Abstract
The present study was intended to appraise the oxidant and antioxidant status in preeclampsia women. Seventy-seven preeclampsia women with severe variety having average B.P. of 170/140 mmHg with proteinuria; 47 preeclampsia women with mild variety having average B.P. of 138/100 mmHg were compared to 56 healthy pregnant women and 15 non-pregnant women for oxidant and antioxidant status. Lipid peroxidation was assessed by measuring malondialdehyde (MDA), and antioxidant status was assessed by measuring antioxidant enzymes N.B.; superoxide dismutase (SOD), glutathione peroxidase, catalase and vitamins viz; A, E, C and reduced glutathione (GSH). Lipid peroxidation was significantly higher in severe preeclampsia women. Antioxidant status was also compromised as is evident from decreased GSH levels and increased SOD activities not only in severe preeclampsia but also in normal pregnancy and mild preeclampsia women compared to non-pregnant women. Decreased antioxidant enzyme activity viz catalase and glutathione peroxidase was observed in pregnancy as compared to non-pregnant women. The levels of vitamin E which act as an antioxidant were significantly elevated in preeclampsia compared to that of normal pregnancy. These findings conclude that initially the oxidative stress due to pregnancy-induced hypertension is critically combated by the intricate defensive mechanism of natural antioxidant system of the body. It appears that this imbalance between oxidant and antioxidant is the effect of disease and not the causative factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia, a hypertensive disorder of pregnancy, is associated with oxidative stress, hyperlipidemia, obesity, hypertension, diabetes, and polycystic ovarian diseases [1–3]. Preeclampsia exists with an incidence ranging between 5 and 10% [4]. In India, the incidence of preeclampsia is reported in 15.2% of total pregnancies [5]. The oxidative metabolism is increased during pregnancy due to increased oxygen demand of mother and fetus, which leads to free oxygen radical formation [6]. There is a physiological balance between the lipid peroxidation and antioxidative processes in normal pregnancies [7]. It has been suggested that placenta is the source of increased unsaturated fatty acid oxidation and lipid peroxidation [8, 9]. Disturbance of this balance leads to modification of polyunsaturated fatty acids (PUFA) which damages the function and structure of capillary endothelial cells [10].
In preeclampsia increased lipid peroxidation has been observed to be a causative factor for its pathogenesis [8, 10]. The defense system against free oxygen radicals consists of exogenic and endogenic antioxidative systems [6, 7]. Antioxidants prevent lipid peroxidation by inactivating free radicals and quenching activated singlet oxygen molecules and dismutates superoxide radicals. Free radicals produced in preeclampsia are expected to increase the utilization of antioxidants [11]. The levels of dietary and cellular antioxidants are abnormally low in women with preeclampsia. The maternal blood levels of vitamin C, vitamin E, vitamin A, and β-carotene are decreased [7, 12, 13], though not all investigators found decreased level of vitamin E in pregnancy [11, 13, 14].
Though the etiology of preeclampsia is unknown, it is believed to be a complex disorder caused by a series of nutritional, environmental, and genetic factors that lead to imbalance between the free radical nitric oxide (NO), superoxide radical (O −2 ), and peroxynitrite in the vascular endothelium [15]. There are conflicting and scattered results on oxidant and antioxidant levels in preeclampsia women. In view of these facts, the present study was planned to estimate the oxidative stress in pregnant women with preeclampsia by measuring the plasma malondialdehyde level and to establish its correlation with the antioxidative defense system by measuring the levels of antioxidant factors like vitamin C, vitamin A, vitamin E, and reduced glutathione along with antioxidant enzymes like superoxide dismutase (SOD), catalase, and glutathione peroxidase in women with preeclampsia.
Material and method
Subjects
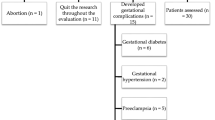
The subjects for this study were categorized into two major groups: fifty-six women with normal pregnancy had average blood pressure range of 120/80 mmHg and seventy-seven women fulfilling the criteria for preeclampsia with severe variety having an average blood pressure of 170/114 mmHg and with proteinuria ≥2+ dipstick and/or fulfilling other clinical or investigative criteria for severe preeclampsia. Two additional groups were also included: one is a group of 47 preeclampsia women with mild variety having an average blood pressure of 145/100 mm Hg and proteinuria ≥1+ dipstick and a group of fifteen normal non-pregnant women as a control group (Table 1). The groups were matched for age and period of gestation (POG). Preeclampsia is best described as a pregnancy-specific syndrome of reduced organ perfusion secondary to vasospasm and endothelial activation, in addition proteinuria is an important sign of preeclampsia. The minimum criteria for preeclampsia is BP ≥140/90 mmHg after 20 weeks of POG with proteinuria ≥1+ dipstick, while the increased certainty of preeclampsia is BP ≥140/90 mmHg after 20 weeks of POG with proteinuria ≥2+ dipstick condition and/or other significant criteria [16]. Written informed consents were taken from all subjects enrolled in this study. Five milliliters of heparinized blood were collected for the assessment of level of malondialdehyde, lipid peroxidation product, an indicator of oxidative stress; antioxidant enzymes like SOD, catalase, glutathione peroxidase, and antioxidants like reduced glutathione, vitamin A, E, and C were measured.
Exclusion criteria
Pregnant women with history of chronic hypertension, diabetes, renal diseases or any other collagen diseases were excluded.
Ethical clearance
Institutional ethical committee has approved the research plan.
Reagents
The reagents used for the study are of analytical grade from commercially available company and HPLC grade chemicals were obtained for HPLC-based experiments.
Assessment of oxidative stress and antioxidant status
Plasma and erythrocytes were separated by the procedure as previously described [17]. Antioxidant enzymes were measured in plasma and RBC using the following protocols.
Measurement of malondialdehyde level as predictor of oxidative stress
The plasma malondialdehyde (MDA) concentration was determined according to the method of Ohkawa et al. [18]. MDA, the lipid peroxidation product reacts with thiobarbituric acid to give a pink chromophore, which was measured spectrophotometrically at 535 nm and expressed as μmole/mg proteins.
Measurement of superoxide dismutase activity
Plasma SOD activity from different groups was measured by the modified method of Nishikimi et al. [19]. This procedure is based on the reduction of nitro blue tetrazolium (NBT) which is mediated by auto-oxidation of hydroxylamine hydrochloride under aerobic conditions. Auto-oxidation of hydroxylamine hydrochloride leads to production of superoxide radicals. The superoxide O −2 anion reduces NBT forming blue formazan which is indicated by increase in absorbance at 560 nm and SOD dismutates the superoxide radicals which lead to inhibition of NBT reduction. This inhibition is directly proportional to the concentration of SOD. Activity of SOD is expressed in units of enzyme/mg protein, where one unit is defined as the amount of SOD required to inhibit the rate of reduction of nitro blue tetrazolium by 50%.
Measurement of catalase activity
The catalase activity in erythrocytes was measured by the method as described previously by Prasad et al. [20]. Catalase catalyzes the breakdown of hydrogen peroxide (H2O2) which was measured spectrophotometrically at 240 nm and is expressed in μmol/min/g Hb.
Measurement of glutathione peroxidase (G-Px) activity
The glutathione peroxidase activity was assayed as per the manufacturer’s instruction provided with RANDOX Ransel kit and the activity of GSH-Px was expressed in units/g Hb.
Measurement of reduced glutathione (GSH)
Total glutathione in RBC was determined according to the procedure described by Beutler et al. [21] that allowed a recovery of GSH >90% and that had no appreciable interference with other thiols present in the plasma or in the reaction mixture. The total GSH content in whole blood was determined. The protein-free filtrate obtained after precipitating with metaphosphoric acid was used to react with DTNB (5,5′-dithiobis-nitrobenzoic acid), which produce a relatively stable yellow-colored solution in proportion to concentration of GSH in the sample, which was measured spectrophotometrically at 412 nm and provides an accurate estimation of GSH in the sample and the values are expressed in mg/g Hb.
Measurement of ascorbic acid
Ascorbic acid level in plasma was measured by the method described by Frei et al. [22]. In this procedure, the acid reacts with 2,4-dinitrophenyl hydrazine. Vitamin C is then oxidized to dehydro ascorbic acid by cupric sulfate. The dehydro ascorbic acid in a strong acidic solution reacts with 2,4-dinitrophenyl hydrazine to form dinitrophenyl hydrazone. The hydrazone in the presence of strong sulfuric acid solution develops yellowish-orange color which is measured spectrophotometrically at 520 nm and the concentration of vitamin C is expressed in mg% in plasma.
Measurement of vitamin A and vitamin E
The levels of vitamin A and E were measured by high pressure liquid chromatography (Waters 2487) using dual wavelength absorbance detector on Spherisorb C-8 column (4.6 mm × 250 mm × 5 μm). Five hundred microliters plasma sample was deproteinized with 500 μl ethanol and mixed properly for 5 min, then 500 μl n-hexane was added and mixed thoroughly for 5 min and centrifuged at 12,000 rpm for 10 min. The organic layer was extracted and the n-Hexane washing process was repeated for three times, and organic layer was collected in the same vial and evaporated to dryness under nitrogen. The dried extract was dissolved in 500 μl of methanol and kept at 4°C for 15 min and centrifuged, and the supernatant was filtered through 0.45 μm syringe filter. HPLC was standardized and calibrated with the standards of Retinol acetate and α-tocopherol of HPLC grade. Following liquid extraction from human plasma samples, these vitamins were successfully separated on the C-8 column using a mobile phase methanol: acetonitrile: n-hexane at a proportion of 46:46:8 (v/v/v) with a flow rate of 1.5 ml/min and detected at 280 and 265 nm. Twenty microliters of standard of different concentrations were injected for preparation of standard curve and 20 μl of prepared samples then injected and quantified on the basis of standard curve. The concentrations of vitamins were expressed in μg/ml.
Statistical analysis
Data of all the groups were statistically analyzed by applying one-way ANOVA and unpaired student’s t-test using the SPSS software for significance of difference among the groups. The unpaired t-test was applied between the groups; a: Group-1 and Group-2; b: Group-1 and Group-3; c: Group-1 and Group-4; d: Group-2 and Group-3; e: Group-2 and Group-4; and f: Group-3 and Group-4. The correlation coefficient was established between the blood pressure and the malondialdehyde concentration, indicator of oxidative stress, by using Pearson’s Correlation of SPSS software. All data were expressed as mean ± standard deviation. P values less than 0.05 and/or 0.01 were considered significant.
Results
The data on oxidative stress, antioxidant enzymes, and antioxidants were analyzed in different groups of subject viz. non-pregnant women (Group-1), normal pregnant women (Group-2), preeclampsia women with mild variety (Group-3), and preeclampsia women with severe variety (Group-4).
The level of MDA, which represents the oxidative stress, was significantly higher in normal pregnant women (P < 0.05), mild preeclampsia (P < 0.01), and severe preeclampsia (P < 0.01) women as compared to non-pregnant women. There was significantly higher level of MDA noticed in severe preeclampsia women to that of normal pregnant women (Fig. 1A). Surprisingly, SOD activity was markedly increased in normal pregnant women, mild preeclampsia, and severe preeclampsia women in comparison to non-pregnant women. However, significant reduction of SOD activity was observed in severe preeclampsia and mild preeclampsia women as compared to normal pregnant women (Fig. 2A) (P < 0.05). Significant reduction in the activity of catalase is observed during pregnancy than that of non-pregnant women (P < 0.05) as shown in Fig. 2B. The catalase activity was further reduced significantly in mild preeclampsia and severe preeclampsia women as compared to normal pregnant women (P < 0.05). The data on glutathione peroxidase activity revealed that there was slightly reduced activity in severe preeclampsia and mild preeclampsia women than that of normal pregnant women, although this difference is not statistically significant (Fig. 2C). Total blood GSH levels as represented in Fig. 1B were found to be significantly higher in non-pregnant women as compared to normal pregnant women, women with severe preeclampsia, and mild preeclampsia, whilst there was no significant difference in the level of GSH among the normal pregnant women, women with severe preeclampsia, and mild preeclampsia. Data on vitamin A, E and C were analyzed in different groups which revealed that the levels of vitamin C were not significantly different between women with severe preeclampsia and normal pregnant women. Surprisingly, the level of vitamin C was significantly higher (P < 0.01) during pregnancy (Fig. 3A). However no significant difference was observed among normal pregnant women, mild preeclampsia, and severe preeclampsia women. On the other hand, there was marked reduction in vitamin E levels in normal pregnant women compared to non-pregnant women. However, vitamin E levels were increased in mild preeclampsia and severe preeclampsia as compared to normal pregnant women. Significant increase in level of vitamin E was observed in women with mild preeclampsia and severe preeclampsia compared to the normal pregnant women as represented in Fig. 3B (P < 0.01). Significant increase in the level of vitamin A in normal pregnant women and mild preeclampsia women as compared to non-pregnant women, although vitamin A levels were reduced in severe preeclampsia women as compared to normal pregnant women (Fig. 3C). A positive correlation at 0.05 level has been observed between systolic blood pressure and MDA. Figure 4A and B shows the correlation between the systolic B.P. versus MDA and diastolic B.P. versus MDA in preeclampsia women, respectively.
Lipid peroxidation and reduced glutathione levels in preeclampsia women compared to control. (A) Malondialdehyde (MDA; product of lipid peroxidation). (B) Reduced glutathione (GSH). * P < 0.01, ** P < 0.05 for unpaired students t-test among a: Group-1 and Group-2; b: Group-1 and Group-3; c: Group-1 and Group-4; d: Group-2 and Group-3; e: Group-2 and Group-4 and f: Group-3 and Group-4
Antioxidant enzyme activity in women with preeclampsia compared to control. (A) Superoxide dismutase (SOD). (B) Catalase. (C) Glutathione peroxidase (GSH-Px). * P < 0.01, ** P < 0.05 for unpaired students t-test among a: Group-1 and Group-2; b: Group-1 and Group-3; c: Group-1 and Group-4; d: Group-2 and Group-3; e: Group-2 and Group-4; and f: Group-3 and Group-4
Plasma vitamin levels in women with preeclampsia compared to control. (A) Vitamin C. (B) Vitamin E. (C) Vitamin A. * P < 0.01, ** P < 0.05 for unpaired students t-test among a: Group-1 and Group-2; b: Group-1 and Group-3; c: Group-1 and Group-4; d: Group-2 and Group-3; e: Group-2 and Group-4; and f: Group-3 and Group-4
Discussion
Preeclampsia remains one of the major causes of maternal and perinatal mortality and morbidity, while the precise etiology remains elusive. Increased numbers of recent reports indicates that maternal plasma and placental lipid peroxides are responsible for damage and dysfunction of the vessels’ endothelium as seen in pregnancy-induced hypertension [23, 24]. Consistent with previous reports, we also found a significant increase in plasma levels of lipid peroxides in women with severe pregnancy-induced hypertension. We also found a positive correlation between systolic blood pressure and diastolic blood pressure with plasma malondialdehyde levels. It is well known that free radicals initiate lipid peroxidation by attacking PUFAs in cell membrane. Peroxidation of fatty acids and cholesterol associated with the cell membrane may alter its fluidity and permeability and thus, damage the membrane [25]. It is possible that increased production of lipid peroxides in severe pregnancy-induced hypertensive women may cause vascular endothelial cell dysfunction. Plasma lipid peroxidation products known as malondialdehyde have been reported to be increased in preeclampsia compared with normal pregnancy [26, 27]. In vitro studies have shown that calcium potentiates the peroxidation of erythrocyte membrane lipids and the lipid loss can be prevented by lipid antioxidants or EGTA [28]. However, hypocalcemia and hypocalciurea were observed in preeclampsia women [29, 30]. Henceforth, there is an evident link between calcium intake, preeclampsia, and calcium status in pregnant women with a view to provide calcium supplementation during pregnancy. Malondialdehyde can form either as a product of lipid peroxidation or as by-product of platelet cycloxygenase turnover, resulting in production of vasoconstrictive eicosanoid known as thromboxane. Serum levels of thromboxane are increased in normal pregnancy, but are elevated to an even greater extent in women with severe preeclampsia [31].
The increase in oxidative stress might not be so important if there was a compensatory increase in antioxidant protection but the opposite occurs. In present study, there was a marked reduction in antioxidant viz reduced glutathione and glutathione peroxidase activity. Glutathione is a major intracellular antioxidant. Red blood cells contain high concentrations of reduced glutathione accounting for almost 98% of total blood content. In addition to their detoxifying function of conjugating with noxious compounds, glutathione and other thiols maintain the redox balance of cells, thereby preventing oxidative damage [32].
In the present study elevated levels of SOD activity in pregnancy could be the result of adaptation to combat oxidative stress during pregnancy. The observed reduction in SOD activity in severe preeclampsia and mild preeclampsia women to that of normal pregnant women could be associated with deleterious effect of hypertension. The deficiency of SOD activity in women with severe preeclampsia may be of particular importance, as the superoxide anion that is being generated continuously by numerous sources throughout the body would not be inactivated effectively and lead to an increase in its concentrations. The increased concentrations of superoxide in conjunction with the increased concentrations of iron would result in greater oxidative stress and lipid peroxidation [33]. The other important aspect of a deficiency in SOD activity is its interaction with NO, a potent vasodilator. Nitric oxide and SOD compete for superoxide. When there is a deficiency in the activity of SOD, NO will react with superoxide to form peroxynitrite a strong oxidizing agent capable of initiating lipid peroxidation [33]. In contrast, catalase activity in blood was not significantly altered among preeclampsia groups, while it was significantly higher in non-pregnant women as compared to preeclampsia women (Fig. 2B). This finding is suggestive of the fact that neutralization of free radicals by catalase in pregnancy is hampered.
Plasma free radicals are buffered by the available extra cellular antioxidants and large number of antioxidants both intracellularly and in the extracellular fluids [34, 35]. In the present study vitamin E levels which act as antioxidant were significantly elevated in pregnancy-induced hypertension as compared to that of normotensive pregnant women (Fig. 3B). Even plasma vitamin C levels were found higher in pregnant women compared to non-pregnant women (Fig. 3A). Albeit, there have been conflicting reports in vitamin E and vitamin C concentration in preeclampsia. Mikhail and Jendryczko reported reduced vitamin C concentrations in pregnancy with a further fall in concentration in preeclampsia [35, 36]. In addition they noted a fall of vitamin E concentration in preeclampsia; however, vitamin C and E concentrations were elevated in patients with mild preeclampsia [14, 37]. It has been shown that vitamin E concentrations increase in normal pregnancy and this rise are fraternized with increase in cholesterol and lipoprotein concentrations seen in pregnancy [11]. Most of the studies conducted have supplemented vitamin E and vitamin C in combination to increased level of antioxidant in pregnancy [38, 39] and showed that supplementation with vitamin C and vitamin E during second trimester does not reduce the risk for preeclampsia.
Interpretation of the plasma oxidant and antioxidant in preeclampsia is the relationship of cause and effect. In the mild preeclampsia, the oxidants’ load entering the circulation was buffered by plasma antioxidants [40]. In moderate overspill, the plasma lipid peroxides and lipid peroxidation remains unchanged, but antioxidants are consumed. In severe preeclampsia conversely, in the present study, plasma vitamin E levels were significantly elevated in severe preeclampsia women. The two possible explanations could be either increased dietary intake or significantly reduced glutathione in sparing vitamin E.
Taken together present study suggests that pregnancy-induced hypertension increases oxidative stress through potential free radical damage. Further, it is observed that raised MDA levels activate intricate defense mechanism to combat oxidative stress due to hypertension through endogenous antioxidant system of the body. There is definite correlation between oxidative stress with initiation and progression of disease process; however, cause and effect relation needs further evaluation. In view of the additive relationship of vitamin C and vitamin E, supplementation of vitamin C would be more appropriate, as significant increase in vitamin E concentration is observed in severe preeclampsia. Further studies are warranted to investigate the use of Vitamin C in severe preeclampsia women and evaluation of genetic variation predisposing certain individual to the natural oxidant-antioxidant imbalance even under stress of pregnancy leading to this morbid condition.
References
Walsh SW (1998) Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol 16:93–104
Gratacos E, Casals E, Sanllehy C, Cararach V, Alonso PL, Fortuny A (1996) Variation in lipid levels during pregnancy in women with different types of hypertension. Acta Obstet Gynecol Scand 75:896–901
Ness RB, Roberts JM (1996) Heterogeneous causes constituting the single syndrome of preeclampsia: A hypothesis and its implications. Am J Obstet Gynecol 175:1365–1370
Sibai BM (1992) Hypertension in Pregnancy. Obstet Gynecol Clin North Am 19:615–632
Dutta DC (1995) Text book of obstetrics, 3rd edn. New Central Book Agency (P) Ltd., Calcutta, pp 230–236
Wisdom SJ, Wilson R, McKillop JH, Walker JJ (1991) Antioxidant system in normal pregnancy and pregnancy induced hypertension. Am J Obstet Gynecol 165:1701–1704
Wang Y, Walsh SW, Gu J, Zhang J (1991) The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol 165:1695–1700
Hubel CA, Roberts JM, Taylor RN, Masci TJ, Rodgers GN, McLaughlin MK (1989) Lipid peroxidation in pregnancy: New perspectives on preeclampsia. Am J Obstet Gynecol 161:1025–1034
Ohel G, Kisselevitz R, Margalioth E, Schenker J (1985) Ascorbate dependant lipid peroxidation in human placenta and fetal membrane. Gynecol Obstet Invest 19:73–77
Roberts JM, Taylor RM, Goldfein A (1991) Clinical and biochemical evidence of endothelial cells dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens 4:700–708
Kwasniewska A, Tukendorf A, Semczuk M (1998) Serum antioxidant concentration in pregnancy induced hypertension. Clin Invest Med Sci Monit 4:448–452
Mikhail MS, Anyaegbunam A, Garfinkel D, Palan PR, Basu J, Romney SL (1994) Preeclampsia and antioxidant nutrients: decreased plasma levels of reduced ascorbic acid, α-tocopherol, and β-carotene in women with preeclampsia. Am J Obstet Gynecol 171:150–157
Hubel CA, Kagan VE, Kisin ER, McLaughlin MK, Roberts JM (1997) Increased ascorbate radical formation and ascorbate depletion in plasma from women with preeclampsia: implications for oxidative stress. Free Radic Biol Med 23:597–609
Schiff E, Friedman SA, Stampfer M, Kao L, Barrell PH, Sibai BM (1996) Dietary consumption and plasma concentrations of vitamin E in pregnancies complicated by preeclampsia. Am J Obstet Gynecol 175:1024–1028
Lopez-Jaramillo P (2000) Calcium, nitric oxide and preeclampsia. Semin Perinatol 24:33–36
Cunningham FG, Gant NF, Leveno KJ, Gilstrap LC, Hauth JC, Wenstrom KD (2005) Hypertensive disorders in pregnancy. Williams obstetrics, 22nd edn. Mc Graw-Hill, USA, pp 761
Prasad R, Mond R, Jain S, Kaur G, Chugh K S (1996) Modulation of ouabain sensitive sodium-potassium pump of erythrocyte from patients with chronic renal failure: role of acute hemodialysis Biochem Mol Biol Int 40:1087–1094
Ohkawa H, Ohisi N, Yagi N (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun 46:849–854
Prasad R, Kaur G, Nath R, Walia BNS (1996) Molecular basis of pathophysiology of Indian Childhood Cirrhosis: role of nuclear copper accumulation. Mol Cell Biochem 156:25–30
Beutler E, Durion O, Kelly B (1963) Improved method for the detection of blood glutathione. J Lab Clin Med 61:882–888
Frei B, England I, Ames BN (1989) Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci USA 86:6377–6381
Mutlu-Turkoglu U, Ademoglu E, Ibrahimoglu L, Aykec-Toker G, Uysal M (1998) Imbalance between lipid peroxidation and antioxidant status in pre-eclampsia. Gynecol Obstet Invest 46:37–40
Madazli R, Budak E, Calay Z, Aksu MF (2000) Correlation between placental bed biopsy findings, vascular cell adhesion molecule (VCAM-1) and fibronectin levels in pre-eclampsia. Br J Obstet Gynaecol 107:514–518
Ledwozyw A, Michalak J, Stephen A, Kadziolka A (1986) The relationship between plasma triglycerides, total lipids and lipid peroxidation products during human atherosclerosis. Clin Chim Acta 155:275–284
Maseki M, Nishigaki I, Hagihara M, Tomoda Y, Yagi K (1981) Lipid peroxide levels and lipid serum content of serum lipoprotein fractions of pregnant subjects with and without pre-eclampsia. Clin Chim Acta 115:155–161
Jain SK, Wise R (1995) Relationship between elevated lipid peroxides, vitamin E deficiency and hypertension in preeclampsia. Mol Cell Biochem 151:33–38
Jain SK, Shohet SB (1981) Calcium potentiates the peroxidation of erythrocyte membrane lipids. Biochim Biophys Acta 642(1):46–54
Malas NO, Shurideh ZM (2001) Does serum calcium in pre-eclampsia and normal pregnancy differ? Saudi Med J 22(10):868–871
Singh HJ, Mohammad NH, Nila A (1999) Serum calcium and parathormone during normal pregnancy in Malay women. J Matern Fetal Med 8(3):95–100
Fitzgerald DJ, Rocki W, Murray R, Mayo G, Fitzgerald GA (1990) Thromboxane A2 synthesis in pregnancy induced hypertension. Lancet 335:751–754
Raijmakers MTA, Zusterzeel PLM, Roes EM, Steegers EAP, Mulder TPJ, Peters WHM (2001) Oxidized and free whole blood thiols in preeclamptic pregnancies. Obstet Gynecol 97:272–276
Radi R, Beckman JS, Bush KM, Freeman BA (1991) Peroxynitrite-induced membrane lipid peroxidation: the cyto-toxic potential of superoxide and nitric oxide. Arch Biochem Biophys 288:481–487
Di Mascio P, Murphy ME, Sies H (1991) Antioxidant defense system: the role of carotenoids, tocopherols and thiols. Am J Clin Nutr 53(Suppl 1):194S–200S
Mikhail MS, Anyaegbunam A, Garfinkel D, Palan PR, Basu J, Romney SL (1994) Pre-eclampsia and antioxidant nutrients: decreased plasma levels of reduced ascorbic acid, α-tocopherol and β-carotene in women with pre-eclampsia. Am J Obstet Gynecol 171:150–157
Jendryczko A, Tomala J (1995) The total free radical trapping ability of blood plasma in eclampsia. Zentralbl Gynakol 117:126–129
Uotila JT, Tuimala RJ, Aarnio TM, Pyykko KA, Ahotupa MO (1993) Findings on lipid peroxidation and antioxidant function in hypertensive complications of pregnancy. Br J Obstet Gynaecol 100:270–276
Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH (2006) Vitamin C and Vitamin E in pregnant women at risk for preeclampsia (VIP trial): randomized placebo controlled trial. Lancet 367:1145–1154
Rumbold AR, Crowther CA, Haslam RR, Dekker GA, Robinson JS (2006) Vitamins C and E and the risk of preeclampsia and perinatal complication. N Engl J Med 354:1796–1806
Zhao S (1993) The relation between the levels of serum lipid peroxide, superoxide dismutase and atrial natriuretic peptide in placenta, umbilical cord vein and intrauterine retardation in pregnancy induced hypertension. Zhonghua Fu Chan Ke Za Zhi 28:278–280, 314
Acknowledgments
Indian Council of Medical Research, New Delhi, vide Letter No. 5/7/50/03-RHN, Dated 18.08.06 with the concurrence of Finance Section, ICMR, vide RFC No. RHN/Ad-hoc/10/2003-04, Dated 25.11.03 for project entitled “Assessment of Antioxidant Status in Pregnancy-Induced Hypertension.”
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaur, G., Mishra, S., Sehgal, A. et al. Alterations in lipid peroxidation and antioxidant status in pregnancy with preeclampsia. Mol Cell Biochem 313, 37–44 (2008). https://doi.org/10.1007/s11010-008-9739-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-008-9739-z