecular markers for varietal identification in Vitis vinifera L. Theor Appl Genet 98:171–177
Van Der Maesen LJG (1987) Origin, history and taxonomy of chickpea. In: Saxena MC, Singh K (eds) The Chickpea. CAB Inter, Wallingford, pp 11–34
Vavilov NI (1926) Studies on the origin of cultivated plants. Inst Appl Bot Plant Breed, Leningrad
Winter P, Benko-Iseppon AM, Hüttel B, Ratnaparkhe M, Tullu A, Sonnante G, Pfaf T, Tekeoglu M, Santra D, Sant VJ, Rajesh PN, Kahl G, Muehlbauer FJ (2000) A linkage map of chickpea (Cicer arietinum L.) genome based on recombinant inbred lines from a C. arietinum × C. reticulatum cross: localization of resistance genes for Fusarium wilt races 4 and 5. Theor Appl Genet 101:1155–1163
Acknowledgments
We wish to present our special thanks to International Centre for Agricultural Research in the Dry Areas (ICARDA), Aleppo, Syria and Seed and Plant Improvement Institute (SPII) of Iran, Karaj, for kindly supplying seeds of chickpea accessions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaffari, P., Talebi, R. & Keshavarzi, F. Genetic diversity and geographical differentiation of Iranian landrace, cultivars, and exotic chickpea lines as revealed by morphological and microsatellite markers. Physiol Mol Biol Plants 20, 225–233 (2014). https://doi.org/10.1007/s12298-014-0223-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-014-0223-9