Abstract
Preeclampsia is a multisystem disorder involves altered homeostasis of oxidants–antioxidants, inflammatory process and endothelial dysfunction. The present study aim was to determine the levels of oxidative stress parameters (malondialdehyde, protein carbonyl, ischemia modified albumin and xanthine oxidase), nutrient antioxidants (vitamin C and vitamin E), enzyme antioxidants (catalase, superoxide dismutase, glutathione peroxidase glutathione reductase), total antioxidant status (TAS) and its association with nitric oxide. The study population consists of three groups, non pregnants (Group 1, n = 57), normotensive pregnants (Group 2, n = 57) and Preeclampsia (Group 3, n = 57). Group 2 and 3 were followed after delivery within 48 h. In preeclampsia xanthine oxidase, malondialdehyde and uric acid levels were significantly increased (p < 0.001), while TAS decreased (p < 0.05) when compared to normotensive pregnant and non pregnant. Catalase, glutathione reductase levels were increased (p < 0.005) and vitamin E, super oxide dismutase levels were decreased (p < 0.001) in preeclampsia when compared to normal pregnants. Receiver operating characteristics curve analysis showed area under curve for xanthine oxidase (0.8), malondialdehyde (0.804), Uric acid (0.84), ischemia modified albumin (0.92) and catalase (0.88) which indicated as good markers in preeclampsia. Amongst, ischemia modified albumin is a better marker of intrauterine hypoxic reperfusion risk with sensitivity 87.7 % and specificity 91.2 %. The increased hydrogen peroxide from xanthine oxidase adds to oxidative stress and increased catalase activity in preeclampsia represents combating action. Increased oxidative stress, decreased TAS and its apparent reversible changes evinced within 48 h after delivery in preeclampsia illustrated that placental abnormality is the contributing factor in the pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia is an obstetric problem associated with hypertension, proteinuria and edema after 20 weeks of pregnancy. The symptoms include persistent headache, blurred vision, Vomiting and abdominal pain. The complications of preeclampsia may result in fetal uterine growth restriction, preterm delivery, maternal and fetal morbidity and mortality [1]. In the developing and developed countries, approximately eight hundred women die from pregnancy and child birth related complications every day. However, from this data nearly 10–25 % of preeclampsia cases results in maternal death [2].
The complications of Preeclampsia decreases after delivery. Although, its exact mechanism is unknown, it has been suggested that free radicals generated during oxidative stress implicated in promotion of maternal vascular malfunction by affecting endothelial cells. Oxidative stress is prominent when the balance between the reactive oxygen species (ROS) overrides the antioxidant capacity of the target cell. Thus, altered equilibrium leads to tissue injury and damages cellular biomolecules. Therefore, a homeostasis between oxidants and antioxidants is crucial in health and disease [3].
Membrane lipid peroxidation markers, protein markers and markers denoting nucleic acid damage are increased in plasma of preeclamptic women. Defensive role of nutrient antioxidants such as carotenoids, vitamin E, L-ascorbic acid and enzyme antioxidants status minimize oxidative damage by virtue of their capacity to scavenge free radicals generated during cellular metabolic process [4]. Karacy et al. [5] reported the oxidative stress in terms of malondialdehyde (MDA) and antioxidants in preeclampsia. In our previous study we reported that xanthine oxidase (XO) can be considered as a good marker for preeclampsia but it was not statistically assessed and compared with other oxidative stress parameters. And also activity was not evaluated in the non pregnant basal group. As an extension of this previous research work we measured xanthine oxidase activity compared with other oxidative stress parameters and antioxidants in large sample size. The possibility of considering xanthine oxidase as an enzyme marker of oxidative stress along with other oxidative stress markers in preeclampsia in pre and post-delivery has become the requisite [6]. Therefore, the present study was conducted to evaluate xanthine oxidase (XO) as a proxidant enzyme and also, ischemia modified albumin (IMA) as a hypoxic risk factor in preeclampsia with its clinical importance by comparing with other known oxidative stress markers. Similarly, serum catalase as an antioxidant enzyme with other antioxidants in preeclampsia during pre and post-delivery conditions within 48 h needs to be elucidated. This research gap necessitates a reason for this study.
Materials and Methods
The study was conducted by the Department of Biochemistry in collaboration with the Department of Obstetrics and Gynecology R.L. Jalappa Hospital and Research Center, Kolar, India after obtaining Institutional Ethical Committee approval. The enrollment of the study population was commenced after obtaining individual Informed Consent. The study was conducted between August 2013 to December 2015.
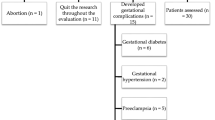
The study population was divided into 3 groups. Group 1 (n = 57) comprising of non-pregnants as control population; group 2 (n = 57) as normotensive pregnants and group 3 (n = 57) as preeclampsia cases. G2 and G3 were in 30–39 weeks of gestation before delivery and same subjects followed after delivery within 48 h. G2 and G3 subjects were clinically diagnosed from Obstetrics and Gynecology. The age matched control populations (G1) were randomly recruited from the healthy volunteers of Sri Devaraj Urs Medical College and were in 20–30 years.
Pre-eclampsia was diagnosed based on the criteria of National High Blood Pressure Education Programme working group involving blood pressure ≥140/90 mm Hg and proteinuria (≥300 mg/24 h) after 20 weeks of gestation.
Inclusion criteria were singleton pregnancy, no fetal anatomical anomaly; nonsmokers were included in the study. Exclusion criteria were patient with any history of renal disease, thyroid disorder, chronic hypertension, gestational diabetes, epilepsy, and hypertensive encephalopathy and cardio vascular disease.
Four mL of fasting blood sample were collected from subjects under the study groups using appropriate vacutainer under aseptic condition. Samples for vitamin E assay were centrifuged, clear plasma obtained was transferred to screw capped vials wrapped with aluminum foil in order to minimize the exposure to light and prevent vitamin E loss. The samples were stored frozen at −80 °C until analysis.
Fine chemicals like xanthine, xanthine oxidase, glutathione peroxidase, glutathione reductase, Nitro blue tetrazolium, Phenazenemethosulphate, Sulphanilamide, NADH, Glutathione (reduced and oxidized), NADPH, Vitamin E, Ascorbic acid, metaphosphoric acid were obtained from Sigma Aldrich. All other reagents used were of analytical grade.
Methods
Malondialdehyde reacts with thiobarbituric acid to form a pink colored complex; absorbance was measured at 535 nm spectrophotometrically [7].
Protein carbonyl reacts with 2, 4, di-nitro phenyl hydrazine (DNPH) forming a Schiff base to produce yellow hydrazine. The absorbance was measured spectrophotometrically at 370 nm [8].
Xanthine oxidase catalyzes the conversion of xanthine to uric acid and hydrogen peroxide. The increase in absorbance (∆A) was measured at 290 nm against control [9].
The concentration of ischemia modified albumin was determined by addition of a known amount of cobalt (II) to a serum specimen and measured unbound cobalt (II) by colorimetric assay using dithiothreitol. The absorbance of the intensity of the color produced measured against control at 470 nm [10].
Uric acid is converted by uricase to allantoin and hydrogen peroxide, which under the catalytic influence of peroxidases, oxidizes 3, 5-dichloro-2 hydroxy benzene sulphonic acid and 4 amino phenazone to form red violet quinine imine absorbance measured at 505 nm spectrophotometric ally [11].
Nitric oxide (NO) in plasma measured by the reduction of nitrate into nitrite by copper coated cadmium granules as a reducing agent using sodium nitrite as standard (NaNO2). The nitrite produced is estimated by diazotization of Sulfanilamide in acidic medium and then coupling with Napthyl ethylene Diamine to produce pink colored compound. The absorbance was measured spectrophotometrically at 540 nm [12]. Ferric Reducing Ability of Plasma (FRAP) reduces ferric ions to ferrous ion at low pH. The absorbance of violet colored ferrous tripyridyltriazine complex measured at 593 nm [13].
Superoxide dismutase (SOD) is measured based on the inhibition of the formation of Phenazenemethosulphate-Nitro blue tetrazoliumformazon complex. The color formed at the end of the reaction can be extracted into butanol and measured at 560 nm [14].
Glutathione peroxidase (GPx) catalyzes the oxidation of reduced glutathione by hydrogen peroxide. In the presence of glutathione reductase and NADPH, the oxidized glutathione is converted to the reduced form with simultaneous oxidation of NADPH measured as decrease in absorbance at 340 nm [15].
Catalase rapidly breaks down hydrogen peroxide leading to decrease in absorbance. A difference in the absorbance at 240 nm per minute is measure of catalase activity [16].
Plasma ascorbic acid oxidized by Cu2+ to form dehydroascorbic acid which reacts with 2, 4, DNPH to form a red color bis-hydrazone. The absorbance is measured at 520 nm [17].
Vitamin E measurement based on Emmerie-Engel reaction, which is based on reduction of ferric iron to ferrous iron by tocopherols which then forms red colored complex with α, α1-dipyridyl. Tocopherols and carotenes were first extracted into petroleum ether and absorbance was read at 460 nm to measure the carotenes. A correction was made for this after adding ferric chloride and read at 520 nm [18].
Statistical Analysis
The results were expressed as mean ± standard deviation and analysed using one way ANOVA test with post hoc Bonferroni analysis to compare the values between the three groups. Pearson correlation analysis was used to find the correlation between the various parameters. Receiver operating characteristics (ROC) curve analysis was done to assess diagnostic utility of a parameter in the study. A probability p value of <0.05 was considered as statistically significant. Statistical analysis was performed with the licensed version of SPSS 20.
Results
The demographic data and results of the study were tabulated in Table 1. The subgroups of the study were age matched. The biochemical parameters under evaluation belong to oxidative stress group (MDA, IMA, protein carbonyl and XO) were significantly higher in preeclamptic cases in pre delivery, whereas the same parameters significantly decreased in post-delivery within 48 h. Similarly, antioxidant status representing parameters were Vitamin C, vitamin E, FRAP, and enzyme antioxidants were SOD, GR, GPx, Catalase. Results indicated inverse relation between oxidative stress and antioxidant status in preeclampsia patients. Declined antioxidant power (FRAP) with preeclampsia in pre delivery gradually increased after delivery that represents the possible reversible changes. In the post-delivery condition antioxidant data represents improvement in pregnancy induced hypertension on removal of placenta towards normal.
Statistical significance and correlation analysis between the parameters of the groups studied were depicted in Tables 2, 3, and 4. Data analysis evinced significantly positive correlation between NO versus GPx, FRAP versus MDA, FRAP versus XO and significantly negative correlation observed between IMA versus GPx, GR versus SOD.
ROC curve analysis for significant parameters was presented in Table 5. The data shows the sensitivity specificity and also the superiority of the IMA as a marker when compared to XO, uric acid, MDA and catalse in preeclampsia.
Discussion
Preeclampsia is associated with various etiological factors which implicated in the pathogenesis. Hypoxic risk is a major cause for the development of oxidative stress that affects integrity of trophoblastic tissues which lead to elevated xanthine and hypoxanthine and also uric acid. The association between preeclampsia and high serum uric acid concentration was reported during the beginning of this century. Reduced uric acid clearance observed in preeclamptic women associated with increased rate of reabsorption amounts to hyperuricemia [19].
The present study showed elevated uric acid level before delivery and decreased after delivery in preeclampsia. But in normal pregnants before and after delivery Uric acid level was unaltered. This indicates possible involvement of oxidative stress on placenta by means of contributing xanthine/hypoxanthine and xanthine oxidase activity.
Increased conversion of XDH into xanthine oxidase by oxidative stress further adds to increased production of hydrogen peroxide which in turn affects the trophoblast cell function [6, 20]. Thereby oxidative stress has become one of the causative factor for preeclampsia complications. The present study reported increased xanthine oxidase activity as unique observation with concomitant rise of uric acid in preeclampsia.
Malondialdehyde is a lipid peroxidation marker produced from the peroxidation of polyunsaturated fatty acid was significantly elevated in preeclampsia compared to normotensive pregnant and non-pregnant which is consistent with the previous work [5]. Increased lipid peroxidation products cause peroxidation damage to endothelial membrane which may result in endothelial dysfunction which is associated with reduced nitric oxide [21]. Our study results justifies this observation. The Significant decrease of MDA levels after delivery in normotensive pregnant and preeclampsia indicated down trend of MDA values after placental removal within 48 h.
Protein carbonyl is a stable indicator of protein damage in biological system. Reactive oxygen species oxidizes amino acid residues like glutamate, histidine and tryptophan in proteins to form product with carbonyl group. Protein carbonyl content was significantly increased in preeclampsia (p < 0.001) when compared to non pregnants. However the increase of protein carbonyls was found non-significant between normotensive pregnant and preeclampsia (p > 0.05). The data from normotensive pregnants and preeclampsia in comparison with control group suggests increase of oxidative stress. Even though, protein carbonyl content in after delivery of normotensive pregnants was non-significant, 64 % of protein carbonyls decreased in preeclampsia groups. Results evinced that decreased protein damage maker indicates revocable changes in after delivery with preeclampsia. The present study showed same pattern as reported by Zusterzeel et al. [22].
Superoxide Dismutase converts superoxide to water and it acts as first line of defense against free radical scavenging. SOD activity was significantly decreased in normotensive pregnant when compared to non pregnant. Decreased activity was also noticed in preeclampsia compared to normotensive pregnant. In after delivery, SOD activity was significantly decreased in preeclampsia and normotensive pregnant groups. Studies conducted by Bakacak et al. [23] showed decreased SOD activity may be due to increased Cu/Zn ratio. This altered ratio inactivates Cu/Zn containing antioxidant enzyme superoxide dismutase which may lead to decreased superoxide dismutase.
Catalase is a heme protein catalyzes cleaving of hydrogen peroxide into water and oxygen, thus it protects the cell from oxidative damage. In preeclampsia there was decreased enzyme activity when compared to non-pregnant. Its activity did not show significant difference between before and after delivery in normotensive pregnant and preeclampsia. Elevated xanthine oxidase and declined catalase activity indicated the severity of the oxidative stress in terms of hydrogen peroxide and hydroxyl radical in preeclampsia is evident in our study. Decrease in catalase activity may be due to inhibition of enzyme by hypochlorite and peroxy nitrite free radicals which were more perhaps in preeclampsia [21].
GPx is a selenium dependent enzyme eliminates hydrogen peroxide and organic hydro peroxides. There was non-significantly decreased GPx activity seen in preeclampsia before delivery when compared to healthy control and normotensive pregnant. In after delivery Gpx activity was non significantly decreased in normotensive pregnant and significantly increased in preeclampsia when compared to before delivery. Decreased glutathione peroxidase may lead to increased generation of reactive oxygen species. Decreased selenium level was associated with decreased glutathione peroxidase activity which may be involved in pathophysiology of preeclampsia [24].
Glutathione reductase replenishes cellular reduced glutathione. Decreased activity in normal Pregnants and increased activity in preeclampsia equivalent to healthy control (p < 0.001) observed in our study. In preeclampsia, activity reduced by two fold in post-delivery indicates the role of placenta. Suhail et al. [25] found that non-significant decrease of GR activity in preeclampsia compared to non pregnants. Unlike these reports, significant two fold increase of glutathione reductase is seen in our study during preeclampsia under the study condition compared to normal pregnancy (p < 0.05) group. Attaining GR activity equivalent level to non-pregnant could be an in vivo defensive response to restore the reduced glutathione level during altered oxidative and antioxidant system may be a compensatory mechanism in response to increased oxidative stress.
FRAP was significantly decreased in normal pregnant and preeclampsia before delivery (p < 0.001) when compared to healthy control. FRAP levels were significantly decreased in preeclampsia when compared to normotensive pregnant (p < 0.05) similar with the observations of Zusterzeel et al. [22]. FRAP levels did not show significant difference between before and after delivery in preeclampsia as well as normal pregnant Karacy et al. [5] also observed decreased FRAP level in preeclampsia. Vitamin C levels were significantly decreased in normal pregnant and preeclampsia when compared to healthy control (p < 0.001). But there was no significant difference between before and after delivery in normal pregnant and preeclampsia.
Vitamin E Levels were significantly increased in normal pregnant when compared to healthy control (<0.05) but there was non-significant decrease in preeclampsia when compared to healthy control. Significantly decreased vitamin E level was seen in preeclampsia when compared to normotensive pregnant (<0.001). In normotensive pregnant and preeclampsia there was no significant difference between before and after delivery. Reduction in vitamin C and E were consistent as shown by other studies [26].
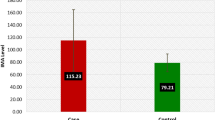
Ischemia modified albumin known as hypoxic risk indicator in various diseases and also in preeclampsia during first trimester [27]. In support of this finding, our study results indicated high IMA in preeclampsia. An observation of decreased maternal serum levels of IMA in post-delivery evidenced clinical improvement. Elevation of MDA and XO activity represents oxidative stress and showed weak relationship with ferric reducing ability of plasma. FRAP is contributed by super oxide dismutase, glutathione peroxidase, catalase, bilirubin, uric acid, reduced glutathione, vitamin E, vitamin C, free cu2+, zn2+, Mn2+, Fe2+, and selenium [28]. Uric acid concentration is known as contributing factor to reducing ability in vitro, but biological significance of uric acid contribution to the antioxidant potential is unclear in vivo [29] and generates research gap. The possible reason for FRAP decline may be due to altered antioxidants in single or in combination.
Positive correlation is also observed between GPx and NO which indicates endothelial dysfunction and proliferates increased formation of super oxide radicals which can inhibit Glutathione peroxidase enzyme [30]. Reduced SOD and glutathione peroxidase activity will cause increase in superoxide anion which reacts with nitric oxide to form peroxy nitrite. This in turn decreases the availability of NO [13].
The negative correlation between IMA and GPx noticed in the study. The study rise the probable illustration that normal pregnancy demands high oxygen requirement and evidence increased oxidative stress. Inadequate supply of oxygen to trophoblastic cells results in preeclampsia. The hypoxia and established oxidative stress alters serum albumin with N-terminal modification into ischemia modified albumin. Therefore in the study context IMA evolved as good predictive marker of preeclampsia related risk. The impact of superoxide radicals has inhibitory effect on enzymes. Hence GPx activity decreased. Vanderlelie et al. [31] found that decreased glutathione reductase gene expression in preeclampsia without significant change in glutathione peroxidase. This indicated possible importance of plasma glutathione and preeclampsia toxemic condition on glutathione reductase. In contrast to other studies we found increase of glutathione reductase in preeclampsia compared to normotensive pregnants. A weak linear correlation was seen between protein carbonyl and MDA in preeclampsia which was not significant.
In ROC curve analysis, area under curve for ischemia modified albumin (0.92), Catalase (0.88), xanthine oxidase (0.8), malondialdehyde (0.804), Uric acid (0.84) with sensitivity of 87.7, 96.5, 61.4, 84.21, 71.93 and specificity of 91.2, 84.2, 89.5, 68.4, 89.5 respectively. Results indicated ischemia modified albumin as a good marker of intrauterine hypoxic reperfusion risk and abnormal placental development by dysfunction of trophoblastic cells under oxidative stress. ROC analysis showed that there is increased production of hydrogen peroxide due to elevated xanthine oxidase activity in preeclampsia and catalase is combating it. Limitations of the study were measurement of IMA and XO level from time of pregnancy to all level of trimesters to understand whether or not gradual increase of IMA levels and XO activity as a marker to denote the number of chances of pregnancy translated into pre-eclampsia. Culturing trophoblastic cells and exposing to free radical stress environment to measure XO activity.
Conclusion
Our research findings generated knowledge about IMA as an intrauterine risk factor and XO as an enzyme oxidant marker. Increased xanthine oxidase activity and uric acid seen in preeclampsia with decreased total antioxidant status. Distinctive observation of elevated catalase activity in pre and post-delivery of preeclampsia within 48 h noticed despite of gradual reduction of oxidative stress. Endothelial dysfunction evidenced by reduction of nitric oxide level in preeclampsia during pre and post-delivery. The inverse relation between XO and NO in our study represents an indication of trophoblastic cell destruction and endothelial dysfunction. Therefore, the over-all study concludes an inverse relation between oxidative stress and antioxidant status in preeclampsia in comparison with normal pregnant and non pregnant suggests restoration of plasma antioxidants level.
References
Sibai BM. Hypertensive disorders of pregnancy: the United States prospective. Curr Opin Obstet Gynecol. 2008;20:102–6.
Betran AP, Wojdyla D, Posner SF, Gülmezoglu AM. National estimates for maternal mortality: an analysis based on the WHO systematic review of maternal mortality and morbidity. BMC Public Health. 2005;5:131.
Kurlak LO, Green A, Loughna P, Broughton Pipkin F. Oxidative stress markers in hypertensive states of pregnancy: preterm and term disease. Front Physiol. 2014. doi:10.3389/fphys.2014.00310.
Bilodeau JF. Review: maternal and placental antioxidant response to preeclampsia—impact on vasoactive eicosanoids. Placenta. 2014;35:S32–8.
Karacay O, SepiciDincel A, Karcaaltincaba D, Sahin D, Yalvaç S, Akyol M, et al. A quantitative evaluation of total antioxidant status andoxidative stress markers in preeclampsia and gestational diabetic patients in 24–36 weeks of gestation. Diabetes Res Clin Pract. 2010;89(3):231–8.
Bambrana V, Dayanand CD, Kotur PP. Is xanthine oxidase, a marker in pre-eclampsia? A case–control study. J Clin Diagn Res. 2015;9(10):BC 1–3.
Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10.
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–78.
Bergmeyer HU, Gawehn K, Grassl M. Glutathione reductase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. New York: Academic Press; 1974. p. 465–6.
Bar Or D, Lau E, Winkler JV. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—a preliminary report. J Emerg Med. 2000;19(4):311–5.
Trivedi RC, Rebar L, Berta E, Stong L. New enzymatic method for serum uric acid at 500 nm. Clin Chem. 1978;24(11):1908–11.
Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem. 1990;36:1440–3.
Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239(1):70–6.
Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21(2):130–2.
Mavis RD, Stellwagen E. Purification and subunit structure of glutathione reductase from bakers’ yeast. J Biol Chem. 1968;243(4):809–14.
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6.
Roe JH. Comparative analyses for ascorbic acid by the 2, 4-dinitrophenylhydrazine method with the coupling reaction at different temperatures: a procedure for determining specificity. J Biol Chem. 1961;236:1611–3.
Martinek RG. Method for the determination of vitamin E (total tocopherols) in serum. Clin Chem. 1964;10:1078–86.
Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol. 1996;174:288–91.
Murata M, Fukushima K, Takao T, Seki H, Takeda S, Wake N. Oxidative stress produced by xanthine oxidase induces apoptosis in human extravillous trophoblast cells. J Reprod Dev. 2013;59(1):7–13.
Kaur G, Mishra S, Sehgal A, Prasad R. Alterations in lipid peroxidation and antioxidant status in pregnancy with preeclampsia. Mol Cell Biochem. 2008;313:37–44.
Zusterzeel PL, Mulder TP, Peters WH, Wiseman SA, Steegers EA. Plasma protein carbonyls in nonpregnant, healthy pregnant and preeclamptic women. Free Radic Res. 2000;33(5):471–6.
Bakacak M, Kılınç M, Serin S, Ercan Ö, Köstü B, Avcı F, et al. Changes in copper, zinc, and malondialdehyde levels and superoxide dismutase activities in pre-eclamptic pregnancies. Med Sci Monit. 2015;21:2414–20.
Khera A, Vanderlelie JJ, Perkins AV. Selenium supplementation protects trophoblast cells from mitochondrial oxidative stress. Placenta. 2013;34(7):594–8.
Suhail M, FaizulSuhail M, Khan H. Role of vitamins C and e in regulating antioxidant and pro-oxidant markers in preeclampsia. J Clin Biochem Nutr. 2008;43(3):210–20.
Ikpen MA, Eigbefoh J, Eifediyi RA, Isabu PA, Okogbenin S, Okogbo FO, et al. Determination of antioxidant status of pre-eclamptic and normotensive sub-rural Nigerian pregnant women at the Irrua Specialist Teaching Hospital, Irrua, Edo State. J Matern Fetal Neonatal Med. 2012;25(10):2046–50.
Papageorghiou AT, Prefumo F, Leslie K, Gaze DC, Collinson PO. ThilaganathanB. Defective endovascular trophoblast invasion in the first trimester is associated with increased maternal serum ischemia-modified albumin. Hum Reprod. 2008;23(4):803–6.
Soto ME, Soria-Castro E, Lans VG, Ontiveros EM, Mejia BI, Hernandez HJ, et al. Analysis of oxidative stress enzymes and structural and functional proteins on human aortic tissue from different aortopathies. Oxid Med Cell Longev. 2014;. doi:10.1155/2014/760694.
Kenet G, Freedman J, Shenkman B, Regina E, Brok-Simoni F, Holzman F, et al. Plasma glutathione peroxidase deficiency and platelet insensitivity to nitric oxide in children with familial stroke. Arterioscler Thromb Vasc Biol. 1999;19(8):2017–23.
Fabbrini E, Serafini M, Colic Baric I, Hazen SL, Klein S. Effect of plasma uric acid on antioxidant capacity, oxidative stress, and insulin sensitivity in obese subjects. Diabetes. 2014;63(3):976–81.
Vanderlelie J, Gude N, Perkins AV. Antioxidant gene expression in preeclamptic placentae: a preliminary investigation. Placenta. 2008;29(6):519–22.
Acknowledgments
We would like to thank the authorities of Sri Devaraj Urs Academy of Higher Education and Research for supporting this doctoral study.
Funding
Self-funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Mrs. Vanishree Bambrana, Dr. C. D. Dayanand and Dr. Pushpa Kotur declare that they have no conflict of interest. Financial support for this work borne by authors.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Research Involving Human Participants and/or Animals
This is non interventional study. Sample collection from human participants under taken after obtaining Institutional Ethical Committee approval. An appropriate individual patient informed consent used for sample collection. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Bambrana, V., Dayanand, C.D. & Kotur, P. Relationship Between Xanthine Oxidase, Ischemia Modified Albumin, Nitric Oxide with Antioxidants in Non Pregnants, Pre and Post-delivery of Normal Pregnants and Preeclampsia. Ind J Clin Biochem 32, 171–178 (2017). https://doi.org/10.1007/s12291-016-0599-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-016-0599-0